Phytochemical Profiles of Albizia coriaria, Azadirachta indica, and Tylosema fassoglensis Used in the Management of Cancers in Elgon Sub-Region
Article Information
Ali Kudamba1,2,* , Hussein M. Kafeero1, Muhammad Lubowa5 , Shaban A. Okurut2, Hakim Nsubuga3, 4, Twaha Abiti3, Joweria Kayendeke2, Abdul Walusansa1,2 and Jamilu E. Ssenku1
1Faculty of Health Sciences, Habib Medical School, Islamic University in Uganda, Kampala
2Faculty of Science, Department of Biological Sciences, Islamic University in Uganda, Mbale
3Faculty of Science, Department of Chemistry and Biochemistry, Islamic University in Uganda, Mbale
4Faculty of Science, Department of Chemistry, Muni University
5Faculty of Science, Department of Food Science and Nutrition, Islamic University in Uganda, Mbale
*Corresponding author: Ali Kudamba, Faculty of Health Sciences, Habib Medical School, Islamic University in Uganda, Kampala.
Received: 17 July 2022; Accepted: 28 July 2022; Published: 20 August 2022
Citation: Ali Kudamba, Muhammad Lubowa, Hussein M. Kafeero, Shaban A. Okurut, Hakim Nsubuga, Twaha Abiti, Abdul Walusansa, Joweria Kayendeke, Hasifah Nanyingi, Muhamad S. Mubajje, and Jamilu E. Ssenku. Phytochemical Profiles of Albizia coriaria, Azadirachta indica, and Tylosema fassoglensis Used in the Management of Cancers in Elgon Sub-Region. Fortune Journal of Health Sciences 5 (2022): 461-471.
View / Download Pdf Share at FacebookAbstract
Background: Cancer is one of the serious health burdens in the 21st century with a steady rise in cases and deaths. The current interventions used in cancer management are chemotherapy, radiation, and surgery, but are ineffective, and also marred with several shortcomings. Preliminary studies indicated that residents in the Elgon sub-region use Albizia coriaria, Azadirachta indica & Tylosema fassoglensis as alternative cancer therapies but there is a paucity of data on their phytochemical profiles as a basis of scientific back-up. Therefore, our study documented the phytochemistry of Albizia coriaria, Azadirachta indica & Tylosema fassoglensis.
Materials & Methods: We adopted an experimental study design. The collected plant materials were cleaned, air dried, ground into a powder, and extracted by aqueous and ethanol. Ultraviolet spectrometry and gravimetry methods were used to determine the phytochemical profiles and data was analyzed with MedCalc version 20.008.
Result: The current study revealed that the three plant species exhibited varied phytochemical constituents. The highest mean concentrations were 10174, 748, 17751 & 8060 mg/100ml for polyphenols, flavonoids, tannins, and alkaloids respectively. Flavonoids from root extracts of Tylosema fassoglensis and Albizia coriaria were insignificant (P= 0.1060 & P= 0.4983) respectively and the rest of the phytochemical compositions significantly (P < 0.0001) varied.
Conclusions: The phytochemical profiles of plant species in the current study exhibited higher concentrations than the same species harvested in different parts of Uganda. Therefore, there is a need to carry out a study on the isolation & characterization of the phytochemical constituents; efficacy tests on anticancer activity against cancer cell-line, and safety in animal models.
Keywords
Phytochemical composition, Elgon sub-region, Albizia coriaria, Azadirachta indica, and Tylosema fassoglensis
Phytochemical composition articles, Elgon sub-region articles, Albizia coriaria articles, Azadirachta indica articles, and Tylosema fassoglensis articles
Article Details
1. Introduction
Currently, cancer is one of the leading global health burdens [1, 2]. In East Africa alone, cervical and prostate cancers have been reported as the most pronounced causes of morbidity and mortality, with over 40 cases per 100,000 of the population [3]. In the case of Uganda, cancer incidences in Kyadondo county revealed incidence rates of 132.5 per million and this is so far, the highest recorded in East Africa and second in Africa after Zimbabwe with an incidence rate of 146.2 per million [4]. In the Elgon sub-region of Uganda, the actual cancer statistics are difficult to establish, due to the lack of a regional region registry. However, informal interviews with health workers at Mbale Referral Regional Hospital indicate rising cancer incidences. The current treatment options for cancer are chemotherapy, surgery, and radiotherapy.
All these are associated with several shortcomings such as being expensive, less readily available, and severe side effects leading to the development of secondary cancer in distant organs like the bladder, liver & kidney among others. Above all, those options are not effective in the treatment of the advanced stage of the disease [5]. Due to these shortcomings, it has been asserted that 70 -80 % of the population in developing countries relies on alternative therapies such as herbal medicines to manage cancer-related complications. The prominence in the use of herbal medicine has been associated with their affordability, perceived effectiveness & cultural acceptability among others [6]. In Uganda, over 168 plant species with anticancer potential have been documented [7] but there are limited exploration studies on their phytochemical profiles.
The anticancer potential of medicinal plants has been associated with the presence of several active secondary metabolites such as alkaloids, saponins, tannins, glycosides, terpenes, flavonoids, reducing sugars, pentoses, & general carbohydrates, anthraquinone, its derivative & ketones [8-10]. Meanwhile, studies by [11, 12] revealed that phytochemical constituents in a plant are affected by several factors such as plant species soil mineral content & pH, methods, altitude, annual rainfall & seasonal patterns, storage conditions, annual temperature variations, relative humidity, and extraction. Thus, the phytochemical profiles reported in other parts of the world for the same species may not be a total reflection of those harvested from the Elgon sub-region due to differences in habitat conditions.
