Impact of Switching From Clopidogrel To Ticagrelor on Adverse Cardiovascular Events in Patients With ST-Segment Elevation Myocardial Infarction
Article Information
Evgeniya Tavlueva, Alexey Alekseenko, Olga Gruzdeva, Evgenya Uchasova*, Olga Barbarash
Federal State Budgetary Institution, Research Institute for Complex Issues of Cardiovascular Diseases, 6 Sosnovy Blvd, Kemerovo, 650002, Russian Federation
*Corresponding Author: Evgenya Uchasova, Federal State Budgetary Institution, Research Institute for Complex Issues of Cardiovascular Disease, Laboratory of Research Homeostasis, 6 Sosnovy Blvd, Kemerovo 650002, Russian Federation
Received: 18 April 2018; Accepted: 30 April 2018; Published: 09 May 2018
Citation: Evgeniya Tavlueva, Alexey Alekseenko, Olga Gruzdeva, Evgenya Uchasova, Olga Barbarash. Impact of Switching From Clopidogrel To Ticagrelor on Adverse Cardiovascular Events in Patients With ST-Segment Elevation Myocardial Infarction. Cardiology and Cardiovascular Medicine 2 (2018): 074-085.
View / Download Pdf Share at FacebookAbstract
Aim: to assess the impact of switching from clopidogrel to ticagrelor on clinical endpoints in the in-hospital period and 1 year post-discharge in patients with ST-segment elevation myocardial infarction (STEMI).
Methods: Eighty patients with STEMI were included in the study. All patients received a loading dose of aspirin (250 mg) and clopidogrel (600 mg) in the ambulance. Groups 1 and 2 patients received a maintenance dosage of 75 mg clopidogrel daily and 90 mg ticagrelor twice daily, respectively. Clinical endpoints were assessed on day 8 in the in-hospital period and 1 year post-discharge. The platelet aggregation and plasma levels of interleukin-6 (IL-6) and C-reactive protein (CRP) were measured before and on day 7 after switching from clopidogrel to ticagrelor.
Results: A trend toward fewer clinical endpoints was found in the ticagrelor group compared with the clopidogrel group. There was no significant increase in the rate of bleeding in the in-hospital period and within 1 year after STEMI in the ticagrelor group compared with the clopidogrel group. On day 8 of the in-hospital period, platelet aggregation was significantly higher in the clopidogrel group than that in the ticagrelor group (p = 0.00). The CRP and IL-6 levels on day 8 of the in-hospital period were significantly higher in the clopidogrel group than those in the ticagrelor group (p = 0.04 vs. p = 0.01, respectively).
Conclusion: Switching from clopidogrel to ticagrelor on day 1 after myocardial infarction was associated with lower rate of endpoints during the 1-year follow-up.
Keywords
Myocardial infarction; Clopidogrel; Ticagrelor
Article Details
1. Introduction
Platelet activation plays a significant role in the pathogenesis of acute coronary syndrome (ACS) [1]. The platelet surface is packed with functional receptors, such as P2Y12, to facilitate platelet adhesion and aggregation, resulting in altered rheological properties of the blood. Moreover, platelet receptors can actively modulate immune responses by activating various pro- and anti-inflammatory factors. Subclinical inflammation has been considered as a key pathophysiological reaction that induces the development and progression of major cardiovascular diseases, including ACS [2].
New-generation antiplatelet drugs differ in the mechanisms of action, specific interference in the platelet activation process, and potential impact on biological, clinical, and adverse effects. All antiplatelet drugs commonly alter the activity of platelet receptors. Thus, the use of two antiplatelet agents with different mechanisms of action after ACS allows reducing the risk of adverse outcomes compared with monotherapy with aspirin. Dual antiplatelet therapy elicits more rapid and stronger antiplatelet effect but results in higher rates of bleeding. However, in most cases, the benefit exceeds the risk. Therefore, recent clinical guidelines consider prolonged dual antiplatelet therapy to be the standard of care for patients with ACS and emphasize the negative effects of its early permanent discontinuation [3].
