Plasma Levels of Selenium (SE) and Glutathion Peroxydase (GSH-PX) and their Relationship to Supplementation of Selenium in Patients with Chronic Renal Failure (CRF) on Hemodialysis (HD)
Article Information
Popov I1, Manolov IV2, Atanasova B2, Vasilev V2, Dimitrova V3, Arabadjieva D3, Velkova N3, Yonova D3*
1Nephrology Department, Medical University, Stara Zagora, Bulgaria
2Clinical Laboratory Department, Medical University, Sofia, Bulgaria
3Nephrology Department, Sofia University “St. Kliment Ohridski”, Sofia, Bulgaria
*Corresponding Author: Diana Yonova, University Hospital “Lozenetz”, “Koziak” street 1, Sofia 1404, Bulgaria
Received: 20 February 2019; Accepted: 04 March 2019; Published: 11 March 2019
Citation: Popov I, Manolov IV, Atanasova B, Vasilev V, Dimitrova V, Arabadjieva D, Velkova N, Yonova D. Plasma Levels of Selenium (SE) and Glutathion Peroxydase (GSH-PX) and their Relationship to Supplementation of Selenium in Patients with Chronic Renal Failure (CRF) on Hemodialysis (HD). Archives of Nephrology and Urology 2 (2019): 013-019.
View / Download Pdf Share at FacebookAbstract
Introduction: Plasma levels of Se and GSH-Px decrease with the progression of renal impairment and is particularly pronounced at the end stage of chronic kidney disease (CKD) as kidney proximal tubular cells are the main source of pGSH-Px activity. A number of authors found that Se supplementation increases its plasma levels in patients on HD, but publications about influence of Se supplementation on plasma GSH-Px are controversial-from a lack of any effect to a significant increase of the enzyme. The study aims to monitor levels of plasma selenium (pSe) and glutathione peroxidase (pGSH-Px) in patients on HD treatment with Se deficit, orally supplemented with Se for 3 months, and to evaluate the supplementation on pSe and pGSH-Px.
Material and Methods: 61 patients on regular hemodialysis, divided into two groups (1st group-33 pts. with pSe deficit and, 2nd group-28 with normal pSe levels) were tested at the beginning of dialysis sessions for serum hemoglobin, total protein, albumin, creatinine, urea, uric acid, C-reactive protein (CRP), phosphorus, potassium plasma Se and pGSH-Px activity. The same parameters were re-examined at the end of the study. The patients of the 1st group were supplemented with oral Se for 3 months and the results in the two groups were compared.
Results: All parameters (except pSe and pGSH-Px in the 1st group) showed no significant differences in the beginning and the end of the follow-up period in both patients groups. The levels of pSe and pGSH-Px in the 2nd group also showed no significant change, but in the 1st group both parameters increased significantly at the end of the 3-months period. We found a significant correlation between pSe/pGSH-Px (p<0.01), and between pGSH-Px/Creatinine (p<0.01) at the start and the end of the study.
Conclusion: Our study suggests that supplementation of oral
Keywords
Chronic kidney disease, Chronic renal failure, Hemodialysis, Selenium, Glutathione peroxidase
Chronic kidney disease articles, Chronic renal failure articles, Hemodialysis articles, Selenium articles, Glutathione peroxidase articles
Chronic kidney disease articles Chronic kidney disease Research articles Chronic kidney disease review articles Chronic kidney disease PubMed articles Chronic kidney disease PubMed Central articles Chronic kidney disease 2023 articles Chronic kidney disease 2024 articles Chronic kidney disease Scopus articles Chronic kidney disease impact factor journals Chronic kidney disease Scopus journals Chronic kidney disease PubMed journals Chronic kidney disease medical journals Chronic kidney disease free journals Chronic kidney disease best journals Chronic kidney disease top journals Chronic kidney disease free medical journals Chronic kidney disease famous journals Chronic kidney disease Google Scholar indexed journals Chronic renal failure articles Chronic renal failure Research articles Chronic renal failure review articles Chronic renal failure PubMed articles Chronic renal failure PubMed Central articles Chronic renal failure 2023 articles Chronic renal failure 2024 articles Chronic renal failure Scopus articles Chronic renal failure impact factor journals Chronic renal failure Scopus journals Chronic renal failure PubMed journals Chronic renal failure medical journals Chronic renal failure free journals Chronic renal failure best journals Chronic renal failure top journals Chronic