Our preliminary study documented the phytochemical constituent of Albizia coriaria Welw. ex. Oliver Azadirachta indica and Tylosema fassoglensis Schweinf, Torre & Hillc, are believed to the be most commonly used plant species by residents in the Elgon sub-region in the management of cancers [13]. Nonetheless, there is a paucity of data on the phytochemical profiles of these species to give a scientific backup on their anticancer potential. Therefore, our study documented the phytochemical profiles of the three most used commonly plant species in the management of cancers in the Elgon sub-region of Uganda. The findings may lead to the development of newer and more effective anticancer products, reduce the exorbitant treatment costs and expound on the government revenue through the sale of formulae to potential investors.
2. Methods & Materials
The plant materials we used in our study were collected from the Sironko and Bulambuli districts located on the slopes of mountain Elgon. These two districts are 24.7 km & 55. 4 km and 275.9 & 306.8 km from Mbale city and Kampala, the Capital City of Uganda respectively (Google Map). Both districts lie within the geographical coordinates of 1o17’ N &1o24 N and 34o15 E & 34o45 E respectively and an average elevation of 3996 ft (1,218 m) above sea level (Google Map, 2020). The average annual rainfall ranges from 920 mm to 1650 mm and the temperature of 24.4 o C per annum[14]. These conditions alongside fertile volcanic soils are known to influence the rate of synthesis, degradation, interconversion, oxidation, and reduction of the phytochemistry of these herbal plants harvested from the Elgon sub-region[15].
2.2 Study Design
We adopted an experimental study design. The three medicinal plant species, in the current study, were selected based on their high frequency of use, which was revealed after in-depth interviews with renowned herbalists in this area as revealed in our earlier preliminary study conducted. Plant identification was done by an expert in the Department of Plant Sciences, Microbiology & Biotechnology, College of Natural Sciences, Makerere University. Gravimetry and UV Spectrometry methods were used in the determination methods for the phytochemical analysis. MedCalc version, 20.008 was used to analyze the generated was transformed into categorical data and was subjected to the non-parametric test, the chi-square test [16]. The analyzed data was presented in tables and figures for easy interpretation.
2.3 Authentication of the Plant Species Used
The aerial plant parts and root tubers, used in our study were identified in-situ by an expert in the Department of Plant Sciences, Microbiology and Biotechnology, College of Natural Sciences, Makerere University. The plant materials were detached from parent plants, labeled, wrapped in the old newspaper, and then placed in the plant placement. They were then transferred to the Makerere University Herbarium laboratory for further scrutiny. The identified plant species names were confirmed using the standard protocol of comprehensive databases, plantlist. org[17].
2.4 Pretreatment and Processing of Plant Materials
The fresh plant materials were well labeled, washed with running tap water, and air-dried in the biology laboratory at the Islamic University in Uganda. These plant materials were then transported to Natural Chemotherapeutic laboratories and were crushed into a fine powder by use of a wood powder machine. Serial extraction was used first by ethanol and then followed by water. Finally, both extracts were subjected to both qualitative and quantitative phytochemical analysis.
2.5 Phytochemical Analysis
2.5.1 Qualitative Phytochemical Analysis
Procedure for phytochemical test
The current study adopted the quantitative analysis procedure laid down by[6],[18] &[19] with modifications as described below:
Test for Phenol: Ferric chloride test: 10mg extracts were treated with a few drops of ferric chloride solution. The formation of bluish-black color indicates the presence of phenol.
Test for Tannins: A small quantity of extract was mixed with water and heated in a water bath. The mixture was filtered and ferric chloride was added to the filtrate. A dark green color was formed. It indicates the presence of tannins.
Test of saponins: Foam test: About 0.5mg of the extract was shaken with five ml of distilled water. Formation of frothing (appearance of creamy miss of small bubbles) shows the presence of saponins.
Test for Phlobatannins: HCl test: 2 mL extract was added to 2 mL 1% HCl and boiled. A red precipitate shows the presence of phlobatannins.
Test for alkaloids: Iodine test: 3ml of the extract’s solution was added few drops of iodine. A blue color that disappears on boiling and reappears on cooling show the presence of alkaloids.
Test for flavonoids: Alkaline reagent test: 1 mL extract was added to 2 mL of 2 % of sodium hydroxide solution. An intense yellow color becomes colorless with the addition of diluted acid showing the presence of flavonoids.
Tests for anthraquinone: Bontrager’s test: 10 mL of 10 % ammonia solution was added with a few drops of the filtrate (shake vigorously for 30 seconds). The appearance of pink, violet, or red-colored solution indicates the presence of anthraquinone.
Test for terpenoids: 0.5g of the extracts was added to 2 ml of chloroform and evaporated to dryness and then 3 ml of concentrated sulphuric acid was added. The appearance of reddish-brown color in the interphase indicates the presence of terpenoids.
Test for steroids and sterols: 5 mg of extract was dissolved in 2 ml of chloroform and an equal volume of concentrated sulphuric acid was added along the sides of the test tube. The upper layer turns red and the lower layer turns yellow with green fluorescence, indicating the presence of the steroids and sterols compound, in the extract.
Test for Proteins and Amino acids: Millon’s test: 2 mL extract was added few drops of millon reagent and once the white precipitate is formed indicates the presence of proteins and amino acids.
Test for reducing sugar: Benedict test: 0.5 mL extract was added to 0.5 mL benedict reagent and boiled for 1 minute and the appearance of green/yellow/ red color indicates the presence of sugar.