The benefits of dual antiplatelet therapy after ACS have already been proven. The large randomized Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial suggested that adding clopidogrel to aspirin is associated with lower rates of cardiovascular death, myocardial infarction (MI), or stroke [4]. The CURE investigators concluded that adding clopidogrel to aspirin allowed preventing extensive MI. The multicenter PLATO trial reported beneficial effects of the combination of aspirin and ticagrelor in patients with ACS. Notably, the therapeutic superiority of ticagrelor over clopidogrel increases within a year [5,6]. The PEGASUS trial reported reduced rates of cardiovascular death, MI, or stroke in patients receiving aspirin and ticagrelor for 3 years [7]. However, the reduced rate of adverse cardiovascular events is accompanied by an increased rate of major bleeding. Various multicenter trials (PLATO, PEGASUS) have proven the superiority of the combination of ticagrelor and aspirin in preventing recurrent cardiovascular events in MI patients [5,7].
However, specific concerns, such as the effects of switching antithrombotic drugs on the rate of adverse events, that need to be assessed in future studies have been raised. Comparison of the impact of switching antiplatelet drugs on inhibition of platelet aggregation and pro-inflammatory effects of platelets is important to estimate the rate of adverse events and assess the safety of this switching with regard to the resultant risk of bleeding.
Therefore, this study aimed to assess the effects of switching from clopidogrel to ticagrelor on the clinical endpoints in the in-hospital period and 1 year post-discharge in patients with ST-segment elevation myocardial infarction (STEMI).
2. Methods
2.1 Study Subjects
A total of 80 patients with STEMI admitted to the Kemerovo Cardiology Dispensary were included in the study. The study protocol was approved by the local ethics committee. A simple random sampling was used to allocate patients in the study groups.
Patients diagnosed with STEMI up to 12 h from the onset according to the criteria of ESC for the diagnosis of STEMI (2017) and those who provided written informed consent were included in the study. Patients who had concomitant diseases (cancer, terminal renal/hepatocellular failure, acute infectious diseases or exacerbation of chronic diseases, and mental illnesses); current bleeding; thrombocytopathy; moderate and severe anemia; periprocedural MI after elective myocardial revascularization (percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)); elective CABG within 1 year; pre-hospital thrombolysis; Killip III-IV acute heart failure; and MI without myocardial revascularization were excluded from the study. Patients who refused to participate in the study and required triple antiplatelet therapy were also excluded.
All patients were given a loading dose of aspirin (250 mg) and clopidogrel (600 mg) in the emergency department. At the time of admission, all patients underwent emergency coronary angiography (CAG) and PCI with bare-metal stent implantation in the infarct-related artery. The patients were assigned into two groups 12-24 h after hospital admission. Group 1 patients (clopidogrel group) continued to receive a maintenance dosage of 75 mg clopidogrel daily (n = 31). Group 2 patients (ticagrelor group) were switched to 90 mg ticagrelor twice daily without a loading dose (n = 49). All patients received a maintenance dosage of 100 mg aspirin daily.
The main clinical and demographic parameters of STEMI patients and current MI clinic presentation are shown in Tables 1 and 2. No significant differences were found in the current MI clinical presentation and main clinical and demographic parameters between the study groups.
