Plasma Prolidase Level is a Reliable Biomarker to Predict Polycystic Ovary Syndrome
Article Information
Moushumi Akhter1,*, A. K. M Shahidur Rahman2, Tania Patwary3, Laila Israt Jahan4, Rehnuma Tasnim5, Rokshana Begum4, Mst. Shaila Yesmin4, Sheuly Ferdoushi4, Md. Saiful Islam4, Shahjada Selim6, Debatosh Paul4
1Department of Laboratory Medicine, BIRDEM General Hospital, Dhaka, Bangladesh
2Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
3Department of Microbiology, Mugda Medical College and Hospital, Dhaka, Bangladesh
4Department of Laboratory Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
5Automation Lab, Lab-Aid Limited Diagnostics, Dhaka, Bangladesh
6Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
*Corresponding author: Dr. Moushumi Akhter, Department of Laboratory Medicine, BIRDEM General Hospital, Dhaka, Bangladesh.
Received: 05 November 2022; Accepted: 11 November 2022; Published: 17 November 2022
Citation: Akhter M, Rahman AKMS, Patwary T, Jahan LI, Tasnim R, Begum R, Yesmin MS, Ferdoushi S, Islam MS, Selim S, Paul D. Plasma Prolidase Level is a Reliable Biomarker to Predict Polycystic Ovary Syndrome. Fortune Journal of Health Sciences 5 (2022): 573-578.
View / Download Pdf Share at FacebookAbstract
Background: Polycystic Ovary Syndrome (PCOS) is characterized by a combination of signs and symptoms of androgen excess and ovarian dysfunction. Plasma prolidase is a member of matrix metallo-proteinase family, which is altered during extracellular matrix remodeling of ovary. Evaluation of plasma prolidase level may be helpful in diagnosis of PCOS.
Objective: To evaluate the role of plasma prolidase in the diagnosis of polycystic ovary syndrome.
Methods: This cross-sectional study was conducted at the Department of Laboratory medicine Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, from March 2021 to February 2022. A total of 70 study subjects were selected by purposive sampling technique. Among them 35 were diagnosed patients of PCOS (group I) and 35 were healthy individuals as control subjects (group II). All study subjects were evaluated by history, physical examination and relevant investigations. Their plasma prolidase levels were measured by enzyme-linked immunosorbent assay (ELISA) following standard procedure. Data were analyzed and compared by statistical tests.
Results: The median plasma prolidase level was 620.0 U/L in Group I and that was 440 U/L in group II. It was observed that plasma prolidase level was significantly high in group I as compared to group II (p<0.001). In receiver-operator characteristics (ROC) curve analysis; cut-off value of plasma prolidase for PCOS patients was found 505U/L with sensitivity and specificity were 91.4% and 74.3% respectively. The area under the curve (AUC) of plasma prolidase was 0.865 (95% CI: 0.8832-0.9702).
Conclusion: Plasma prolidase level is significantly higher in PCOS patients than healthy individuals. A cut-off value of plasma prolidase for Bangladeshi PCOS patients is 505 U/L with significant sensitivity and specificity. Therefore plasma prolidase level ma
Keywords
Biomarker; Evaluation; Plasma Prolidase; Polycystic Ovary Syndrome (PCOS)
Biomarker articles; Evaluation articles; Plasma Prolidase articles; Polycystic Ovary Syndrome (PCOS) articles
Article Details
1. Introduction
Polycystic ovary syndrome (PCOS) is regarded as a major public health issue due to its high prevalence [1]. It is a heterogenous disorder characterized by hyperandrogenism associated with chronic anovulation, hirsutism, obesity and insulin resistance [2]. The World Health Organization (WHO) estimates that approximately 3.4% of women are affected by PCOS in each year [3]. The global prevalence of PCOS ranges from 2.2% to 26% [4]. Although the prevalence of PCOS varies depending on environmental, genetic, and ethnic factors as well as the criteria used for diagnosis [5]. PCOS has multifactorial risk factors including- family history of PCOS particularly first-degree relatives, fast food diet habits, physical activity, body mass index (BMI) and waist circumference [6] [7]. Infertility is a common issue among PCOS women [1, 3, 5]. PCOS could be associated with secondary amenorrhea, type 2 diabetes mellitus (DM), cardiovascular disease, dyslipidemia, endometrial carcinoma etc [1, 3, 5, 8].