Test for Glycosides: Bontrager’s test: 2mL hydrolysate was added to 3 mL chloroform and shaken well, in the chloroform layer is separated and then 10 % ammonia was added and a pink-colored solution indicates the presence of glycosides.
2.5.2 Quantitative Phytochemical Determination
The quantification of the different phytochemical compounds was done by gravimetric and spectrophotometric methods, following the already prescribed procedure laid down by [20], [6] & [18] with modifications as described below:
2.5.3 Determination of total Phenolic Compounds
The total phenolic compounds were determined using Folin- Ciocalteu reagent. A total of 100 mg of samples were introduced into test tubes and 10 ml of 50% methanol was added into two portions of 5 ml each. Each sample was extracted by manually shaking for 30 minutes & 40 minutes, respectively. After each extraction, sample extracts were kept at -20 oC for 10 minutes, and thereafter, it was centrifuged at 300 rpm for 10 minutes. The extracts were decanted into a test tube and 5.0 ml of Folin-Ciocalteu reagent (2N) was diluted with 5 ml of distilled water. 0.1 ml of each the samples was diluted with 0.4 ml distilled water and diluted with Folin-Ciocalteu reagent (0.25 ml) and 1.25ml of 20% sodium carbonate was added to each sample and shaken with the help of a vortex. The tubes were vortexed for 15 minutes and then allowed to stand for 35 minutes and were measured at the absorbance of 725 nm. Different concentrations of garlic acid 10, 50, 100, 250, and 500 mg/ml, were prepared in methanol for the preparation of a standard curve. The results were expressed in mg gallic acid equivalents g-1 dried extract (General analytical evaluation, GAE/g dry fruit weight).
2.5.4 Determination of total Tannins:
500 mg of each of the powdered samples was weighed and put into a 100ml plastic bottle. 50ml of distilled water was added and shaken for 1hr in a mechanical shaker. The mixture was then filtered into a 50ml conical flask and made up to the mark. Then, 5ml of the filtrate was pipetted out into a tube and mixed with 3ml of 0.1M FeCl3 in 0.1N hydrochloric acid and 0.008M potassium ferrocyanide. The absorbance was measured in a spectrophotometer at 530nm wavelength, within 10 minutes. A calibration curve was developed using garlic acid and results were expressed as General analytical evaluation, GAE/g.
2.5.5 Determination of total Flavonoids:
Total flavonoid was determined spectrophotometrically using a method reported by [21]. 2.0 ml of the ethanol extracts were put in test tubes and an equal volume (2ml) of 2% AlCl3 solution was added to each. The solution was incubated at room temperature for 1 hour and the absorbance was measured at 420 nm. A standard curve of rutin was prepared by dissolving 100 mg of rutin in 100 ml of methanol as the stock solution that is the concentration of 1mg/ml. The stock solution was diluted to a concentration ranging from 0, 1.5, 0.3, 0.6, and 1.2 mg/ml with distilled water. The results were then expressed as rutin equivalents (mg rutin equivalents per g). Then total flavonoid contents were calculated as rutin (mg/g) using the following equation based on the calibration curve.
2.5.6 Determination of total Alkaloids:
100mg of the powdered samples were weighed and put into a 250ml beaker and 200ml of 20% acetic acid in ethanol was added and covered to stand for 4hrs. This was filtered and the extracts were concentrated using a water bath to one-quarter of their original volume. Concentrated ammonium hydroxide was then added dropwise to the extract until the precipitation was complete. The whole solution was allowed to settle, and the precipitate was collected by filtration using filter paper and weighed. The percentage weight of alkaloid could be derived using the formula stated below:
(W2-W1)/ W3 * 100; Where W1= weight of filter paper, W2= weight of residue + filter paper, W3= weight of the sample).
2.5.7 Data Analysis & Presentation
The collected data was cleaned, transformed to categorical data as per the procedure laid down by[22] [22] and entered into excel and it was then exported to MedCalc(version 20.008) for analysis following the procedure previously described by [23] with modifications. The continuous data were transformed to categorical data by rounding off the nearest whole number. The difference in the mean concentration of the phytochemical compositions was tested chi-square test. a non-parametric of (95 % Cl). The analyzed data was presented in the tables and figures for easy interpretation.