|
Parameters |
Clopidogrel, n = 31 |
Ticagrelor, n = 49 |
p |
|
Age, years (Me: 25; 75) |
54.3 (50.1; 62.4) |
56.1 (48.6; 64.1) |
0.65 |
|
Females, n (%) |
9 (29) |
7 (14) |
0.09 |
|
Prior type 2 diabetes, n (%) |
1 (3.2) |
6 (12.2) |
0.16 |
|
BMI (Me: 25; 75) |
27.2 (24.5; 31) |
28.4 (24.1; 32.1) |
0.89 |
|
Prior MI, n (%) |
7 (22.5) |
5 (10.2) |
0.11 |
|
Prior ACVA, n (%) |
1 (3.2) |
0 |
- |
|
Prior PCI, n (%) |
2 (6.5) |
4 (8.2) |
0.57 |
|
Prior CABG, n (%) |
0 |
0 |
- |
|
Previously diagnosed PAD, stenoses >50%, n (%) |
0 |
0 |
- |
|
Prior CHF, n (%) |
12 (38.7) |
15 (30.6) |
0.45 |
|
Prior COPD, n (%) |
4 (12.9) |
2 (4.1) |
0.15 |
|
Prior ulcer, n (%) |
9 (29) |
7 (14) |
0.09 |
|
Hb level at the time of admission, g/l (Me: 25; 75) |
139.6 (127; 151.5) |
143.1 (136; 154) |
0.38 |
|
GFR, ml/min/1,73 m at the time of admission (Me: 25; 75) |
58 (49.9; 67.6) |
61.4 (48.1; 74.5) |
0.1 |
|
Troponin T, pg/ml (Me: 25; 75) |
0.71 (0; 1.3) |
0.42 (0; 0.65) |
0.09 |
|
Glucose level at the time of admission, mmol/l (Me: 25; 75) |
7.14 (6.1; 8.1) |
6.47 (5.2; 7.5) |
0.56 |
Table 1: Clinical and demographic parameters in STEMI patients
Note: ACVA: Acute cerebrovascular accident; BMI: Body mass index; CABG: Coronary artery bypass grafting; CHF: Congestive heart failure; GFR: Glomerular filtration rate MI: Myocardial infarction; PCI: Percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
|
Parameters |
Clopidogrel, n = 31 |
Ticagrelor, n = 49 |
p |
|
LVEF at admission, % (Me: 25; 75) |
48.18 (41; 57) |
52.78 (46; 62) |
0.11 |
|
Localization of MI: anterior |
10 (32.3) |
20 (40.8) |
0.27 0.31 0.64 |
|
AHF, Killip II, n (%) |
5 (16.1) |
1 (2) |
0.058 |
|
Developing acute LV aneurism (based on the ECHO-CG findings), n (%) |
2 (6.4) |
1 (2) |
0.33 |
|
Heart rhythm and conduction disorders, n (%) |
13 (41.9) |
11 (22.4) |
0.055 |
Table 2: STEMI clinical presentation
Note: AHF: Acute heart failure; ECHO-CG: Echocardiography; LVEF: Left ventricular ejection fraction; MI: Myocardial infarction; STEMI: ST-segment elevation myocardial infarction.
In addition to dual antiplatelet therapy, all patients received the drugs listed in Table 3. Group 2 patients were more likely to receive dihydropyridine calcium channel blockers (amlodipine) because of their higher blood pressure levels (p = 0.000). Aspirin, statins, and B-adrenoblockers are known to affect the levels of pro-inflammatory markers, particularly C-reactive protein (CRP) and interleukin-6 (IL-6). No significant differences were found among a number of these drugs consumed by the study groups.
|
|
In-hospital period |
p |
1 year post-discharge |
? |
||
|
Clopidogrel, n = 31 |
Ticagrelor, n = 49 |
Clopidogrel, n = 31 |
Ticagrelor, n = 49 |
|||
|
?-blockers, n (%) |
31 (100) |
49 (100) |
1.0 |
22 (73.3) |
41 (83.6) |
0.20 |
|
ACE inhibitors, n (%) |
27 (87) |
48 (97.9) |
0.07 |
20 (66.6) |
36 (73.5) |
0.34 |
|
ARBs, n (%) |
1 (3.2) |
1 (2) |
0.62 |
3 (10) |
3 (6.1) |
0.42 |
|
Statins, n (%) |
31 (100) |
49 (100) |
1.0 |
4 (13.3) |
- |
- |
|
Nitrates, n (%) |
2 (6.5) |
5 (10.2) |
0.44 |
3 (10) |
4 (8.2) |
0.55 |
|
Diuretics, n (%) |
8 (25.8) |
- |
- |
25 (83.3) |
41 (83.7) |
0.60 |
|
Aldosterone receptor antagonists, n (%) |
3 (9.7) |
10 (20.4) |
0.17 |
29 (96.6) |
48 (98) |
0.61 |
|
Dihydropyridine calcium channel blockers, n (%) |
6 (19.3) |
29 (59.2) |
0.000* |
22 (73.3) |
41 (83.6) |
0.20 |
|
Antiarrhythmic drugs, n (%) |
4 (12.9) |
3 (6.1) |
0.25 |
20 (66.6) |
36 (73.5) |
0.34 |
Table 3: Concomitant therapy in the in-hospital period and 1 year post-discharge
Note: ACE inhibitor: Angiotensin converting enzyme inhibitor; ARBs: Angiotensin II receptor blockers; * p < 0.05.