About 70% of affected women remain undiagnosed or have long delays before the condition is recognized [9]. Although polycystic ovary syndrome (PCOS) is an extremely normal endocrine condition, the etiology and pathogenesis of this disorder are not yet completely understood [9, 10]. Women with PCOS have abnormalities in androgen and estrogen production [10]. Female reproductive cycle is regulated by various hormones, growth factors and cytokines [11]. These growth factors are released from the extracellular matrix [12]. Prolidase is a cytosolic enzyme that belongs to the matrix metalloproteinase family and is responsible for the extracellular matrix degradation of the ovary [13]. Prolidase is released from erythrocytes, leukocytes, dermal fibroblasts and keratinocytes [14]. Prolidase regulates collagen metabolism and extracellular matrix remodeling [15]. In women with PCOS, elevated matrix metalloproteinase levels may be associated with abnormalities in ovarian extracellular matrix remodeling, multiple cyst formation and chronic anovulation [16]. Transvaginalsonography is used to diagnose PCOS with ovarian volume and stroma [17]. However, transvaginalsonography has some limitations in diagnosing PCOS [17]. Teenagers and women of reproductive age are unwilling to undergo transvaginal examinations due to their virginity or discomfort [17]. Obesity is another issue that creates problems in transvaginalsonography imaging [17]. Moreover, it needs experts to measure follicle number in 3D stroma and ovarian volume specifically [2]. Therefore, it is necessary to find out an easy, reliable biomarker for the diagnosis of PCOS. It was reported that there is strong and positive association between plasma prolidase and PCOS [2, 11, 16]. But there is scarce evidence to evaluate the role of plasma prolidase in diagnosis of PCOS in Bangladesh. In this background, this study was aimed to evaluate the role of plasma prolidase in diagnosis of polycystic ovarian syndrome among Bangladesh population.
2. Methodology
This cross sectional study was conducted at the Department of Laboratory medicine Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, from March 2021 to February 2022. The study protocol was approved by the Institutional Review Board (IRB), BSMMU, Dhaka, Bangladesh. A total of seventy (70) study subjects were selected by purposive sampling technique. Among them 35 were diagnosed patients of polycystic ovary syndrome (PCOS) in reproductive age (group I) and 35 were age matched healthy individuals as control subjects (group II). Patients with any systemic disease (like- liver disease, kidney disease, heart disease or any other systemic diseases), patients having hypothyroidism or androgen producing tumors or hyperprolactinemia or active thyroid disease or cushing’s syndrome and pregnant subjects were excluded from the study. In this study PCOS was diagnosed according to the international evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018 [18]. According to this guideline, PCOS is diagnosed by menstrual history, clinical and biochemical hyperandrogenism and ultrasonography for polycystic ovarian morphology [18]. All study subjects were evaluated by history, physical examination and relevant investigations.
2.1 Blood sample collection and analysis
About 5ml of venous blood sample was collected from antecubital vein of each study subject with all aseptic precaution. The blood sample were collected into a violet tube containing 0.5% chlorhexidinegluconate. Each tube was labeled with the patient’s identification number. Then blood was centrifuged by 3000 rpm for 5 minutes at room temperature (22°C - 24°C) and separated into eppendorf tube for analysis and storage.Separated plasma was storage at -20°C temperature until analysis was done and for further used. All samples were tested within one month of collection. Plasma prolidase level was measured by human prolidase (peptidase D) ELISA (enzyme linked immunosorbent assay) kit (MBS9714914) using the principle of Sandwich-ELISA method.
2.2 Statistical analysis
Data were collected in a pre-designed data collection sheet. All data were checked for normal distribution. Then statistical analysis was performed by using statistical package for social sciences (SPSS) version- 26. Data was expressed as frequency, percentage (%), mean value, median and standard deviation (SD). Unpaired t-test, Chi-square test and Mann-Whitney U test were performed accordingly. Sensitivity, specificity, positive predictive value and negative predictive value were determined from receiver-operator characteristics (ROC) curve analysis. A probability (p) value of <0.05 was considered as statistically significant.
