Plasma Viral Load of HIV-1 in Microbial Infection and Antimicrobial Resistance among Heterosexual Serodiscordant Couples in Nigeria
Article Information
Otuonye MN1, Luo Ma2, Chinweokwu C3, Aniedobe MN4, Okoye RN4, Enya VNV5, Ogbonna FN4, Uwandu M6, Adedeji A7, Ponmark J6, Nduaga S4 Akindele SK4, Liboro GO6, Ayoola JB1, Adewole EO6, Adesesan AA8, Ojetunde MM1, Musa AZ7 and Oduukwe NN9
1Central Research Laboratory, Nigerian Institute of Medical Research, Yaba, Lagos
2National Microbiology Laboratory Winnipeg, Manitoba, Canada
3Immunology Department, Nnamdi Azikiwe University Nnewi Campus
4Clinical Diagnostic Department, Nigerian Institute of Medical Research, Yaba, Lagos
5Biochemistry and Nutrition department, Nigerian Institute of Medical Research, Yaba
6Centre for Human Virology and Genomics, Nigerian Institute of Medical Research, Yaba, Lagos
7Monitoring and Evaluation Unit, Nigerian Institute of Medical Research, Yaba, Lagos
8Centre for TB and Research, Nigerian Institute of Medical Research, Yaba, Lagos
9Clinical Science Department Nigerian Institute of Medical Research, Yaba
*Corresponding author: Ngozi Mirabel Otuonye, Central Research Laboratory, Nigerian Institute of Medical Research, PMB 2013, Yaba, Lagos Nigeria
Received: 19 March 2022; Accepted: 28 March 2022; Published: 10 May 2022
Citation: Otuonye MN, Luo Ma, Chinweokwu C, Aniedobe MN, Okoye RN, Enya VN, Ogbonna FN, Uwandu M, Adedeji A, Ponmark J, Nduaga S, Akindele SK, Liboro GO, Ayoola JB, Adewole EO, Adesesan AA, Ojetunde MM, Musa AZ and Oduukwe NN. Role of Microbial Infections and Antibiogram in Serodiscordant Couples. Fortune Journal of Health Sciences 5 (2022): 254-270
View / Download Pdf Share at FacebookAbstract
Background: This study investigated microbial infections, antimicrobial susceptibility and resistance associated with Heterosexual HIV-1 transmission among serodiscordant couples in Nigeria. Methods: A total of 271 serodiscordant and concordant couples (HIV positive and negative) were enrolled, blood samples were collected from the subjects by venipuncture. Plasma viral load, CD4+ analysis were done on the blood samples collected from the participants. Endocervical/urethral swabs and early morning urine samples were collected from participants who presented with Pelvic Inflammatory Diseases, profuse vaginal discharge (greenish and grey colour), itching, and painful urination. By standard microbiological methods, samples were screened by microscopy, culture, antibiogram, and biochemical tests with a view to identify microbial agents of co-infections with HIV.
Results: The Participants age ranged from ≥ 21- < 50years. The index whose plasma viral loads were 10,001-100,000 copies/ml had microbial infections of 32(60.9% p=0.059). Staphylococcus aureus and Escherichia coli (22.1%) were isolated from the index (HIV positive subject) while 14.5% of Staphylococcus aureus and 27.2% of E coli were isolated from their partners (HIV negative). Staphylococcus aureus from the index showed more sensitivity to Amoxillin/clavulanate (95.4%/90.4%) compared to the partners (55.1%/73.5%) and more resistant to Ceftazidime (81.4%) compared to the partners (69.8%).
Conclusion: The microbial infections isolated from the index were associated with high viral loads and are independent makers to HIV-1 transmission among serodiscordant couples. Amoxillin/clavulanic acid showed more sensitivity to S. aureus and more resistance to Cephalosporin antibiotic among the index addressing the impact of antibiotic-sensitivity and resistance in microbial infections on clinical outcomes.
Keywords
HIV-1, Microbial infections, Antimicrobial resistance, Plasma viral load, Heterosexual Serodiscordant couples and Nigeria
HIV-1 articles, Microbial infections articles, Antimicrobial resistance articles, Plasma viral load articles, Heterosexual Serodiscordant couples and Nigeria articles
Article Details
1. Introduction
About 36.7 million people globally are currently living with HIV infection [1, 2].. Nigeria has the 2nd largest HIV epidemic in the world with prevalence of 3.1% and has the highest new infection rates in sub Saharan Africa [3]. HIV is a public health problem; over 65% of the world’s HIV infection is found in sub Saharan Africa with heterosexual exposure as the primary mode of HIV transmission [4]. Approximately 80 percent of HIV infections in Nigeria are as a result of heterosexual sex4. However, many couples do not know their HIV status and so; this has given room to most heterosexual HIV-1 transmission among serodiscordant couples [3, 4]. Again, an estimated 70% HIV -1 transmission occur between married partners, making cohabiting African couples the largest HIV risk group [5,6].