Clinical endpoints were assessed on day 8 after admission and 1 year after STEMI through telephone calls. Only 79 (100%) patients were interviewed 1 year after STEMI. The clinical endpoints were death; MI (repeated or recurrent); acute cerebrovascular accident; early post-infarction angina pectoris; stent thrombosis; repeated hospitalization for decompensation of heart failure; life-threatening heart rhythm disorders; and unstable angina. A composite endpoint is a clinically relevant endpoint that is constructed from combinations of the above-mentioned endpoints.
Minor and major bleeding constituted a primary endpoint for the evaluation of safety of switching antiplatelet drugs. In this study, minor bleeding was defined as minimal bleeding according to the TIMI criteria, and major bleeding as minor and major bleeding according to the TIMI scale.
2.2 Laboratory Assays
Platelet aggregation was measured with the Helena Laboratories kits (Beaumont, Texas). Platelet aggregation was assessed before and on day 7 after switching from clopidogrel to ticagrelor (i.e., on day 8 after STEMI) in platelet-rich plasma using the following agonists: adenosine diphosphate (ADP, 1.25 and 2.5 mcg/ml), epinephrine, collagen, and ristocetin. Platelet aggregation was measured using light transmission aggregometry. The sample was processed with laser light (650 nm). Platelets decrease light transmission, resulting in the amount of light reaching the photodetector being proportional to the number of platelets in the solution. The photodetector converts the light intensity into an analog signal, which is digitized and sent to the CPU for processing. Blood (5 ml) was collected by venipuncture into the VACUETTE tube containing buffered 3.2% sodium citrate. Platelet-rich plasma (PRP) was prepared by centrifugation of citrate samples at 1000 rpm for 7 min. Platelet-poor plasma (PPP) was used as the control sample, and it was prepared by centrifugation at 3000 rpm for 15 min. The absorbance of the unreacted PRP mixed with the aggregation reagent represents 0% aggregation, and the absorbance of a PPP control represents 100% aggregation. As platelets aggregate, the number of floating platelets decreases, reducing the light absorbed by the PRP. Light transmission platelet aggregation (%) from a healthy subject in response to different agonists is presented in Table 4.
|
Agonist |
??? % aggregation |
|
ADP 1.25 mkg/ml |
20-40 |
|
ADP 2.5 mkg/ml |
40-60 |
|
Epinephrine |
40-60 |
|
Collagen |
40-60 |
|
Spontaneous |
Up to 10 |
Table 4: Platelet aggregation in response to different agonists, %
Note: ADP: Adenosine diphosphate.
Plasma levels of IL-6 and CRP were measured 1 day after hospital admission (before switching from clopidogrel to ticagrelor) and on day 7 after switching (i.e., on day 8 of the in-hospital period). Levels of IL-6 and CRP were assessed with ELISA using commercially available kits (Biomerica kits, USA). The assay system utilizes a unique monoclonal antibody directed against a distinct antigenic determinant on the on the CRP molecule. The reference values for IL-6 and CRP levels were <4.1 pg/ml and <0.5 mg/l, respectively.
2.3 Statistical analysis
Statistical analysis was performed using STATISTICA 6.0. The Shapiro-Wilk test was used to assess the normality of distribution. Skewed data were presented as a median and interquartile range (Me: 25; 75). The Mann-Whitney test was used to qualitatively compare two independent groups. The chi-square test of independence was performed to examine for a statistically significant relationship between two categorical variables. p < 0.05 was considered statistically significant.