renal failure free medical journals Chronic renal failure famous journals Chronic renal failure Google Scholar indexed journals Hemodialysis articles Hemodialysis Research articles Hemodialysis review articles Hemodialysis PubMed articles Hemodialysis PubMed Central articles Hemodialysis 2023 articles Hemodialysis 2024 articles Hemodialysis Scopus articles Hemodialysis impact factor journals Hemodialysis Scopus journals Hemodialysis PubMed journals Hemodialysis medical journals Hemodialysis free journals Hemodialysis best journals Hemodialysis top journals Hemodialysis free medical journals Hemodialysis famous journals Hemodialysis Google Scholar indexed journals Selenium articles Selenium Research articles Selenium review articles Selenium PubMed articles Selenium PubMed Central articles Selenium 2023 articles Selenium 2024 articles Selenium Scopus articles Selenium impact factor journals Selenium Scopus journals Selenium PubMed journals Selenium medical journals Selenium free journals Selenium best journals Selenium top journals Selenium free medical journals Selenium famous journals Selenium Google Scholar indexed journals Glutathione peroxidase articles Glutathione peroxidase Research articles Glutathione peroxidase review articles Glutathione peroxidase PubMed articles Glutathione peroxidase PubMed Central articles Glutathione peroxidase 2023 articles Glutathione peroxidase 2024 articles Glutathione peroxidase Scopus articles Glutathione peroxidase impact factor journals Glutathione peroxidase Scopus journals Glutathione peroxidase PubMed journals Glutathione peroxidase medical journals Glutathione peroxidase free journals Glutathione peroxidase best journals Glutathione peroxidase top journals Glutathione peroxidase free medical journals Glutathione peroxidase famous journals Glutathione peroxidase Google Scholar indexed journals continuous ambulatory peritoneal dialysis articles continuous ambulatory peritoneal dialysis Research articles continuous ambulatory peritoneal dialysis review articles continuous ambulatory peritoneal dialysis PubMed articles continuous ambulatory peritoneal dialysis PubMed Central articles continuous ambulatory peritoneal dialysis 2023 articles continuous ambulatory peritoneal dialysis 2024 articles continuous ambulatory peritoneal dialysis Scopus articles continuous ambulatory peritoneal dialysis impact factor journals continuous ambulatory peritoneal dialysis Scopus journals continuous ambulatory peritoneal dialysis PubMed journals continuous ambulatory peritoneal dialysis medical journals continuous ambulatory peritoneal dialysis free journals continuous ambulatory peritoneal dialysis best journals continuous ambulatory peritoneal dialysis top journals continuous ambulatory peritoneal dialysis free medical journals continuous ambulatory peritoneal dialysis famous journals continuous ambulatory peritoneal dialysis Google Scholar indexed journals Hemodialysis articles Hemodialysis Research articles Hemodialysis review articles Hemodialysis PubMed articles Hemodialysis PubMed Central articles Hemodialysis 2023 articles Hemodialysis 2024 articles Hemodialysis Scopus articles Hemodialysis impact factor journals Hemodialysis Scopus journals Hemodialysis PubMed journals Hemodialysis medical journals Hemodialysis free journals Hemodialysis best journals Hemodialysis top journals Hemodialysis free medical journals Hemodialysis famous journals Hemodialysis Google Scholar indexed journals glutathione peroxidase articles glutathione peroxidase Research articles glutathione peroxidase review articles glutathione peroxidase PubMed articles glutathione peroxidase PubMed Central articles glutathione peroxidase 2023 articles glutathione peroxidase 2024 articles glutathione peroxidase Scopus articles glutathione peroxidase impact factor journals glutathione peroxidase Scopus journals glutathione peroxidase PubMed journals glutathione peroxidase medical journals glutathione peroxidase free journals glutathione peroxidase best journals glutathione peroxidase top journals glutathione peroxidase free medical journals glutathione peroxidase famous journals glutathione peroxidase Google Scholar indexed journals plasma selenium articles plasma selenium Research articles plasma selenium review articles plasma selenium PubMed articles plasma selenium PubMed Central articles plasma selenium 2023 articles plasma selenium 2024 articles plasma selenium Scopus articles plasma selenium impact factor journals plasma selenium Scopus journals plasma selenium PubMed journals plasma selenium medical journals plasma selenium free journals plasma selenium best journals plasma selenium top journals plasma selenium free medical journals plasma selenium famous journals plasma selenium Google Scholar indexed journals