Factors contributing to this includes: Lack of knowledge about sexual health, non to inconsistent condom use, untreated ulcerative and non-ulcerative sexually transmitted diseases, reproductive tract infections, non-disclosure of partner HIV status, multiple sexual partners and vaginal washing [3, 7, and 8]. However, gender inequality among women has been identified as a strong factor to HIV epidemic in African women [8].
Vaginal infections which are caused by bacterial vaginosis (BV), bacterial pathogens (BP) and yeast infections (YI) are common among HIV-infected men and women especially during the reproductive age. This may be a marker for increased transmissibility to sexual partners, infants at delivery, significant morbidity, underscoring their importance from a public health perspective. Severe co- infections have been reported in serodiscordant couples. In a recent study, 564 patients presented to the Nigerian Institute of Medical Research (NIMR) HIV clinic with symptoms of lower waist pain, pelvic inflammatory diseases (PID), painful urination, vaginal/ urethral itching, discharge, certain foul-smelling fluid from the vagina and/or a balloon-like substance from the vagina.
Results presented showed fifty-five bacterial isolates co-infected with Candida species, while 5 Trichomonas vaginalis co-infected with candida species, 23 bacterial vaginosis (BV) co-infected with other bacterial pathogens [8]. About 10 patients had triple infection of BV, yeast and bacterial pathogens. Co-existence of reproductive tract infections (RTIs) was associated with increased HIV transmission through heterosexual contact (p<0.002) [8]. Though, traditionally, reproductive tract infections have been viewed as a lesser public health importance. In another study on lower genital tract infections in HIV-seropositive women in India, the laboratory findings showed high prevalence of BV (30%), mixed infection (30%), and candidiasis (10%) among HIV-seropositive women (P < 0.001) [9].
Another study in India identified sexually transmitted infections in 57% of HIV positive women compared to 34% of HIV negative women (p=0.0037). Vaginal candidiasis was the most common infection followed by Trichomonas vaginalis. Human papilloma virus infection was seen in nine HIV positive women and none was seen in HIV negative women [10]. Symptomatic vaginal yeast infections are increased in HIV-infected women with low CD4 cell count if they are not on antifungal prophylaxis. Significantly, vaginal infections may cause significant morbidity, especially among HIV-infected women, and may contribute to increased risk of HIV transmission among sexual partners. Serodiscordant couples involved in oral sexual practices can be of high risk in HIV-1 transmission if there are open sores in the mouth.
The sexual practices with the highest risks are those that cause mucosal trauma, during intercourse while anal-receptive intercourse poses the highest risk. Mucous membrane inflammation facilitates HIV transmission. Sexually Transmitted Diseases, such as gonorrhea, chlamydial infection, trichomoniasis, cause ulceration. Chancroid, herpes, and syphilis, increase HIV risk acquisition in several folds. In heterosexuals, the estimated risk per coital act is about 1/1000 [11]. However, the risk is increased in early and advanced stages of HIV infection when HIV concentrations in plasma and genital fluids are higher, in younger people with ulcerative genital diseases [12].
The risk of HIV acquisition in a single sexual exposure is estimated to be very low [13]. However, the key factor in determining the viral load burden is the exposing dose of the virus from an Index to the partner [14]. A study conducted in Uganda on heterosexual HIV serodiscordant couples showed a direct relationship between the risk of HIV acquisition and donor plasma viral load (pVL) [15]. No HIV transmissions were observed when the pVL was below a critical threshold of 1500 copies HIV RNA/ml [15]. In this study, we therefore;
- Investigated the microbial infections and plasma viral load (HIV-1 RNA) in association with HIV-1 transmission among serodiscordant couples
- Identified the antibiotic susceptibility and resistance patterns of the microbial infections predominant in serodiscordant couples
2. Subjects, Materials and Methods
2.1 Recruitment of study population
The study population comprised of: Serodiscordant couples (Index and Partner). The positive partner may or may not be on antiretroviral drugs (ART). Concordant couples were also recruited: in this case, the couple can be concordant HIV positive and or concordant HIV negative. These couples were registered with the Nigerian Institute of Medical Research (NIMR) HIV clinic Yaba, Lagos and Nnamdi Azikiwe University Teaching Hospital Nnewi Anambra State (NAUTH) HIV clinics. Other clients were recruited from NIMR HCT Unit (HIV Counseling and Testing Unit). HIV negative couples were recruited among NIMR Workers Couples (NIMRWC) and Divine Grace Evangelical Church Couple (DGECC) for control measures. Only couples who gave informed consent and were recruited.