3. Results
No significant differences were found in the number of clinical endpoints that occurred on day 8 between the study groups. One case (3.2%) of stent thrombosis with recurrent MI and subsequent death was reported in the clopidogrel group. Clinical endpoints were assessed 1 year post-discharge (Figure 1). The rate of decompensation of chronic heart failure in both groups was comparable (p = 0.57). No cases of acute cerebrovascular accidents, unstable angina, and heart rhythm disorders in both groups were reported. Significant differences were not found in the rate of repeat MI between the clopidogrel and ticagrelor groups (p = 0.39). One patient in the clopidogrel group died because of stent thrombosis.
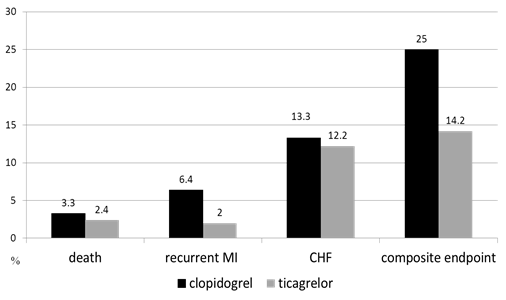
Figure 1: Clinical endpoints 1 year after STEMI
Patients were surveyed on their medication history to the recommended treatment regimen to assess the effects of concomitant therapy for coronary artery disease (Table 3). No significant differences were found in the number of drugs consumed by patients in the clopidogrel and ticagrelor groups. Most patients continued to take the recommended therapy within 1 year post-discharge. The duration of clopidogrel and ticagrelor therapies was evaluated according to the adherence to the recommended treatment regimen at 3 months, 6 months, and 1 year after STEMI (Table 5). Significant differences were not found in the duration of clopidogrel and ticagrelor therapies between the study groups. One patient in Group 1 prematurely discontinued clopidogrel within 1 month after hospital discharge because of gastrointestinal bleeding, which required hospital admission and blood transfusion. This patient had no previously recorded ulcers and did not receive appropriate gastroprotection. One patient stopped taking clopidogrel within 3-6 months, and 1 patient within 6-12 months. Notably, no adverse cardiovascular events were reported in patients who prematurely discontinued clopidogrel during the 1-year follow-up. Two patients prematurely discontinued ticagrelor within 3 months. Four patients stopped taking ticagrelor because of unusual bruising. Thus, ticagrelor was replaced with 75 mg clopidogrel daily in these patients. No adverse cardiovascular events occurred in patients who prematurely discontinued ticagrelor.
|
Duration of therapy |
Clopidogrel, n = 30 |
Ticagrelor, n = 49 |
p |
|
within 3 months, n (%) |
29 (96.6) |
47 (95.9) |
0.67 |
|
within 6 month, n (%) |
28 (93.3) |
43 (87.7) |
0.34 |
|
within one year, n (%) |
27 (90) |
43 (87.8) |
0.53 |
Table 5: Duration of clopidogrel and ticagrelor therapy
The rates of minor and major bleeding were compared in the study groups on day 8 of the in-hospital period and 1 year post-discharge to assess the safety of switching antiplatelet drugs. No major and minor bleeding was reported, neither in the clopidogrel group nor in the ticagrelor group in the in-hospital period. However, 3 patients (6.1%) in the ticagrelor group and 1 patient (3.2%) in the clopidogrel group had minor bleeding (p = 0.49). One year after STEMI, 8 patients (26.6%) in the clopidogrel group and 12 patients (24.5%) in the ticagrelor group suffered from minor bleeding. No significant differences were found between the study groups (p = 0.48). One patient (3.3%) in the clopidogrel group had gastrointestinal bleeding (Figure 2).
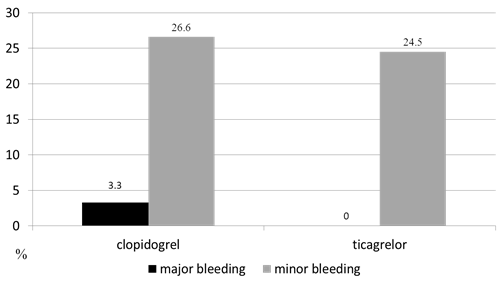
Figure 2: Rate of bleeding 1 year after STEMI
Based on the results of the assessment of platelet aggregation before switching from clopidogrel to ticagrelor (12-24 h after hospital admission), no significant differences were found in hypoaggregation in either group with either of the agonists (ADP 1.25 and 2.5 ?g/ml, epinephrine, collagen, ristocetin). However, the ADP-induced platelet aggregation when applied at 1.25 ?g/ml was 41.2% in the clopidogrel group and 40.6% in the ticagrelor group (p = 0.86). The ADP-induced platelet aggregation applied at 2.5 ?g/ml was 50.5% and 39.3%, respectively (p = 0.13).