Article Details
1. Introduction
Numerous studies have shown that the compounds containing trace elements (mainly enzymes), but not trace elements “per se”, are critical for biological activities in the human body [1-5]. Compounds with (Se), cuprum (Cu), and zinc (Zn) play a major role in the antioxidant protectiv system. They perform their antioxidant functions through the proteins in which they are included [1-3]. Se is a component of about 25 enzymes, including glutathione peroxidase family (GSH-Px), thioredoxin reductases and selenoprotein P. Selenium is a trace element that participates as a cofactor in a number of enzyme processes-regulating thyroid hormone metabolism, modulating immune processes, and enhancing antioxidant protection for the human body [4-7]. Although there are not too many studies of plasma selenium levels in patients with chronic kidney disease (CKD) on dialysis treatment, they prove a deficiency of this important element of vital human function, both in hemodialysis (HD) and in continuous ambulatory peritoneal dialysis (CAPD) patients [4-8]. Plasma levels of Se and GSH-Px decrease with the progression of renal impairment and is particularly pronounced at the end stage of chronic kidney disease (CKD), as the kidney proximal tubular cells are the main source of pGSH-Px activity [5, 7, 9]. A number of authors found that Se supplementation increases its plasma levels in patients on HD, but publications about influence of Se supplementation on plasma GSH-Px are controversial-from a lack of any effect to a significant increase of the enzyme [4, 5, 9].
1.1 Aim of the study
To monitor levels of plasma selenium (pSe) and glutathione peroxidase (pGSH-Px) in patients with Se deficit on HD, orally supplemented for 3 months with Se, and to evaluate its effect on pSe and pGSH-Px.
2. Material and Methods
61 patients on regular hemodialysis (34 men and 27 women, aged 19 to 67 years), divided into two groups (1st group-33 pts. with pSe deficit and, 2nd group-28 with normal pSe levels) were tested at the beginning of dialysis sessions for serum hemoglobin, total protein, albumin, creatinine, urea, uric acid, C-reactive protein (CRP), phosphorus, potassium and calcium by routine procedures with automatic analyzer. Plasma Se was determined by atomic absorption spectrometry (AAS), using a HITACHI® Z-500 spectrometer and pGSH-Px activity-with a CX7 Synchron system (Beckman Instruments, CA, USA, kit Ransel; Randox, Ireland). The same parameters were re-examined at the end of the study in the both groups of patients. The patients of the 1st group were supplemented with 200 µg/day oral Se for 3 months and the results in the two groups were compared. The statistical analyses were performed using SPSS19.0 software (Chicago, IL, USA). Statistical accepted significance is p<0.05.
The study protocol was reviewed and approved by the Ethics Committees of the all participating University Hospitals and all the patients gave a written informed consent.
3. Results
Hemoglobin, total protein, albumin, creatinine, urea, uric acid, C-reactive protein (CRP), phosphorus, potassium and calcium showed no significant differences in the beginning and the end of the follow-up period in both patient groups. The levels of pSe and pGSH-Px in the 2nd group also showed no significant change, but in the 1st group both parameters increased significantly at the end of the 3-months period, despite plasma total proteins and albumins were unchanged: pSe-1 rises from: 497.5 +/- 132.6; to pSe-2: 1012.4 +/- 269.8 mcg / l; pGSH-Px-1 rises from: 7.8 +/- 1.6; to pGSH-Px-2: 24.8 ± 6.8 U/gHb (Table 1).
|
Laboratory |
1st Group Supplementd by |
1st Group /Study’s end / |
P< |
2nd Group Unsupplementd |
2nd Group /Study’s end/
|
|
pSe (µg/L) |
497.5 ± 132 |
1012.4 ± 269 |
0.01 |
814.2 ± 254 |
832.4 ± 198 |
|
pGSH-Px (U/gHb) |
7.8+/-1.6 |
24.8+/-6.8 |
0.01 |
20.9+/-8.8 |
19.7+/-9.2 |
|
T-protein (g/L) |
65.5 ± 3.3 |
64.3 ± 3.1 |
N.S. |
65.5 ± 3.3 |
66.1 ± 2.3 |
|
Albumin (g/L) |
36.5 ± 2.1 |
37.2 ± 3.3 |
N.S. |
37.5 ± 2.1 |
36.9 ± 2.7 |
Table 1: Plasma Se, GSH-Px and proteins in Se supplemented and un-supplemented HD patients at the start and end of the study.