3. Result
Table 1: Summary of Qualitative Analysis
|
Plant |
Parts |
Phytochemical |
Results |
Conclusion |
||
|
Aqueous Ethanol extracts extracts |
Aqueous extracts |
Ethanol extracts |
||||
|
Azadirachta indica (Neem tree (Murubaine) |
Leaves |
Alkaloids |
+ |
+ |
Present |
Present |
|
Flavonoid |
+ |
+ |
Present |
Present |
||
|
Tannins |
+ |
+ |
Present |
Present |
||
|
Phenols |
+ |
+ |
Present |
Present |
||
|
Steroids |
- |
+ |
Absent |
Absent |
||
|
Terpenoids |
+ |
+ |
Present |
Present |
||
|
Phlobatannins |
+ |
+ |
Present |
Present |
||
|
Anthraquinones |
+ |
+ |
Present |
Present |
||
|
Saponin |
+ |
+ |
Present |
Present |
||
|
Glycosides |
+ |
- |
Present |
Absent |
||
|
Reducing sugars |
+ |
+ |
Present |
Present |
||
|
Proteins |
- |
- |
Absent |
Absent |
||
|
Stem bark |
Alkaloids |
+ |
+ |
Present |
Present |
|
|
Flavonoid |
+ |
+ |
Present |
Present |
||
|
Tannins |
+ |
+ |
Present |
Present |
||
|
Phenols |
+ |
+ |
Present |
Present |
||
|
Steroids |
- |
+ |
Absent |
Present |
||
|
Terpenoids |
+ |
+ |
Present |
Present |
||
|
Phlobatannins |
+ |
+ |
Present |
Present |
||
|
Anthraquinones |
+ |
+ |
Present |
Present |
||
|
Saponin |
+ |
+ |
Present |
Present |
||
|
Glycosides |
- |
+ |
Absent |
Present |
||
|
Reducing sugars |
+ |
+ |
Present |
Present |
||
|
Proteins |
- |
- |
Absent |
Absent |
||
|
Roots |
Alkaloids |
+ |
+ |
Present |
Present |
|
|
Flavonoid |
+ |
+ |
Present |
Present |
||
|
Tannins |
+ |
+ |
Present |
Present |
||
|
Phenols |
+ |
+ |
Present |
Present |
||
|
Steroids |
- |
+ |
Present |
Present |
||
|
Terpenoids |
+ |
+ |
Present |
Present |
||
|
Phlobatannins |
+ |
+ |
Present |
Present |
||
|
Anthraquinones |
+ |
+ |
Present |
Present |
||
|
Saponin |
- |
- |
Absent |
Absent |
||
|
Glycosides |
- |
- |
Absent |
Absent |
||
|
Reducing sugars |
+ |
+ |
Present |
Present |
||
|
Proteins |
- |
- |
Present |
Present |
||
|
Albizia coriaria (Kiluku) |
Roots |
Alkaloids |
+ |
+ |
Present |
Present |
|
Flavonoid |
+ |
+ |
Present |
Present |
||
|
Tannins |
+ |
+ |
Present |
Present |
||
|
Phenols |
+ |
+ |
Present |
Present |
||
|
Steroids |
- |
+ |
Absent |
Present |
||
|
Terpenoids |
+ |
+ |
Present |
Present |
||
|
Phlobatannins |
+ |
+ |
Present |
Present |
||
|
Anthraquinones |
- |
+ |
Absent |
Present |
||
|
Saponin |
+ |
+ |
Present |
Present |
||
|
Glycosides |
- |
- |
Absent |
Absent |
||
|
Reducing sugars |
+ |
+ |
Present |
Present |
||
|
Proteins |
+ |
- |
Present |
Absent |
||
|
Stem barks |
Alkaloids |
+ |
+ |
Present |
Present |
|
|
Flavonoid |
+ |
+ |
Present |
Present |
||
|
Tannins |
+ |
+ |
Present |
Present |
||
|
Phenols |
+ |
+ |
Present |
Present |
||
|
Steroids |
- |
+ |
Absent |
Present |
||
|
Terpenoids |
+ |
+ |
Present |
Present |
||
|
Phlobatannins |
+ |
+ |
Present |
Present |
||
|
Anthraquinones |
+ |
+ |
Present |
Present |
||
|
Saponin |
+ |
+ |
Present |
Present |
||
|
Glycosides |
- |
- |
Absent |
Absent |
||
|
Reducing sugars |
+ |
+ |
Present |
Present |
||
|
Proteins |
+ |
- |
Present |
Absent |
||
|
Tylosema fassoglensis (Kikayi) |
Root tuber |
Alkaloids |
+ |
+ |
Present |
Present |
|
Flavonoid |
+ |
+ |
Present |
Present |
||
|
Tannins |
+ |
+ |
Present |
Present |
||
|
Phenols |
+ |
+ |
Present |
Present |
||
|
Steroids |
- |
+ |
Absent |
Absent |
||
|
Terpenoids |
+ |
+ |
Present |
Present |
||
|
Phlobatannins |
+ |
+ |
Present |
Present |
||
|
Anthraquinones |
+ |
+ |
Present |
Present |
||
|
Saponin |
+ |
+ |
Present |
Present |
||
|
Glycosides |
+ |
- |
Present |
Absent |
||
|
Reducing sugars |
+ |
+ |
Present |
Present |
||
|
Proteins |
- |
- |
Absent |
Absent |
||
The results indicate that all the three plant species under the present study were highly embodied with a variety of bioactive compounds. Interestingly, variations existed in some instances in the phytochemical constituents within the same parts and species. Generally, all three plant species contain several bioactive molecules like alkaloids, flavonoids, phenols, tannins, phlobatannins, saponins, glycosides, reducing sugars, anthraquinone, terpenoids, and glycosides. Proteins were only present in the aqueous stem bark extracts of Albizia coriaria. Aqueous leaf and stem bark extracts of Azadirachta indica contained no steroids and glycosides. Furthermore, the findings indicated that both aqueous and ethanol stem bark extracts of Albizia coriaria lacked glycosides and steroids & anthraquinone in roots aqueous extracts while both root tuber extracts of Tylosema fassoglensis lacked steroids and glycosides respectively.