3. Results
This cross-sectional study was intended to evaluate of the plasma prolidase level in diagnosis of polycystic ovary syndrome (PCOS). A total of 70 subjects were enrolled according to the selection criteria. Of them 35 women in reproductive age who diagnosed as PCOS patients were categorized as group I and another 35 were age matched healthy women categorized as control group (group II). Mean (±SD) age of the group I patients was 23.7±5.1 years and that was 24.4±3.5 years in group II (p=0.476). It was observed that patients with family history of PCOS and patients having diabetes mellitus (DM) were significantly higher in group I (p<0.001). The mean (±SD) body mass index (BMI) of the patients in group I was significantly high (p=0.038) (Table- 1).
Table 1: Basic characteristics of the study participants (N=70)
|
Variables |
Group I (PCOS patients) (n=35) |
Group II (Healthy individuals) (n=35) |
p-value |
|||
|
Number (n) |
Percentage (%) |
Number (n) |
Percentage (%) |
|||
|
Age (years) |
23.7±5.1 |
24.4±3.5 |
|
|||
|
Marital status |
||||||
|
Married |
17 |
48.6 |
13 |
37.1 |
0.334ns |
|
|
Unmarried |
18 |
51.4 |
22 |
62.9 |
||
|
Family history of PCOS |
8 |
22.9 |
0 |
0.0 |
<0.001s |
|
|
Family history of DM |
26 |
74.3 |
0 |
0.0 |
<0.001 s |
|
|
Family history of hypertension |
7 |
20.0 |
2 |
5.7 |
0.153ns |
|
|
Family history of obesity |
10 |
28.6 |
3 |
8.6 |
0.065ns |
|
|
Family history of hirsutism |
5 |
28.6 |
0 |
0.0 |
0.063ns |
|
|
Family history of infertility |
5 |
14.3 |
1 |
2.9 |
0.200ns |
|
|
BMI (kg/m2) |
27.5±6.9 |
24.7±3.6 |
0.038s |
|||
Unpaired t-test was performed to compare between two groups and chi-square test was performed to see the association within qualitative data, s= significant, ns= not significant
Analysis of the clinical presentations in PCOS (group I) patients showed that acne, and hirsutism was present in 9(25.7%) patients and 19(54.3%) patients respectively. It was observed that, 27(77.1%) patients had menstrual irregularity which included oligomenorrhea in 24(88.9%) patients and amenorrhea in 3(11.1%) patients among group I patients (Table- 2).
Table 2: Distribution of the patients by clinical presentations in group I (n=35)
|
Variables |
Number of patients (n) |
Percentage (%) |
|
Acne |
9 |
25.7 |
|
Hirsutism |
19 |
54.3 |
|
Menstrual irregularity |
27 |
77.1 |
|
Oligomenorrhea |
24 |
88.9 |
|
Amenorrhea |
3 |
11.1 |
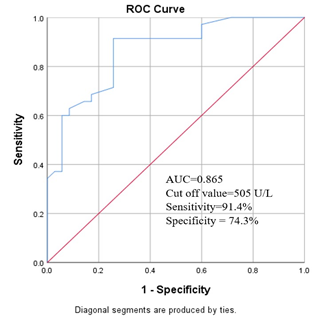
Among the study population we found that, median plasma prolidase level was significantly high in group I patients than group II subjects (620.0 U/L versus 440 U/L, p<0.001) (Table- 3). Figure- 1 shows the receiver operated curve (ROC) which displaying area under the curve (AUC) of plasma prolidase was 0.865 [95% confidence interval (CI): 0.782-0.949]. The best cut-off point of plasma prolidase for detecting PCOS was 505U/L (Figure- 1).
Table 3: Comparison of plasma prolidase level between two groups (N=70)
|
Plasma prolidase (U/L) |
Group I (n=35) |
Group II (n=35) |
p-value |
|
Mean±SD Median Range (minimum- maximum) |
674.6±225.4 620.0 410-1360 |
456.9±82.3 440 340-650 |
<0.001s |
Mann-Whitney U –test was performed to analyze the data, s=significant
Figure- 1 shows the receiver operated curve (ROC) which displaying the area under curve (AUC) of plasma prolidase was 0.865 [95% confidence interval (CI): 0.782-0.949]. The best cut-off point of plasma prolidase for detecting PCOS was 505U/L.