The rise of plasma Se due to the Se supplementation is well visualized in the next Figure 1.
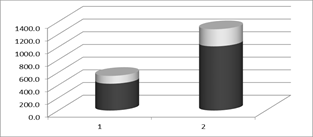
Figure 1: pSe (µg/L) (MD+/-SD) in 1st group before (1) and after (2) Se supplementation.
The improvement of plasma enzyme activity of GSH-Px in Se-supplemented patients is shown on the next Figure 2.
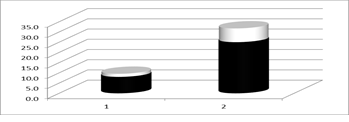
Figure 2: pGSH-Px (MD+/-SD) in 1st group before (1) and after (2) Se supplementation.
We found a significant correlation between pSe/pGSH-Px (at the start-p<0.01) and (at the end-p<0.01) and between pGSH-Px/Creatinine (at the start-p<0.01) and (at the end-p<0.05), but not between pSe/CRP and between pGSH-Px/CRP at the start and the end of the study.
4. Discussion
The likely mechanisms for the emergence and augmentation of pSe deficit in hemodialysis patients are reduced dietary intake, impaired intestinal absorption of selenium, decreased serum levels of selenium-binding protein, increased oxidative stress and loss of selenium by urine or in dialysis procedure [1-7]. Low Se intake and low Se soil concentration are also an important cause of this deficiency [8-10]. Bioavailability of Se in soils varies greatly in different regions. Fish and mammal organs, such as kidney and liver, are known to accumulate significant levels of Se and are, potentially, good dietary sources of this essential element for humans. In general, fruits and vegetables contain low Se levels, however, depending on Se bioavailability cereals may constitute an important source of Se [11-12]. It is well-known that Brazil nuts are very rich in selenium and only a few nuts a day would provide the necessary amount of selenium in deficiency, but they also contain too much phosphate, which makes them unsuitable for dialysis patients [12-14]. However, it has been indicated that Se deficiency might occur in HD patients in nonspecific areas where environmental Se is sufficient. Deficiency of selenium causes pathological abnormalities in a number of organs and systems in the human body presented in the following points [1, 8, 15-17]:
- Grought retardation
- Reproductive disturbances
- Muscle degeneration
- Hepatocyte necrosis
- Pancres degeneration
- Cardiomyopathy
- Disturbed cell membrane
The presence of oxidative stress, which is manifested by an excess of oxidative radicals and decreased levels of antioxidants, has been demonstrated in dialysis patients [3, 8, 15, 17]. In our study, oxidative stress is confirmed by abnormally reduced levels of plasma levels of glutathione peroxidase (P-GSH-Px) and Se. Plasma glutathione peroxidase (pGSH-Px) is also decreased due to the fact that selenium is required for its synthesis and activation, and the localization of the enzyme formation process is in proximal renal tubules that are significantly damaged by CRF [4, 5]. Our finding of a positive correlation between increase of pSe and pGSH-Px suggests a rise of synthesis of pGSH-Px due to Se supplementation, but the publications about this issue in the medical journals are very heterogeneous [4, 5, 14, 17]. The undisputed rise to normalizing levels of pSe and the beneficial effect of selenium supplementation on one of the important antioxidant enzymes as well as maybe of the other beneficial activities of Se “per se” is encouraging. The lack of correlation between pSe/CRP and pGSH-Px/CRP suggests that the acute inflammation “per se” could not be an important factor on the Se turnover in the human body. For us it was especially interesting to find out whether, despite the irreversibly reduced renal parenchyma in terminal CRF of hemodialysis patients, the body of these patients has compensatory mechanisms to restore the enzyme activity of this antioxidant enzyme (GSH-Px), when supplementing selenium, and the answer was positive. At the other hand, our study presented several limitations. The sample sizes were small, we did not have a control with healthy subjects, and we did not have meaning data from the diet of patients, because the foods have different concentrations of Se and there is no accurate software to calculate the diets and, the major problem in dietary assessment was inaccuracy in patients recordings of dietary intake.