Table 2: Relative concentration of the phytochemicals in the different plant parts
|
Plant |
Parts |
Phytochemical |
X2 |
95 % Cl |
P value |
|
Aqueous extracts |
|||||
|
Azadirachta indica (Neem tree (Murubaine) |
Leaves |
Alkaloids |
172.772 |
0.785 to 2.826 |
P < 0.0001 |
|
Flavonoids |
24.329 |
70.568 to 399.912 |
P < 0.0001 |
||
|
Tannins |
217.05 |
736.966 to 1080.034 |
P < 0.0001 |
||
|
Polyphenols |
264.198 |
9964.848 to 10384.152 |
P < 0.0001 |
||
|
Stem bark |
Alkaloids |
148.772 |
0.785 to 2.826 |
P < 0.0001 |
|
|
Flavonoids |
90.768 |
250.547 to 280.533 |
P < 0.0001 |
||
|
Tannins |
168.544 |
1207.571 to 2554.429 |
P < 0.0001 |
||
|
Polyphenols |
103.068 |
520.284 to 3658.716 |
P < 0.0001 |
||
|
Roots |
Alkaloids |
227.611 |
0.551 to 3.094 |
P < 0.0001 |
|
|
Flavonoids |
134.473 |
70.568 to 399.912 |
P < 0.0001 |
||
|
Tannins |
14935.913 |
2105.277 to 2554.429 |
P < 0.0001 |
||
|
Polyphenols |
111.184 |
1268.881 to 1527.119 |
P < 0.0001 |
||
|
Albizia coriaria (Kiluku) |
Roots |
Alkaloids |
901.408 |
-1.210 to 4.022 |
P < 0.0001 |
|
Flavonoids |
0.459 |
268.598 to 406.842 |
P =0.4983 |
||
|
Tannins |
361.029 |
2995.814 to 3758.186 |
P < 0.0001 |
||
|
Polyphenols |
65.358 |
757.183 to 7173.817 |
P < 0.0001 |
||
|
Stem barks |
Alkaloids |
92.801 |
2.276 to 3.846 |
P < 0.0001 |
|
|
Flavonoids |
94.635 |
70.568 to 399.912 |
P < 0.0001 |
||
|
Tannins |
391.658 |
4477.498 to 13794.541 |
P < 0.0001 |
||
|
Polyphenols |
56.641 |
520.284 to 3658.716 |
P < 0.0001 |
||
|
Tylosema fassoglensis (Kikayi) |
Root tuber |
Alkaloids |
1852.178 |
-0.680 to 2.292 |
P < 0.0001 |
|
Flavonoids |
53.097 |
258.280 to 348.240 |
P =0.1060 |
||
|
Tannins |
2.613 |
734.057 to 3160.943 |
P < 0.0001 |
||
|
Polyphenols |
14.052 |
757.183 to 7173.817 |
P = 0.0002 |
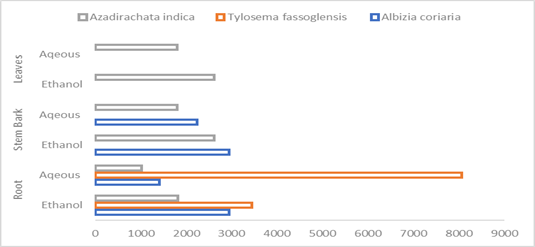
We also found out that all the three plant species exhibited a variety of phytochemical compositions of total flavonoids, polyphenols, tannins, and alkaloids. Total tannins and flavonoids exhibited the highest and lowest mean concentrations in the root extracts of Azadirachta indica roots were 17751 mg/100ml and 42.4 mg/100ml respectively. The highest mean concentration of flavonoid differed significantly (X2 = 10633.584, P < 0.0001) with respect for all other phytochemical constituents. The mean concentrations of flavonoids, tannins, alkaloids & polyphenols were significantly varied (P < 0.0001). Meanwhile the mean concentrations of total flavonoids and tannins in the root extracts of Albizia coriaria and Tylosema fassoglensis respectively were insignificant (X2= 0.459, P = 0.4983 & X2 = 2.613; P = 0.1060). Therefore, the mean concentrations of the different phytochemical constituents varied significantly in the different extracts in the same & different plant parts and species.
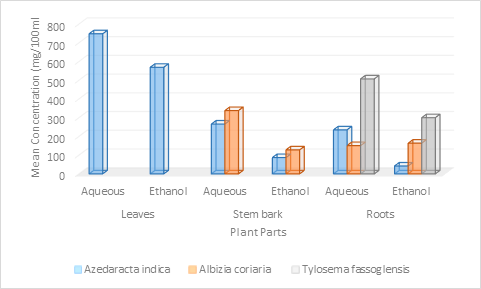
The present study also showed a varied concentration of flavonoids, in the same & different plant parts species. The highest and lowest flavonoids mean concentration was 748 mg/100ml & 42 mg/100 ml in aqueous leaf & ethanol root extracts of Azadirachta indica respectively. The highest mean concentrations of flavonoids were significant (X2= 630.932; P < 0.0001) with respect to the rest. The mean concentration of flavonoids in aqueous & ethanol extracts from the same plant part also varied significantly (P < 0.0001) except for root extracts of Albizia coriaria which was insignificant (X2 =0.45; p=0.4983). Meanwhile, the mean concentration of flavonoids in the root extracts of Albizia coriaria was insignificant (X2= 0.459; P=0.4983). Generally, the mean concentration of flavonoids significantly varied for different or the same plant extracts and species grown under the same conditions in the Elgon sub-region.
Our results further indicate that the highest and lowest mean concentration of polyphenols (1018mg/100ml and 1947mg/100ml) in root extracts Albizia coriaria and root tubers of Tylosema fassoglensis and significantly (X2 = 6008.922; P < 0.0001) varied with the respect to the rest. There was also a significant (P < 0.0001) difference in the mean concentration of polyphenols for the plant extracts obtained from the same or different parts and species.