Table- 4 shows validity testing of plasma prolidase level in evaluation of polycystic ovary syndrome (PCOS). These were evaluated by sensitivity, specificity from ROC curve analysis. Positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated from sensitivity and specificity. Plasma prolidase showed 91.43% sensitivity, 74.29% specificity, 78,05% positive predictive value, 89.66% negative predictive value and 82.86% accuracy for detecting PC0S (Table- 4).
Table 4: Validity testing of plasma prolidase level among PCOS patients
|
Parameters |
Values |
|
Sensitivity |
91.43% |
|
Specificity |
74.29% |
|
Positive Predictive Value |
78.05% |
|
Negative Predictive Value |
89.66% |
|
Accuracy |
82.86% |
4. Discussion
Polycystic ovary syndrome (PCOS) has become an important public health concern worldwide. Severe diagnostic criteria were used to detect PCOS in last few decades where morphology of ovary was done by Transvaginalsonography or Ultrasonography [17]. There is no established blood biomarker is available for diagnosis of PCOS. Plasma prolidase is a member of matrix metallo-proteinase family which is responsible for extracellular remodeling of ovary [16]. Therefore, there is a possible relation between plasma prolidase and PCOS. In this background the aim of this study was to evaluate the plasma prolidase level in diagnosis of PCOS patients. Total 70 women in reproductive age were included as study subjects by purposive sampling technique following selection criteria. Of them 35 were diagnosed patients with polycystic ovary syndrome (PCOS) and rest 35 subjects were age matched healthy women as control group. In this study, it was observed that mean(±SD) age of PCOS patients was 23.4(±5.4) years and that was 24.4(±3.5) years in healthy individuals. A couple of previous study reported the mean age of PCOS was 26.50(± 2.023) years [2] and 24.13(±6.26) years [11]. These findings were nearly consistent with the finding of current study.
In this study, it was observed that family history of PCOS was significantly high in PCOS group in comparison to healthy subjects (22.9% versus 0.00%, p<0.001), but that finding was not consistent with a similar previous study (60.8% and 58.5% respectively) [7]. Difference of this data might be due to sample size variation and ethnic diversity. In this present study, family history of diabetes mellitus (DM) was found 74.3% in PCOS group and 17.1% among healthy individuals. The difference was statistically significant (p<0.001). A related study showed 28.2% had family history of diabetes mellitus (DM) in PCOS group and 19.20% had family history of DM among healthy individuals (p= 0.01) [19]. Another study reported 75% had family history of DM in PCOS group and 19% had family history of DM in healthy individuals group (p<0.001) [20]. Our finding was supported by these previous studies [19, 20]. In this present study, mean(±SD) body mass index (BMI) of PCOS patients was 27.5±6.9 kg/m2 and mean(±SD) BMI of healthy individuals was 24.7±3.6 kg/m2. The difference between two groups was statistically significant (p= 0.038). Similar results were reported in a couple of related study [2, 20]. Regarding clinical presentations; acne was found in 25.7% PCOS patients and hirsutism was present in 54.3% PCOS patients. On the other hand, among the PCOS patients having menstrual disturbances- 88.9% had oligomenorrhea and 11.1% had amenorrhea in this current study. These findings were not consistent with similar previous studies [1, 8, 20]. This might be due to environmental differences and different genetic makeup among the study subjects. In present study, it was observed that the median plasma prolidase level was 620U/L in PCOS patients and in healthy individuals that was 440U/L. There was significantly increase in plasma prolidase level in PCOS as compared to healthy individuals (p<0.001). Increased prolidase level in PCOS patients has been identified in a previous study [2].
The receiver-operating characteristics (ROC) curve of plasma prolidase for the diagnosis of PCOS was depicted in this study. The area under the curve (AUC) for predicting the patients was 0.865 (95% CI: 0.782-0.949). Another study found the AUC to predict the development of PCOS was 0.926 (95% CI: 0.8832-0.9702) [2]. These findings were nearly consistent with present study. Using the receiver-operating characteristics (ROC) curve, the potential cut- off value to diagnose PCOS patients was calculated. An optimal cut-off value of plasma prolidase for PCOS patients was found 505U/L. This was determined by ROC curve to see sensitivity and specificity of test. In this study, sensitivity and specificity of plasma prolidase was 91.4% and 74.3% respectively at cut-off value 505 U/L. A similar previous study found the cut-off point of plasma prolidase for diagnosis of PCOS was 621.54U/L with sensitivity was 93.76% and specificity was 89.34% [2]. The sensitivity of our study was nearly consistent with this previous study; however the specificity was not consistent with that study [2]. It may be due to sample size difference and demographic variations.This study demonstrated a significant difference of plasma prolidase level in PCOS compared to healthy individuals. Therefore, plasma prolidase exhibit a good diagnostic performance for the evaluation of PCOS.