2.2 Study design
This is a cross sectional study design compromising of participants living with HIV/AIDS and their HIV negative partners (serodiscordant couples), concordant HIV positive couples and concordant HIV negative couples (control) with previous or current history of cervicitis, pelvic inflammatory disease (PID), painful urination, itching and foul-smelling vaginal and urethral infections were enrolled for the study.
2.3 Study Sites
Samples were collected and processed in two government-owned health institutions namely: Nigerian Institute of Medical Research Yaba, Lagos State and Nnamdi Azikiwe University Teaching Hospital Nnewi, Anambra State.
2.4 Sample size Calculation
The prevalence of Serodiscordant couple is in Nigerian is 7.7% [16]. Therefore, the formula N = z2pq/d2 for a cross sectional study was used. It was multiplied by the design effect of 1.5 and attrition of 10%. Total sample size was approximately 271.
2.5 Inclusion and Exclusion Criteria
The participants included in this study were: (i) Couples that have discordant HIV test result (ii) Couples that have concordant HIV results (iii) Couples who have co-habited for at least 3 months and or may have more than one partner (iv) The women are aged between 20-60 and the men 20- 65 years (v) The HIV positive partners can be or not be on ARV (vi) Willingness to participate and given informed consent Also excluded were (i) Those over/or under the required age group (ii) Those, who have not co-habited for three months (iii) Those who were not willing to participate and give informed consent.
2.6 Ethical Approval: Approval was obtained from NIMR IRB (IRB/12/176) and NAUTH Ethical Committee (CS/66/7/79).
2.7 Administration of Structured Questionnaire using In-depth Interview (IDI) Procedures and face to face interviews
Structured questionnaires were administered to a total of 271 consenting sero-discordant, concordant HIV positive and HIV negative couples. Information on socio-demographic characteristics, knowledge of partners’ HIV status, knowledge of STIs, laboratory tests and treatment status, sexual behaviours and practices, were required for the completion of the questionnaire. This procedure was done during couples counseling and testing.
2.8 Sample collection methods
A total of 476 blood samples, 123 endocervical swabs, 11 urethral swabs, 109 urine samples were collected from 236 couples from Nigerian Institute of Medical Research. Seventy (70) blood samples, 28 urine samples and 3 endocervical swabs were also collected from 35 couples from Nnamdi Azikiwe University Teaching Hospital Nnewi Anambra State. Endocervical swabs and early morning urine samples were obtained from the participants who presented with: vaginal discharge, pelvic inflammatory diseases (PID) itching, painful urination and foul smelling vaginal discharge. The swabs and the urine samples were cultured using standard microbiological methods.
2.9 Isolation and Identification of Microbial Infections
2.9.1 Culture
Endocervical/urethral swabs were cultured on chocolate and blood agar under (10% CO2), and Sabouraud Dextrose agar plates and placed at 37 0C for 24 hours. Additionally, urine samples were cultured on MacConkey, CLED and blood agar plates, placed at 370C for 24 hours, using standard microbiological techniques. Characterization and identification were done on the microbial isolates using standard microbiological techniques such as cultural, morphological and biochemical characteristics accordingly [17].
2.9.2 Wet Preparation
This was done on endocervical swabs and urine samples to determine the presence of clue and yeast cells and T. vaginalis using standard microbiological methods [18].
2.9.3 Gardnerella vaginalis detection (Bacterial Vaginosis {BV})
BV diagnosis was done using the Amstel criteria which include vaginal pH, greater than 4.5, positive whiff test, milky discharge, and the presence of clue cells on microscopic examination of vaginal fluid [19].