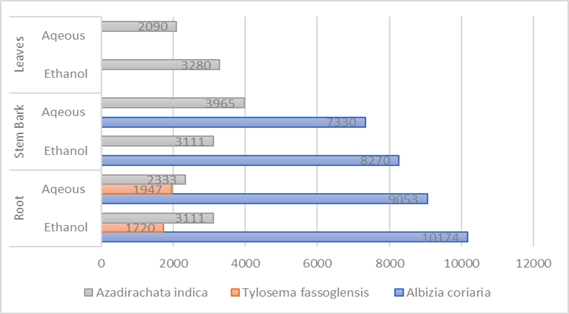
Interesting and varied results were obtained regarding the mean concentration of tannins in all the three plant species and different parts under the current study. Albizia coriaria and Azadirachta indica of ethanol root & leaf extracts respectively exhibited the highest and lowest mean concentration (17751 mg/100ml & 909 mg/100ml) respectively and was significant (X2 = 15203.745; P < 0.0001). Both ethanol and aqueous extracts from the same or different plant parts were significant (P < 0.0001). Therefore, the mean concentration of tannins in the three plant species and parts, harvested from the slopes of mountain Elgon differed.
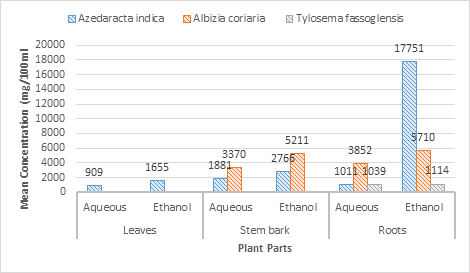
The highest and lowest mean concentration of alkaloids were 8060 mg/100ml & 803 mg/100ml from aqueous root tuber & leaf extracts of Tylosema fassoglensis & Azadirachta indica respectively and was a significant (X2= 5942.012; P < 0.0001). Similarly, both extracts differed significantly (P < 0.0001) in the mean concentration of alkaloids for both extracts from the same or different plant parts and species. Therefore, alkaloids significantly differed in the mean concentrations across plant species harvested under the same habitat conditions of the Elgon sub-region.
4. Discussions
We documented variations in the mean concentrations of flavonoids, phenols, tannins, phlobatannins, saponins, glycosides, reducing sugars, anthraquinone, terpenoids, and glycosides detected present in plant species. With exception of aqueous stem bark extracts of Albizia coriaria, all the plant species in our study lacked proteins in both aqueous and ethanol extracts. However, there existed some differences like the phytochemical composition within the same plant species and even parts extracted by different solvents. For example, aqueous extracts leaf and stem bark of Azadirachta indica lacked steroids and glycosides but were present in the ethanol leaf extracts. Ethanol stem bark extracts of Albizia coriaria lacked glycosides, steroids, and anthraquinone but were present in the aqueous roots extracts. Further, still, the results also showed that the aqueous & ethanol extracts from the root tuber of Tylosema fassoglensis lacked steroids and glycoside respectively. All three plant species exhibited a variety of bioactive molecules like alkaloids, flavonoids, tannins, polyphenols, anthraquinones, saponins, steroids, glycosides, and phlobatannins & reducing sugars. Flavonoids, phenols, anthraquinones & terpenoids have been documented in the leaves of aqueous & methanol Azadirachta indica (neem tree) and so agree with findings in the study[7]. On the contrary [20] showed differences in the phytochemical composition in extract for Azadirachta indica. This difference could be attributable to variations in plant habitat conditions of the plant harvested from the Elgon sub-region and those from Kogi State in Nigeria. For example, the average environmental temperatures in the area where their plants were harvested were much higher than in the current study. In addition to this, differences exist in altitude, rainfall & seasonal patterns, which are factors well documented for influencing the rate of synthesis, degradations, oxidations, reductions, and interconversion of the phytochemical compositions [24] & [25] and so the observed variations.
Our finding also indicated that all the three plant species exhibited varied quantities of total flavonoids, polyphenols, tannins, and alkaloids. Azadirachta indica registered the highest (17751 mg/100g) and lowest (42 mg/100g) mean concentration of the total tannins and flavonoid respectively in the ethanol root extracts. The highest and lowest concentration of total were tannins and flavonoids in the ethanol root extracts of Azadirachta roots were 17751 mg/100ml and 42.4 mg/100ml respectively, significant (X2 = 10633.584, P < 0.0001) differed with respect the rest. Generally, the mean difference in concentrations for flavonoids, tannins, alkaloids & polyphenol extract from the same or different plant parts was significant (P < 0.0001). However, the mean concentrations of total flavonoids of and tannins for the root extracts of Albizia coriaria and Tylosema fassoglensis was insignificant (X2= 0.459, P =0.4983 & X2 = 2.613; P = 0.1060) respectively. Therefore, the mean concentrations of different phytochemical constituents varied significantly in the different extracts from the same or different plant parts and species. The mean concentration of 210mg/ml, 27mg/100ml, 1450mg/100 & 3mg/100ml for flavonoid, glycosides for alkaloid Azadirachta documented by[26] was much lower than the one in the current study. This could be attributed to habitat conditions from which the plant species used in their study were harvested. For example, the plant used in their studies were harvested from an area of lower altitude and higher temperatures will a small amount of annual rainfall than the Elgon sub-region. These could have affected the rate of synthesis, degradation, and interconversion of the different phytochemical compounds.