5. Conclusion
Plasma prolidase level is significantly higher in polycystic ovary syndrome (PCOS) patients than healthy individuals. Area under the cure (AUC) has showed very good discrimination ability in detection of PCOS. Because of significant sensitivity and specificity, plasma prolidase level may be used as an important tool for the diagnosis of polycystic ovary syndrome.
Limitations
It was a single centre study with relatively small sample size. Moreover the sample was taken purposively; therefore there might be chance of bias which can influence the results.
Recommendation
A multicenter prospective cohort study with large sample size is required to confirm the findings of this present study. Follow up study is recommended for better evaluation of plasma prolidase with prognosis and outcome of polycystic ovary syndrome.
Acknowledgement
The authors greatly acknowledge Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh for their technical support.
Conflicts of interest
The authors declared no conflicts of interest regarding this publication.
References
- Shermin S, Noor A, Jahan S. Polycystic ovary syndrome: a brief review with recent updates. Delta Medical College Journal7 (2019):84-99.
- Bhatnager R, Nanda S, Dang AS. Plasma prolidase levels as a biomarker for polycystic ovary syndrome. Biomarkers in Medicine12 (2018):597-606.
- Kabel AM. Polycystic ovarian syndrome: insights into pathogenesis, diagnosis, prognosis, pharmacological and non-pharmacological treatment. Pharmaceutical Bioprocessing 4 (2016):7-12.
- Bharathi RV, Swetha S, Neerajaa J, Madhavica JV, Janani DM, Rekha SN, Ramya S, Usha B. An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertility Society Journal 22 (2017):313-6.
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Human reproduction 31 (2016):2841-55.
- Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. Journal of pediatric and adolescent gynecology 24 (2011):223-7.
- Begum GS, Shariff A, Ayman G, Mohammad B, Housam R, Khaled N. Assessment of risk factors for development of polycystic ovarian syndrome. diabetes1 (2017).
- Fatema K, Das TR, Kazal RK, Mahamood S, Pervin HH, Noor F, et al. Prevalence and characteristics of polycystic ovarian syndrome in women attending in outpatient department of obstetrics and gynecology of Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 10 (2021):830-6.
- Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. The Journal of Clinical Endocrinology & Metabolism 102 (2017):604-12.
- Yildirim B, Sabir N, Kaleli B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertility and sterility 79 (2003):1358-64.
- Bhatnager R, Nanda S, Dang AS. Increased Prolidase Level and Altered Hormonal Profile in Women with Poly Cystic Ovarian Syndrome. growth9 (2016):10.
- Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reproductive sciences 14 (2007):6-10.
- Tsuruda T, Costello-Boerrigter LC, Burnett JC. Matrix metalloproteinases: pathways of induction by bioactive molecules. Heart failure reviews 9 (2004):53-61.
- Hui KS, Lajtha A. Prolidase activity in brain: comparison with other organs. Journal of neurochemistry 30 (1978):321-7.
- Surazynski A, Miltyk W, Palka J, Phang JM. Prolidase-dependent regulation of collagen biosynthesis. Amino acids 35 (2008):731-8.
- Hilali N, Vural M, Camuzcuoglu H, Camuzcuoglu A, Aksoy N. Increased prolidase activity and oxidative stress in PCOS. Clinical endocrinology 79 (2013):105-10.
- Sahmay S, Atakul N, Aydogan B, Aydin Y, Imamoglu M, Seyisoglu H. Elevated serum levels of anti-Müllerian hormone can be introduced as a new diagnostic marker for polycystic ovary syndrome. ActaobstetriciaetgynecologicaScandinavica92 (2013):1369-74.
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human reproduction 33 (2018):1602-18.
- Moini A, Eslami B. Familial associations between polycystic ovarian syndrome and common diseases. Journal of assisted reproduction and genetics 26 (2009):123-7.
- Begum F. Clinical and hormonal profile of polycystic ovary syndrome. Journal of SAFOG1 (2009):22-5.