2.9.4 Antibiotic Susceptibility Testing
The disk-diffusion agar method tests the effectiveness of antibiotics on a specific microorganism.The inocula were prepared directly from overnight cultures adjusted to 0.5 McFarland standard. Whatman Antibiotic Assay Single Discs (9mm) already impregnated with a known volume of appropriate concentration of antimicrobial, representing Gram positive and negative antibiotics compromising of: Ofloxacin (5µg), Gentamycin (10µg), Amoxicillin/Clavulanate (10µg), Amoxicillin (10µg), Ciprofloxacin (5µg), Nitrofurantoin (100µg) Nitrofurantoin is basically used to treat UTI, Erythromycin (10µg), Cefotaxime (30µg), Nalidixic acid (30µg), were placed aseptically on the inoculated plates. The plates were incubated for 24 hours at 370C. Clear Zones of Inhibition was measured in millimetres using a ruler on the underside of the plate. Zone measurements (+/-2 mm) with 96% of the tests were accepted as the right zone of inhibition according to the Bauer-Kirby procedure [20]. Isolates showing resistance to three or more categories of antibiotics were considered as multidrug resistance bacteria.
2.9.5 HIV-1 RNA Assay (plasma viral load)
Determination of the Amplicor HIV-1 MONITOR Test version 1.5 is an in vitro nucleic acid amplification test for the quantitation of RNA copies/ml of plasma of HIV-1 RNA in human plasma. Five stages were involved: Pre-PCR reagent, Pre-PCR standard specimen preparations, amplification, detection, calculation and interpretation of results. The HIV-1 RNA copies/ml of plasma were calculated as copies/PCR x 40 = HIV-1 RNA copies/mL [21].
2.9.6 CD4 Count
Twenty (20µl) microlitre of whole blood, 20µl of CD4 mAb PE was added to Partec test tubes and incubated for 15 mins in the dark. Subsequently, 800 µl of no lyse buffer was added and vortexed gently. Total counting of CD4+ was done by Partec devise Cyflow which is a desktop flow cytometer. Result was displayed on the computer screen and calculated in cells/mm3. Normal range in HIV negative individual is 400-1600cells/mm3 as described [22]..
2.10 Statistical Analysis
Data generated was entered into IBM SPSS statistics version 20. Cross-sectional analysis using Chi Square was used to identify association between plasma viral load, microbial infections, and other co-factors associated with HIV -1 transmission from Index to their partners. Confidence interval was set at 95% with level of significance set at p value less than 0.05.
3. Results
3.1 The Sociodemographic Characteristics
The socio demographic characteristics of the 271 (542) study participants are shown on table 1. Their ages ranged from 20 to 60 years with median age of 38 years. Sixteen couples 16 (3.0%) out of 271 couples were engaged to be married but have been co-habiting and are serodiscordant. The ethnicity showed that 54.9% were Igbos, 20.9% Yoruba, 9.3% Hausa and 14.9% were of other ethnic extractions. HIV status showed 224 (82.7%) serodiscordant, 26(9.6%) concordant HIV positive and 21(7.7%) concordant HIV negative couples as shown on table 1.
Table 1: Sociodemographic Characteristics of Study Participants (N= 271)
3.2 The impact in HIV-1 RNA burden (plasma viral load (pVL), Biological and behavioural Risk factors
The burden of plasma viral load and other risk factors on heterosexual HIV-1 transmission is shown on table 2. The heterosexual index whose pVL was 10,001 to 100,000 had higher number of microbial infections (P = 0.059).
Table 2: Impact of Baseline HIV-1 RNA (Viral load) and Other Risk Factors in HIV-1 Transmission in the Index
Low viral load is 40-500 copies/ml.
High viral load -5,000-10,000 copies/ml
Baseline viral load was restricted to Index spouse who were involved in risk factors such as: male and female condone use, microbial infections, oral, anal and vaginal sex (n=69).
3.3 Microbial organisms isolated from people living with HIV (Index spouse) and HIV negative (partners)
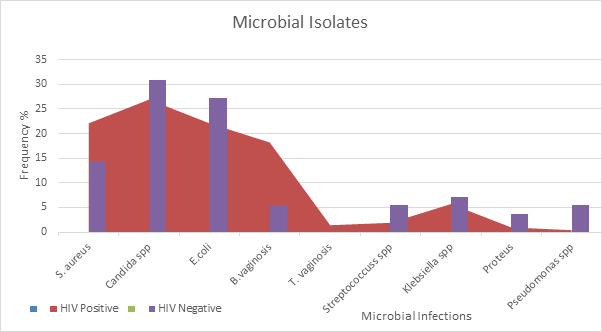
Most organisms isolated occurred as mixed infections in the population of HIV negative subjects which is shown on table 3a. A total number of 55 microorganisms were isolated and out of which, Candida spp. 17(30.9%) was the most prevalent followed by Escherichia coli 15(27.2%) and Proteus spp. 2 (3.6%) being the least. Escherichia coli (11) and Candida spp. (11) mostly co-infected followed by Staphylococcus aureus (6) and the least is Streptococcus spp. (1). Escherichia coli and Candida spp. co-infected with each other 5 and 7 times respectively.