No significant differences were found in CRP and IL-6 levels on day 1 after STEMI between the clopidogrel and ticagrelor groups. The CRP levels were 12.9 (2.9, 20.7) mg/l in the clopidogrel group and 12.8 (4.1, 18.8) mg/l in the ticagrelor group (p = 0.82). The IL-6 levels were 3.7 (3.35, 8.83) pg/ml and 3.2 (2.1, 5.5) pg/ml in the clopidogrel and ticagrelor groups, respectively (p = 0.27).
On day 8 after STEMI (i.e., on day 7 after switching antiplatelet drugs), the ADP-induced platelet aggregation (1.25 ?g/ml, 2.5 ?g/ml) was significantly lower in the ticagrelor group than that in the clopidogrel group. The ADP-induced platelet aggregation when applied at 1.25 ?g/ml was 34.8% (23, 47.9) in the clopidogrel group and 20.45% (11.8, 28.4) in the ticagrelor group (p = 0.001). The ADP-induced platelet aggregation when applied at 2.5 ?g/ml was 45.61% (32.7, 56) in the clopidogrel group and 30.3% (13.3, 41.6) in the ticagrelor group (p = 0.008; Table 6).
|
|
ADP-induced platelet aggregation (1.25 mkg/ml), % |
ADP-induced platelet aggregation (2.5 mkg/ml), % |
CRP, |
IL-6, |
|
Clopidogrel, n = 31 |
34.8 |
45.61 |
25.3 (4.6; 46.4) |
7.03 (2.7; 11.3) |
|
Ticagrelor, n = 49 |
20.45 |
30.3 |
17.5 (4.6; 20.9) |
2.8 |
|
p |
0.001* |
0.008* |
0.04* |
0.01* |
Table 6: Platelet aggregation and pro-inflammatory markers (CRP and IL-6) on day 7 after switching from clopidogrel to ticagrelor.
Note: ADP: Adenosine diphosphate; CRP: C-reactive protein; IL-6: Interleukin-6. * p < 0.05.
The CRP levels increased in both study groups on day 8 after STEMI (i.e., on day 7 after switching antiplatelet drugs). The increase in CRP levels before and after switching antiplatelet drugs in the clopidogrel group was statistically significant (12.9 (2.9, 20.7) mg/l vs. 25.3 (4.6, 46.4) mg/l, respectively, p = 0.01). By contrast, the increase in CRP levels in the ticagrelor group was not statistically significant (12.8 (4.1, 18.8) mg/l vs. 17.5 (4.6, 20.9) mg/l, respectively, p = 0.11). Thus, the CRP levels on day 8 after STEMI were significantly higher in the clopidogrel group than those in the ticagrelor group (p = 0.04; Table 6).
On day 8 after STEMI (i.e., on day 7 after switching antiplatelet drugs), the IL-6 levels before and after switching antiplatelet drugs remained within the reference range in the ticagrelor group (3.2 (2.1, 5.5) pg/ml vs. 2.8 (1.8, 4.2) pg/ml, respectively, p = 0.89). The IL-6 levels increased twofold in the clopidogrel group, but the difference did not reach reliable values (3.7 (3.35, 8.83) pg/ml vs. 7.03 (2.7, 11.3) pg/ml, p = 0.09). Thus, the IL-6 levels were significantly higher in the clopidogrel group than those in the ticagrelor group (p = 0.01; Table 5).