The study revealed variations in flavonoids, in different plant parts and species. The highest mean concentration of 748 mg/100 ml and 42 mg/100ml in the aqueous and ethanol leaf extracts of Albizia coriaria and Azadirachta indica respectively. The highest mean concentration of flavonoids significantly (X2= 630.932; P < 0.0001) varied with respect to the rest. Furthermore, the mean concentration of flavonoids of both extracts from the same part or different plant parts also differed significantly (P < 0.0001). Our study revealed a higher concentration of flavonoids than those reported by [7] where their highest and lowest mean concentration of flavonoids was 13 ± 0.01 mg/100ml and 0.55 ± 0.01 mg/100 for ethanolic and aqueous extracts for Azadirachta indica collected from Mbarara and Jinja respectively. This could be attributed to the differences in the soil mineral contents & soil pH, rainfall and seasonal patterns, oxygen partial pressure, soil metallic ions, environmental temperatures & altitude among others. For example, in the Elgon sub-region, annual rainfall ranges from 920 mm & to 1650 mmHg, which is much higher than Mbarara (922 mm Hg) and Jinja (999.9 mm Hg. These variations in environmental conditions are responsible for the variations in the rate of synthesis, degradation, oxidation, reduction, interconversion & stability of the phytochemical components.
We found out that the highest and lowest mean concentrations (10175±16mg/100 ml and (1720 mg/100ml) of polyphenol in the ethanol root extract of Albizia coriaria and Tylosema fassoglensis were respectively. The mean concentration of polyphenols differed significantly from the rest (X2 = 6008.922; P < 0.0001) with respect to the rest. The mean concentration documented by [27] was 8.5 mg/100ml for polyphenol and this was much lower than documented in the present study. The present studies also revealed that the highest mean concentration of phenols is 6796 mg/100ml for extracts of Tylosema fassoglensis and this was higher than 8.53 ±57.00 mg/100ml recorded by[28]. This variation could be explained based on the differences in temperatures, altitude, and annual rainfall amount between South Africa, Abuja, and the Elgon sub-region. These variations in environmental conditions affect the rate of phenol synthesis, degradations, oxidation, reductions, stability & interconversion.
Interesting and varied results were documented regarding the concentration of tannins in all three plant species under the present study. Ethanol root and leaf extracts of Albizia coriaria & Azadirachta indica exhibited the highest and lowest mean tannins concentration (5710 mg/100ml & 909 mg/100ml) respectively. The difference in the tannin mean concentration for both extracts of the same or different plant parts and the highest and lowest were significant (X2 = 15203.745; P < 0.0001). Therefore, the mean concentration of tannins varied for the same or different plant parts and species harvested from the Elgon sub-region. Meanwhile, studies conducted by [24] showed observed that the mean concentration of tannins in aqueous and methanol leaf extracts of Azadirachta indica was 1.83±1.24 % and 29.9 mg/100ml and both were lower than in the current study. This difference was attributed to variations in the average environmental temperature of Abuja (29.6 o C), rainfall (4mm) and relative humidity (38 %), and altitude (197 meters). All these differed from those stated in the Elgon sub-region and so could have affected the rate of synthesis, degradation, oxidations, reductions, stability, and interconversion of tannins to other phytochemical constituents.
We found out that the highest and lowest alkaloids mean concentrations (8060 mg/100ml & 803 mg/100ml) aqueous root tuber of Tylosema fassoglensis & leaf extracts of Azadirachta indica respectively. There was a significant (X2= 5942.012; P < 0.0001) difference in the highest mean concentration of alkaloids with respect to the rest. Similarly, both extracts differed significantly (P < 0.0001) in the mean concentration of alkaloids for both extracts from the same plant parts and species. Therefore, alkaloids significantly differed in the mean concentrations from the same or different plant parts and species harvested under environmental conditions of the Elgon sub-region. Meanwhile [28] & [26]found out that the highest and lowest means concentration of alkaloid was 7.1 mg/100ml & 4.3 mg/100ml and this greatly differed from that of our study. This was due to variations in environmental factors that affect the rate of synthesis, degradation, stability, oxidation, reduction, and interconversions that vary significantly with environmental conditions.
5. Conclusions & Recommendations
The findings in the present study revealed that all the three plant species contain: flavonoids, phenols, alkaloids, tannins, steroids, saponins, terpenoids, phlobatannins, glycosides & reducing sugars. The highest mean concentrations of flavonoids, polyphenols, tannins, and alkaloids were 748, 10174, 17751 & 8060 mg/100ml respectively, and significantly differed (P < 0.0001) with respect to the rest. Due to the high concentration of phytochemical constituents in the plant species observed in this study, they could act as a potentially useful source for developing anticancer drugs in the nearby future. Therefore, we recommend that further research on the isolation and characterization of their phytochemical and efficacy tests on the anti-cancer activity against cancer cell-line and safety tests in the animal model be conducted to verify their claimed cancer healing potential & safety.
Ethical Approval and Consent to participate in the study
Approval for this study was provided by the Islamic University in Uganda, Research Review Committee under and was approved under ethical number, RCC/2019/GROUP2/016 Permission to access the communities and collect plant specimens was obtained from Sironko and Bulambuli districts Local leaders including LC1 Chairpersons of the respective villages.
Consent to publish
Not applicable.
Availability of data and materials
Data sets generated and analyzed during this study are available from the corresponding author on reasonable request.
List of abbreviations
WHO: World Health Organization
TM: Traditional medicine
LC: Local council
IUIU: Islamic University in Uganda
IsDB: Islamic Development Bank
References
- WHO, “WHO global report on traditional and complementary medicine 2019,” World Health Organization, (2019).
- Sung et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA. Cancer J. Clin 71 (2021): 209-249.
- Abila DB, Wasukira SB, Ainembabazi P and Wabinga H. “Burden of Risk Factors for Cervical Cancer Among Women Living in East Africa: An Analysis of the Latest Demographic Health Surveys Conducted Between 2014 and 2017,” JCO Glob. Oncol 7 (2021): 1116-1128.
- Stoeter O, et al. “Trends in childhood cancer incidence in sub-Saharan Africa: Results from 25 years of cancer registration in Harare (Zimbabwe) and Kyadondo (Uganda),” J. Cancer 149 (2021): 1002-1012.