A total number of 55 microbial agents were isolated from HIV negative individuals
Table 3a: Microbial Agents Isolated and Occurrence of Mixed Infections in HIV Negative Individuals
Table 3b highlighted the microbial organisms isolated and the occurrence of mixed infections in HIV positive subjects. A total number of 208 microbial organisms were isolated and Candida spp. 56 (26.9%) was the most prevalent followed by Staphylococcus. aureus and Escherichia. coli 46 (22.1%) respectively while Pseudomonas spp. had the least frequency 1(0.4%). Trichomonas vaginalis: (1.4%) were seen in HIV positive females. Candida spp. Co-infected mostly with Klebsiella spp. (6) followed by Staphylococcus aureus, Escherichia coli and Streptococcus spp. (3) respectively. Gardnerella vaginalis co-infected with Staphylococcus aureus (4), followed by Streptococcus spp. and Klebsiella spp. (2) respectively.
A total of 208 Microbial agents were isolated from HIV Positive individuals
Table 3b: Microbial Agents Isolated and Occurrence of Mixed Infections in HIV Positive Individuals
3.4 Antimicrobial Susceptibility Patterns of the Bacterial Isolates
On assessment of the antimicrobial sensitivity patterns of the bacterial isolates of reproductive tract infections in HIV positive and negative individuals, the following were observed: Staphylococcus aureus isolated from HIV positive individuals showed more sensitivity to Amoxicillin/clavulanate (95.4%) followed by gentamycin (90.4%) compared to HIV negative individuals (55.1%/34.4%). Escherichia coli isolated from HIV positive individuals showed more sensitivity to Nitrofurantoin followed by Gentamycin and Ofloxacin (95.4%/98.5%/98.0%) compared to HIV negative individuals (66.1%/73.5% /33.0%). Klebsiella spp. isolated from HIV positive individuals showed more sensitivity to Ofloxacin and Nitrofurantoin (87.7%) respectively compared to HIV negative individuals (27.7% /0.0%). This is shown on table 4a below.
On assessment of the antimicrobial resistant patterns of the microbial organisms isolated from the reproductive tract of people living with HIV and negative individuals, the following were observed: Staphylococcus aureus isolated from HIV positive individuals showed more resistance to Ceftazidime and Gentamycin (81.4%/72.3%) compared to HIV negative individuals (68.9%/6.8%). Escherichia coli isolated from in HIV positive individuals showed more resistance to Tetracycline and Ofloxacin (85.9%/76.9%) compared to HIV negative individuals (11.0%/33.0%). Proteus spp. isolated from HIV negative individuals showed more resistance to Gentamycin, Amoxil, Amoxicillin/clavulanate and Ceftazidime (83.3%) respectively compared HIV positive individuals (0.0%/48.0%/9.6% and 0.0%). This is also shown on table 4b.
Table 4a: Antibiotic Susceptibility Pattern of Microbial Agents Isolated from HIV Negative Individuals and those living with HIV/AIDS (Index spouse)
0- means the isolates showed no sensitivity/resistance to antibiogram
TET = TETRACYCLINE, OFL= OFLOXACIN, GEN= GENTAMYCIN, AMOX = AMOXIL
AUG=AUGMENTIN, CPR= CIPROFLOXACIN, CTR= CEFTRIAXONE, ERY=ERYTHROMYCINE
NIT=NITROFURANTOIN, NAL= NALIDIXIC ACID, CAZ= FORTUM/CEFTAZIDIME,
CRX= CEFUROXIME, CXC= CLOXACILLIN, COT= COTRIMOXAZOLE AND CTR = CEFTRIAXONE
Table 4b: Antibiotic Resistant Pattern of Microbial agents Isolated from HIV Negative Individuals and those living with HIV/AIDS (Index spouse)
4. Discussion
HIV infected subjects acquire new microbial infection at a rate greater than the HIV negative subjects. This is because they are immune compromised and are involved in other risky sexual practices such as: failure to use condom during sexual activities, anal or oral sex and some of the patients are involved in poor ARV adherence which may result in HIV mutation that may lead to increase in opportunistic infections. Sixty-nine (69) index whose HIV-1 plasma viral load was categorized at baseline: <100,000 to > 1,000,000 copies/ml showed a significant association between baseline HIV-1 plasma viral load, heterosexual HIV transmission (p=0.057) and microbial infections (p=0.059).