4. Discussion
Ticagrelor (AZD6140) is the first reversibly binding oral P2Y(12) receptor antagonist that blocks ADP-induced platelet aggregation. [5]. The pharmacological and clinical effects of ticagrelor do not require metabolic activation in vivo. Ticagrelor therapy is associated with rapid onset and faster offset of actions and greater and consistent effects than clopidogrel [8]. The results of the PLATO trial suggested the superiority of ticagrelor over clopidogrel by more pronounced inhibition of platelet aggregation (percentage of platelet aggregation in the clopidogrel and ticagrelor groups was 44 ± 15% and 28 ± 10%, respectively, p < 0.0001) [9]. The findings obtained in the current study are consistent with the PLATO results. The average life span of circulating platelets is 7 days, which suggests that the complete renewal of platelets blocked by clopidogrel takes 7 days [10]. Based on this fact, we selected this period to measure cytokine levels. Inhibition of platelet aggregation on day 7 after switching antiplatelet drugs was more pronounced in patients who continued receiving ticagrelor compared with those who continued receiving clopidogrel.
The results of the PLATO trial demonstrated the superiority of ticagrelor over clopidogrel in reducing the rate of cardiovascular death (21%) and MI (16%) in ACS patients. Significant reduction in the overall mortality was found in the subgroups of MI patients undergoing both conservative and invasive treatments [11]. However, the unprecedented effects of ticagrelor could not be explained only by its antiplatelet effect [12].
The inflammatory theory of atherosclerosis dates back to the 19th century, and the role of inflammation in the development of ACS is actively discussed [13]. This concept is confirmed by elevated levels of inflammatory markers (CRP, IL-6, fibrinogen, etc.) in blood samples of patients with cardiovascular diseases [14,15]. Oh et al. described the deposition of CRP in the ischemic myocardium in a rat acute MI model [16]. Similar results were obtained by Guo et al. in a pig MI model after thrombolysis [17].
The association between CRP and IL-6 levels and prognosis in patients with cardiovascular diseases has been proven in a number of studies. Ridker et al. reported that elevated baseline levels of CRP in healthy men predict the risk of future MI and thromboembolic stroke [18]. A recently published study comprising 60 patients showed that the levels of pro-inflammatory markers (IL-1?, IL-6, tumor necrosis factor) are significantly higher after CABG in patients with previous MI [19].
However, pro-inflammatory factors can affect platelet function, provoking hypercoagulation. Thus, Bester and Pretorius reported that IL-1?, IL-6, and IL-8 are critically involved in platelet hyperactivation [20]. Thomas et al. demonstrated the significant effect of P2Y12 inhibitors on inflammatory factors and prothrombotic mechanism [21]. They demonstrated the critical importance of platelets as central orchestrators of systemic inflammation induced by bacterial endotoxin. P2Y12 inhibitors are associated with lower mortality in patients with sepsis in clinical trials.
Significant inhibition of platelet aggregation was found in the ticagrelor group. Moreover, the levels of CRP and IL-6 on day 7 after switching antiplatelet drugs were lower in the ticagrelor group than those in the clopidogrel group. A trend toward a smaller number of endpoints occurred within 1 year after STEMI was found in the ticagrelor group compared with the clopidogrel group. This trend may be explained by a more pronounced pro-inflammatory effect of ticagrelor compared with clopidogrel. Therefore, the lower total mortality in the ticagrelor group in the PLATO trial may be explained by the fact that ticagrelor effectively blocks platelets involved in the synthesis of pro-inflammatory factors.
Switching from clopidogrel to ticagrelor is safe with regard to hemorrhagic complications. This study did not show any significant increase in the rate of bleeding in the in-hospital period and 1 year after STEMI in the ticagrelor group compared with the clopidogrel group. Ticagrelor and clopidogrel did not differ in the rate of major bleeding (11.6% vs. 11.2%, respectively), and fatal and/or life-threatening hemorrhages according to the PLATO criteria (5.8% in both groups). However, the incidence of major and minor bleeding in the PLATO trial was higher in the ticagrelor group (16.1%) than that in the clopidogrel group (14.6%, p = 0.0084) [13].
5. Conclusion
Switching from clopidogrel to ticagrelor on day 1 after MI was associated with fewer clinical endpoints that occurred within 1 year after STEMI. This replacement is safe with regard to the risk of hemorrhagic complications. It is also associated with lower platelet aggregation and inflammation activity assessed on day 8 after MI.
Conflicts of interest
None.