5. Conclusion
Our study suggests that supplementation of oral Se in patients with CKD on regular HD, significantly increases not only pSe, but pGSH-Px as well, despite the patients on regular HD have proved bilateral nephrosclerosis. The phenomenon is probably due to the activation of small islets of renal parenchyma and, to a greater extent, the presence of outbreaks of GSH-Px synthesis (upon import of Se) into non-renal body tissues. As a positive effect, at least one of the members of anti-oxidant system of this patient’s contingent is enhanced-plasma GSH-Px.
Conflict of Interest
We declare that we are not in conflict of interests in this study.
References
- Yang CY, Wu ML, Chou YY, et al. Essential trace element status and clinical outcomes in long-term dialysis patients: a two-year prospective observational cohort study. Clin Nutr 31 (2012): 630-636
- Tonelli M, Wiebe N, Hemmelgarn B, et al. Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med 7 (2009): 25.
- Manal A Aziz, Ghanim H Ajeed, Kareem S Diab, et al. The association of oxidant-antioxidant status in patients with chronic renal failure. Journal Renal Failure 38 (2016): 20-26
- Zachara BA, Salak A, Koterska D, et al. Selenium and glutathione peroxidases in blood of patients with different stages of chronic renal failure. J Trace Elem Med Biol 17 (2004): 291-299.
- Zachara BA, Gromadzinska J, Wasowicz W, et al. Selenium supplementation to chronic kidney disease patients on hemodialysis has no effect on superoxide dismutase activity and malonyldialdehyde concentration in blood. Austin Ther 1 (2014): 1-7.
- Drutel A, Archambeaud F, Caron P. Selenium and the thyroid gland: more good news for clinicians. Clin Endocrinol (Oxf) 78 (2013): 155-164.
- Mehdi Y, Hornick JL, Istasse L, et al. Selenium in the environment, metabolism and involvement in body functions. Molecules 18 (2013): 3292-3311.
- Fujishima Y, Ohsawa M, Itai K, et al. Serum selenium levels are inversely associated with death risk among hemodialysis patients. Nephrol Dial Transplant 26 (2011): 3331-3338.
- Salehi M, Sohrabi Z, Ekramzadeh M, et al. Selenium supplementation improves the nutritional status of hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Nephrol Dial Transplant 28 (2013): 716-723.
- Shaltout AA, Castilho IN, Welz B, et al. Method development and optimization for the determination of selenium in bean and soil samples using hydride generation electrothermal atomic absorption spectrometry. Talanta 85 (2011): 1350-1356.
- Lemire M, Philibert A, Fillion M, et al. No evidence of selenosis from a selenium-rich diet in the Brazilian Amazon. Environ Int 40 (2012): 128-136.
- Stockler-Pinto MB, Lobo J, Moraes C, et al. Effect of Brazil nut supplementation on plasma levels of selenium in hemodialysis patients: 12 months of follow-up. J Ren Nutr 22 (2012): 434-439.
- Saeed Amirkhanlou, Hassan Emadi, Anna rashedi, et al. Evaluation of Plasma Selenium Level and its Association with Malnutrition in Hemodialysis Patients in Golestan Province. Iran J of Clinical and Basic Research 1 (2017): 17-21.
- Stockler-Pinto MB, Mafra D, Moraes C, et al. Brazil nut (Bertholletia excelsa, H.B.K.) improves oxidative stress and inflammation biomarkers in hemodialysis patients. Biol Trace Elem Research 158 (2014): 105-112.
- Tiali A, Taleb-Belkadi O, Tbahriti HF, et al. Vitamin E supplementation improves oxidant–antioxidant balance in chronic renal failure patients treated by hemodialysis. Br J Med Med Res 4 (2014): 4169-4177.
- Milena Barcza Stockler-Pinto, Juan Jesus Carrero, Luciene de Carvalho Cardoso Weide, et al. Effect of selenium supplementation via Brazil nut (Bertholletia excelsa, HBK) on thyroid hormones levels in hemodialysis patients: a pilot study. Nutr Hosp 32 (2015): 1808-1812.
- Trendafilov I, Georgieva I, Manolov V, et al. Status and relation to in?ammation of some serum trace elements (TE) in hemodialysis (HD) patients. Nephrology and Renal Diseases Journal 3 (2018): 1-4.