Similarly, 36.3% of Index whose pVL (<1000-10,000 pVL) showed there was no HIV transmission observed between the Index and their partners when the pVL was within the threshold of > 1,500- copies HIV-1 RNA/ml. Our result is consistent with a report from a study that showed reduction in the plasma HIV-1 RNA as an important target for HIV prevention. The study of heterosexual Uganda HIV serodiscordant couples showed an association between the risk of HIV acquisition and donor plasma viral load (pVL). Their study reported that no transmissions were observed when the pVL was below a critical threshold of 1500 copies HIV RNA/m [15]. Furthermore, our result is consistent with a recent study that estimated the risk of HIV-1 acquisition in a sole sexual exposure valued to be very low if the host viral load is low [23].
It is well understood that one of the significant factors that affects the heterosexual transmission of HIV-1 in serodiscordant couples is the exposing dose of the virus in the plasma of the index [24]. Furthermore, the degree of protein binding of a particular antiretroviral drug determines the level of penetration into the genital tract. The protease inhibitor drugs have poor genital tract penetration (between 10 and 50% of the levels found in plasma) whilst drugs such as tenofovir can reach up to a 10-fold higher intracellular concentration in genital tract mononuclear cells than in peripheral blood cells [23].
In this study also, a total number of 208 microbial organisms were isolated from HIV positive individuals compared to 55 isolated from HIV negative individuals. Candida spp. 56 (26.9%) was the most prevalent isolated from HIV positive individuals followed by Staphylococcus aureus and Escherichia coli 46 (22.1%) respectively. This result is
Showing that, immune compromised HIV-1 infected women have increased rates of vaginal candidiasis with increased severity of pelvic inflammatory diseases. Our result is consistent with a study done in Port Harcourt among HIV positive individuals having urethral tract infection [25]. In their report, Staphylococcus aureus had the highest percentage occurrence 49 (29.7%), followed by Escherichia coli 47 (28.5%), Pseudomonas aeruginosa 46 (27.9%) and Klebsiella pneumoniae 23 (13.9%). Another report that is similar to our result was done on lower genital tract of women living with HIV in India [9]. Their laboratory findings showed high prevalence of Bacteria vaginalis (BV) (30%), mixed infection (30%), and candidiasis (10%) among HIV-seropositive women (p <0.001). %). Our findings showed Candida spp. (26.9%), Bacteria vaginosis (18.2%) and 20% mixed infection. Furthermore, the transition of Candida spp.from harmless commensal to unrelenting pathogen in the immune compromised host is attributable to extensive expressed virulence determinants, including hyphal formation, thigmotropism, protease secretion, adherence and phenotypic switching [26]. Another study that showed contrasts with our findings was done in the State of São Paulo [27]. In their study, they reported that Pseudomonas aeruginosa was the most frequently isolated microorganism, followed by Klebsiella pneumoniae in AIDs patients. In our study however, Escherichia coli and Staphylococcus aureus (22.1%) respectively were the most frequently isolated microorganism followed by Klebsiella spp. 12(5.7%) while Pseudomonas spp. was the least frequent 1(0.4%) with low CD4 count.
Another study showing similar pattern to our study was done at llorin North- west Nigeria. Their report documented Staphylococcus aureus accounting for 22% while Klebsiella pneumonia and E. coli were isolated in 11% of the patients whose immunity was compromised [28]. However, our study showed Candida spp. and Gardnerella vaginalis co-infected (20x / 9x) with other organisms in HIV positive subjects compared with HIV negative subjects (11x /5x) consecutively. People living with HIV/AIDS are immunosuppressed and have very high chance of developing opportunistic bacterial, parasitic and or fungal infections. Therefore, it is suggested that their treatments should be personalized and culture specific including proper and consistent use of antiretroviral drugs.
In this report also, antimicrobial sensitivity patterns of the microbial isolates from the reproductive tract of people living with HIV/AIDS revealed that Staphylococcus aureus showed more sensitivity to Augmentin followed by gentamycin (95.4% / 90.4%) compared to HIV negative individuals (55.1% /34.4%). Escherichia coli isolated from HIV positive individuals showed more sensitivity to Nitrofurantoin followed by Gentamycin and Ofloxacin (95.4%/98.5%/98.0%) compared to HIV negative individuals (66.1%/73.5% /33.0%). Furthermore, Klebsiella spp isolated from HIV positive individuals showed more sensitivity to Ofloxacin (87.7%) compared to HIV negative individuals (27.7%) as shown on table 4a. Our findings were similar to a report from a study on antibiotic resistance of bacterial isolates from HIV positive patients with urinary tract infection in Rivers State Nigeria. The authors reported that Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae showed more sensitivity to Oxacillin, (95.0%, 92.81%, 94.9%, and 65.0%) when compared to HIV negative individuals (44.4%, 40.0%, 71.4% and 33.3%). Both drugs (Augmentin and Oxacillin) are in the same drug class (penicillin antibiotic) and have shown from this study to have efficacy in the management of microbial infections in people living with HIV/AIDS.