- El-Hussein A, Manoto SL, Ombinda-Lemboumba S, Alrowaili ZA, Mthunzi-Kufa P. “A review of chemotherapy and photodynamic therapy for lung cancer treatment,” Anti-Cancer Agents Med. Chem. (Formerly Curr. Med. Chem. Agents) 21 (2021): 149-161.
- Obakiro SB et al. “Ethnobotany, ethnopharmacology, and phytochemistry of traditional medicinal plants used in the management of symptoms of tuberculosis in East Africa: a systematic review,” Med. Health 48 (2020): 1-21, 2020.
- Omara T, et al. “Medicinal plants used in traditional management of cancer in Uganda: a review of ethnobotanical surveys, phytochemistry, and anticancer studies,” Evidence-Based Complement. Altern. Med, (2020).
- Omara T, Kiprop AK, Kosgei VJ. “Intraspecific Variation of Phytochemicals, Antioxidant, and Antibacterial Activities of Different Solvent Extracts of Albizia coriaria Leaves from Some Agroecological Zones of Uganda,” Evidence-based Complement. Altern. Med (2021).
- “Review of Phytochemical and Medical Applications of Annona Muricata Fruits,” Chem. Rev 2 (2020): 70-79.
- Shiracko N, Owuor BO, Gakuubi MM, Wanzala W. “A survey of ethnobotany of the AbaWanga people in Kakamega county, Western province of Kenya,” (2016).
- Kokila K, Priyadharshini SD, Sujatha V. “Phytopharmacological properties of Albizia species: a review,” Int J Pharm Pharm Sci 5 (2013): 70-73.
- Raphael “Phytochemical constituents of some leaves extract of Aloe vera and Azadirachta indica plant species,” Glob. Adv. Res. J. Environ. Sci. Toxicol 1 (2012): 14-017.
- Choubey A, Parasar A, Choubey A, Iyer D, Pawar RS, Patil UK. “Potential of medicinal plants in kidney, gall and urinary stones,” J. Drug Dev. Res 2 (2010): 431-447.
- Allan B, Gorettie N, J. J.N, G. Michael, and A. Banana, “Perceptions of Climate Variability in the Mt. Elgon sub-region, Eastern Uganda. Cogent Environmental Science” (2016).
- Calzada F and Bautista E. “Plants used for the treatment of diarrhoea from Mexican flora with amoebicidal and giadicidal activity, and their phytochemical constituents,” Ethnopharmacol 253 (2020): 112676.
- Gachanja AN, Kareru PG, Kenji GM, Keriko JM, Mungai G. “Traditional Medicines Among the Embu and Mbeere Peoples of Kenya,” J. Tradit. Complement. Altern. Med.(Online) (2007): 75-86.
- Köhl KI, Basler G, Lüdemann A, Selbig J, Walther D. “A plant resource and experiment management system based on the Golm Plant Database as a basic tool for omics research,” Plant Methods 4 (2008): 1-11.
- Emaldi U, Nassar JM, Semprun C. “Physicochemical character and food value of two Venezuelan cactus fruits,” Sci 44 (2004): 105-107.
- Phuyal N, Jha PK, Raturi PP, Rajbhandary S. “Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC,” World J (2020).
- Anibijuwon II, Adedokun A, Mustapha SM, Abiola OC, Olayinka OS. “Multi-Drug Resistant Salmonella typhi among Out-Patients in Hospitals within Ilorin, Nigeria and their Susceptibility to Cymbopogon Citratus,” Niger. J. Pure Appl. Sci (2020): 3628-3638.
- Seal BK, Sil H, Mukherjee DC. “Independent determination of equilibrium constant and molar extinction coefficient of molecular complexes from spectrophotometric data by a graphical method,” Acta Part A Mol. Spectrosc 38 (1982): 289-292.
- Csikszentmihalyi Handbook of research methods for studying daily life. Guilford Press (2011).
- Hanusz Z, Tarasinska J, Zielinski W. “Shapiro-Wilk test with known mean,” REVSTAT-Statistical J 14 (2016): 89-100.
- Mapesa WA, Waweru MP, Bukachi F, Wafula KD. “Aqueous Tuber Extracts of Tylosema fassoglense (Kotschy ex Schweinf.) Torre and Hillc.(Fabaceae). Possess Significant In-Vivo Antidiarrheal Activity and Ex-Vivo Spasmolytic Effect Possibly Mediated by Modulation of Nitrous Oxide System, Voltage-Gated Calc,” Pharmacol 12 (2021): 266.
- Qaiser D, et al. “Novel Use of Fluorescein Dye in Detection of Oral Dysplasia and Oral Cancer,” Photodiagnosis Photodyn. Ther 31 (2020).
- Ujah II, Nsude CA, Ani ON, Alozieuwa UB, Okpako IO, Okwor AE, “Phytochemicals of neem plant (Azadirachta indica) explains its use in traditional medicine and pest control,” GSC Biol. Pharm. Sci 14 (2021): 165-171.
- Venmathi Maran BA, Josmeh D, Tan JK, Yong YS, Shah MD. “Efficacy of the Aqueous Extract of Azadirachta indica Against the Marine Parasitic Leech and Its Phytochemical Profiling,” Molecules 26 (2021): 1908.
- Saheed O, Adewale E, Anna A, BH M. “Effect of seasonal changes on the quantity of secondary metabolites from neem and eucalyptus plants in North Central Nigeria,” World J. Biol. Pharm. Heal. Sci 7 (2021): 43-52.