Funding
The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Li Z, Li Y, Zhang T, Miao W, Su G. Comparison of the influence of ticagrelor and clopidogrel on inflammatory biomarkers and vascular endothelial function for patients with ST-segment elevation myocardial infarction receiving emergency percutaneous coronary intervention: study protocol for a randomized controlled trial. Trials 17 (2016): 75.
- Tavlueva EV, Yarkovskaya AP, Alekseenko AV et al. Hypoaggregatory effect of switching clopidogrel to ticagrelor in patients with ST-segment elevation myocardial infarction. Atherothrombosis Journal 2 (2016): 54-60.
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, et al. ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 130 (2014): e344-e426.
- Mehta SR, Yusuf S. Clopidogrel in unstable angina to prevent recurrent events (?URE) trial investigators/effect of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345 (2001): 494-502.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361 (2009): 1045-1057.
- Varenhorst C, Alström U, Braun OÖ, Storey RF, Mahaffey KW, et al. Causes of mortality with ticagrelor compared with clopidogrel in acute coronary syndromes. Heart 100 (2014): 1762-1769.
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 372 (2015): 1791-1800.
- Averkov OV. Dual antiplatelet therapy as a long-term action in secondary prevention after myocardial infarction: focus on ticagrelor. Consilium medicum 17 (2015): 38-43.
- Lindholm D, Varenhorst C, Cannon CP, Harrington RA, Himmelmann A, et al. Ticagrelor versus clopidogrel in patient with non-ST-elevation acute coronary syndrome with or without revascularization: results from the PLATO trial. Eur Heart J 35 (2014): 2083-2093.
- Pleskanovskaja SA, Tachmuhammedova AH. Impact of the Juniperus turcomanica decoction on white blood cells population and population and morphology of platelets in circulating peripheral blood of apparently healthy subjects in vitro. Molodoyi uchenyi 12 (2015): 86-91.
- Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes. The PLATO (PLATelet inhibition and patient Outcomes) PLATELET Substudy. J Am Coll Cardiol 56 (2010): 1456-1462.
- DiNicolantonio JJ, Tomek A. Inactivations, deletions, non-adjudications, and downgrades of clinical endpoints on ticagrelor: serious concerns over the reliability of the PLATO trial. Int J Cardiol 168 (2013): 4076-4080.
- Stone GW. Ticagrelor in ACS: redefining a new standard of care? Lancet 375 (2010): 263-266.
- Sunitha S, Rajappa M, Mohan Thappa D, Chandrashekar L, Munisamy M, Revathy G. Is the ratio of antibodies against oxidized LDL to oxidized LDL an indicator of cardiovascular risk in psoriasis? Oman Med J 31 (2016): 390-393.
- Widén C, Holmer H, Coleman M, Tudor M, Ohlsson O, et al. Systemic inflammatory impact of periodontitis on acute coronary syndrome. J Clin Periodontol 43 (2016): 713-719.
- Oh SJ, Na Kim E, Jai Kim C, Choi JS, Kim KB. The effect of C-reactive protein deposition on myocardium with ischaemia-reperfusion injury in rats. Interact Cardiovasc Thorac Surg 25 (2017): 260-267.
- Guo FM, Han XH, Guo YY, Zhang DM, Tang FJ, Zhao L, Ji LL, Liu WB. Correlation study between interleukin-6 levels and coronary reflow. Eur Rev Med Pharmacol Sci 21 (2017): 1837-1842.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 97 (1998): 425-428.
- Kiris I, Kapan S, Narin C, Ozayd?n M, Cure MC, Sutcu R, Okutan H. Relationship between site of myocardial infarction, left ventricular function and cytokine levels in patients undergoing coronary artery surgery. Cardiovasc J Afr 27 (2016): 299-306.
- Bester J, Pretorius E. Effects of IL-1?, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep 6 (2016): 32188.
- Thomas MR, Outteridge SN, Ajjan RA, Phoenix F, Sangha GK, et al. Platelet P2Y12 inhibitors reduce systemic inflammation and its prothrombotic effects in an experimental human model. Arterioscler Thromb Vasc Biol 35 (2015): 2562-2570.