Our study showed that out of the bacterial isolates that exhibited multidrug resistance (AMR), Staphylococcus aureus and Escherichia coli isolated from HIV positive individuals were more resistant to Ceftazidime (81.4%), Gentamycin (72.3%) and Tetracycline (85.9%). Escherichia coli showed more resistance to Tetracycline and Ofloxacin (85.9%/76.9%) compared to HIV negative individuals (11.0%/33.0%). This result is in contrast to a study that reported antibiotic susceptibility profile of 380 (5.3%)Staphylococcus aureus strains isolated from urine samples of HIV patients in India [27]. The authors reported high percentage (69.5%) ofStaphylococcus aureus strains isolated were resistant topenicillin(95.2%) followed by cephalexin(84.6%).
Emergence of multidrug-resistant of microbial infections in people living with HIV/AIDS limits the treatment options and challenges the clinical management of infections. The high levels of multidrug resistance in this cohort are a serious public Health concern in low income countries. This is because there is a lot of across-the-counter purchase of antibiotics without doctor’s prescription or laboratory test results. In this study, result from questionnaires showed that 70% of the clients who had PID, itching, painful urination, vaginal and urethral discharge have had self- medication of antibiotics mostly of substandard quality and only reports to the hospital when they cannot manage the situation. This is due to ignorance, poverty, poor hygiene practices as well as inconsistent use of condom [28].
5. Conclusion
The microbial agents isolated from the index spouse were associated with high viral loads leading to low immunity and so are independent makers to HIV-1 transmission among serodiscordant couples. The periodic monitoring ofmicrobial infections and its drug resistance profile in people living with HIV/AIDS are of vital importance in clinical management. Furthermore, there is need to intensify health education to create public awareness on the use of low standard antibiotics purchased across the counter and self- medication. This will reduce multi-drug resistance and prevent reemergence of opportunistic infections in the index spouse.
6. Acknowledgement
I deeply appreciate HIV Research Trust Scholarship UK 2012 (HIVRT12-082) for sponsoring the preliminary training I required for this project which included, traveling to Project San Francisco (PSF) Kigali, Rwanda and Zambia Emory HIV Research Project (ZEHRP) Lusaka to get trained on Couples HIV Voluntary Counselling and Testing, White Cell Pellet Harvesting, DNA extraction and PCR. This was done under the supervision of Prof Susan Allen. I am grateful to Dr. Ma Lou. Associate Professor, University of Manitoba, HIV Host Genetics, National Microbiology Laboratories Winnipeg Canada for the provision of reagent and equipment used for the HLA studies as well as the analysis. My warm appreciation goes to Prof Babatunde Salako, the Director General of the Nigerian Institute of Medical Research, who approved my Pre-Doctoral Research Development Grant through its’ Graduate Initiative Programme (GIP) NIMR which was used for the completion of this study. Professors Oliver Ezechi and APIN (granting me approval to have access to PLWHA) and Rosemary Audu (viral load and CD4 count done in CHVG, NIMR) are highly appreciated for the role they played in this project.
Duality of Interest
The authors have declared no duality of interests
References
- Kaiser, H.J. The Global HIV/AIDSEpidemic Global Health Policy (2017): 1-20.
- Trend of New HIV Infections Unaids (2017).
- Webby AIDS and HIV Prevalence in Nigeria Nigeria News (2011).
- Kharsany ABM and Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS Journal 10 (2016): 34-48.
- Allen S, Karita E, Chomba E, Roth D, Telfair J, Zulu I, Clark L. Promotion of couples' voluntary counselling and testing for HIV through influential networks in two African capital cities. BMC Public Health 7 (2007): 349
- Bagala A Married Couples Top HIV Infection Rates The Daily Monitor Kampala (2006).
- Afe AJ, Fadero T, Oluokun O. HIV Serodiscordant Couples in Southwest Nigeria: Prevalence and Associated Risk Factors. Journal of AIDS and HIV Infections 11 (2015): 103-108
- Otuonye NM, Enabulele OI, Aluyi HS and Onwuamah CK. Sexual Transmitted Infections and HIV in Female Commercial Sex Workers in Lagos Nigeria. Nigerian Women Nigerian Journal of Clinical and Biomedical Research 5 (2011): 22-30.
- Goel V, Bhalla P, Sharma A and Mala YM. Lower genital tract infections in HIV-seropositive women in India. Indian Journal Sexually Transmitted Disease 32 (2011): 103-107.
- Archana S, Marfatia YS and Megha M. Reproductive tract infections in HIV positive women: A case control study. Indian Journal of Sexual Transmitted Diseases 30 (2009): 16 18.
- Jin F, Jansson J, Law M, Prestage GP, Zablotska I and Imrie JC. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART AIDS 24 (2010): 907-913.
- Tan DH, Murphy K, Shah P, and Walmsley SL. Herpes simplex virus type 2 and HIV disease progression: a systematic review of observational studies. Journal of Infectious diseases 13 (2013): 502.
- Kesler MA, Kaul R, Liu J, Loutfy M, Gesink D, Myers T, and Remis RS. Actual sexual risk and perceived risk of HIV acquisition among HIV-negative men who have sex with men in Toronto Canada BioMed Central 16 (2015): 254-260.
- AIDS Information. Guidelines for the use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Plasma HIV-1 RNA (Viral Load) and CD4 Count Monitoring.
- Brenner BG, Roger M and Routy JP. Risk of HIV transmission in discordant partners. Journal of HIV Therapy 12 (2007): 19-21.
- Magaji FA, Ocheke AN, Pam VC, Afolaramin T, Musa J et al. HIV Status in Sero-Discordant Couples: Prevalence and Pattern among Pregnant Women in Plateau State, NigeriaJournal of Clinical Research In HIV AIDS and Prevention3 (2018): 24-31.
- NACA/AVAC. NACA launches demonstration on HIV/AID prevention in serodiscordant couples.
- Iwatram-Negrón T, and El-Bassel N. Systematic Review of Couple-Based HIV Intervention and Prevention Studies: Advantages, Gaps, and Future Directions AIDS and Behavior 18 (2014): 1864-1887.
- Cheesbrough M. Medical Laboratory Manual for Tropical Countries (11) Cambridge university press Cambridge UK (2010): 433pp.
- Hainer BL and Gibson MV. Vaginitis: Diagnosis and Treatment. American Family Physician 83 (2011): 807-815.
- Barry AL,Amsterdam D,Coyle MB,Gerlach EH,Thornsberry C and Hawkinson Simple inoculum standardizing system for antimicrobial disk susceptibility tests J Clin Microbiol 10 (1979): 910-8.
- Giordano M, Kelleher T, Colonno RJ, Lazzarin A and Squires K.Affiliations The effects of the Roche AMPLICOR HIV-1 MONITOR® Ultra-Sensitive Test versions 1.0 and 1.5 viral load assays and plasma collection tube type on determination of response to antiretroviral therapy and the inappropriateness of cross-study comparisons. Journal of Clinical Virology 35 (2006): 420-442
- Fox J and Fidler S. Sexual Transmission of HIV-1 Journal of Antiviral Research 88 (2010): 1276-285.
- Aidsmap Undetectable viral load and HIV transmission (2015).
- Kemajou TS, Ajugwo AO, Oshoma CE, and Enabulele OI. Antibiotic Resistance of Bacterial Isolates from HIV Positive Patients with Urinary Tract Infection (UTI) in Portharcourt, Nigeria. Journal of AIDS and Clinical Research 7 (2016): 8.
- Sweet SP, Selection and pathogenicity ofCandida albicansin HIV infection Oral diseases (2013).
- Lopes RE, Canini S, Reinato L and Gir E. Prevalência de bactérias gram-negativas em portadores de HIV internados em serviço especializado. Acta Paulista de Enfermagem 28 (2015): 281-282
- Salami AA, Olatunji PO, Oluboyo PO, Akanbi AA and Fawibe EA. Bacterial pnuemonia in AIDS patients WAJM 25 (2006): 1-5.
- Swathirajan RC, Ramesh kumar MR; Solomon SS Pradeep A, Vignesh R and krishnan PB (2020). Changing antibiotic resistance profile ofStaphylococcus aureusisolated from HIV patients (2012-2017) in Southern India. Journal of Infectious disease and Public Health 13 (2020): 75-79.
- Otuonye NM, Enabulele OI, Aluyi HSA and Onwuamah CK. Sexual Transmitted Infections and HIV in Female Commercial Sex Workers in Lagos Nigeria. Nigerian Women. NJCBR 5 (2011): 1-6.