Phenotypic and Genomic Characterization of Rapidly Growing Non-Tuberculous Mycobacteria Isolated from Surgical Site Infections in a Tertiary Care Hospital of Dhaka
Article Information
Rafia Afreen Jalil*,1, ABM Bayezid Hossain2, Md. Manjur Alam2, Abdullah Al Tarique2, Nabila Khanduker2, Samia Shihab Uddin2, Imtiaz Ahmad2, Mushfique Manjur3, Shaila Akhtar1, Nooriya Haque1
1Department of Microbiology, Green Life Medical College & Hospital Ltd., Green Road, Dhaka, Bangladesh.
2Department of Surgery, Green Life Medical College & Hospital Ltd., Green Road, Dhaka, Bangladesh.
3Department of Orthopedics, Delta Medical College & Hospital, Dhaka, Bangladesh.
*Corresponding Author: Rafia Afreen Jalil, Department of Microbiology, Green Life Medical College & Hospital Ltd., Green Road, Dhaka, Bangladesh.
Received: 12 September 2025; Accepted: 19 September 2025; Published: 25 September 2025
Citation: Rafia Afreen Jalil, ABM Bayezid Hossain, Md. Manjur Alam, Abdullah Al Tarique, Nabila Khanduker, Samia Shihab Uddin, Imtiaz Ahmad, Mushfique Manjur, Shaila Akhtar, Nooriya Haque. Phenotypic and Genomic Characterization of Rapidly Growing Non- Tuberculous Mycobacteria Isolated from Surgical Site Infections in a Tertiary Care Hospital of Dhaka. Fortune Journal of Health Sciences. 8 (2025): 888-896.
View / Download Pdf Share at FacebookAbstract
Background: Chronic surgical site infections (SSIs) caused by rapidly growing non-tuberculous mycobacteria (RGM) are increasingly recognized, yet their epidemiology and antimicrobial profiles remain poorly defined.
Objective: The study aimed to isolate, identify, and characterize rapidly growing RGM from patients with SSIs, and to determine their antimicrobial susceptibility profiles using both phenotypic and molecular techniques.
Methods: In this cross-sectional study at Green Life Medical College & Hospital, Dhaka, 149 patients who developed SSI following laparoscopic or open surgery were evaluated over one year. Clinical specimens (wound swab, pus, and tissue) underwent culture on standard media. Bacterial and mycobacterial isolates were identified by colony morphology, biochemical assays, and GeneXpert MTB/RIF (to exclude M. tuberculosis). RGM species were further characterized by urea hydrolysis, citrate utilization, and susceptibility to Ciprofloxacin and Polymyxin B. Antibiotic susceptibility was assessed by disc diffusion and broth microdilution.
Results: Patients’ ages ranged from 22 to 80 years (mean 51.0±16.74 years); 54.36% were male. Most infections (67.79%) occurred following laparoscopic procedures, with symptoms typically onset at 2-5 weeks postoperatively. Of 149 samples, 84 (56.38%) yielded pathogens: non-tuberculous mycobacteria (17.45%) predominated, followed by Klebsiella spp. (15.44%), Staphylococcus aureus (10.06%) and Escherichia coli (7.38%). Among 26 RGM isolates, Mycobacterium abscessus comprised 80.77% and M. fortuitum 19.23%. Biochemically, all 26 were urea-positive and citrate-negative. Only 19.23% showed susceptibility to both Ciprofloxacin and Polymyxin B by disc diffusion. The broth microdilution method revealed 100% sensitivity of both species to Amikacin; M. fortuitum was uniformly susceptible to Ciprofloxacin, Meropenem, Clarithromycin, Doxycycline, and Linezolid. Conversely, M. abscessus exhibited high resistance to Ciprofloxacin (85.71%), Meropenem (100%), and Doxycycline (61.90%), while 61.90% remained susceptible to Clarithromycin and 76.19% to Linezolid. Both species were resistant to Trimethoprim-Sulfamethoxazole.
Conclusions: RGM, particularly M. abscessus, are significant SSI pathogens with diverse resistance profiles. Species-level identification and tailored antimicrobial susceptibility testing are essential for effective management.
Keywords
Rapidly growing Non-tuberculous Mycobacteria, chronic infection, SSIs, phenotypic and molecular characterization
Rapidly growing Non-tuberculous Mycobacteria articles, chronic infection articles, SSIs articles, phenotypic articles, molecular characterization articles
Article Details
Introduction
Non-tuberculous mycobacteria (NTM) are diverse acid-fast organisms, with rapidly growing mycobacteria (RGM) forming mature colonies within seven days [1]. Modern molecular methods have replaced the traditional Runyon criteria for their identification and classification [2]. NTM, particularly rapidly growing species like Mycobacterium fortuitum and M. chelonae, are emerging opportunistic pathogens [3]. They are increasingly recognized as causes of hospital-acquired infections, including surgical site infections (SSIs), leading to prolonged hospital stays and challenging treatments [4,5]. These infections often present as non-healing wounds unresponsive to standard antibiotics, with sterile routine cultures, warranting suspicion of NTM [6-8]. RGM commonly causes skin, soft tissue, bloodstream, pulmonary, and prosthetic joint infections, particularly in immunosuppressed individuals or those with disrupted barriers [9,10]. With rising immunosuppression, managing these infections has become increasingly important [11].RGM infections can mimic tuberculosis but generally cause less severe disease [12]. Definitive diagnosis relies on culture and DNA sequencing, since these organisms often fail to respond to standard anti-tubercular regimens [13,14]. Enhanced detection through improved culture methods, histopathology, and molecular sequencing has driven increased reporting of NTM infections [15,16].
Surgical site infections (SSIs) are a major cause of hospital-acquired infections, accounting for about 20% of cases [17]. Their occurrence depends on factors like the type of surgery (clean to dirty), the patient’s immune status, presence of foreign bodies or prostheses, and fluctuations in core body temperature [18]. SSIs significantly increase patient readmissions, hospital stays, costs, and mortality [19]. Patients with SSIs have doubled the mortality risk, are 60% more likely to require intensive care, and face five times higher readmission rates [17,20]. While the main causative organisms have remained relatively stable over the past 10-15 years, there has been a rise in reports of rare pathogens such as NTM, contributing to changing infection patterns [21,22]. Minimally invasive laparoscopic surgery is widely preferred for its benefits, including less pain and quicker recovery [7,23]. However, port-site infections caused by NTM, though rare, are increasingly reported and can lead to significant morbidity and prolonged hospitalization. This rise is linked to NTM contamination from hospital water systems, biofilm formation, disinfectant resistance, and lapses in infection control [24,25].
Although South Asia is recognized as a region where both tuberculosis and nontuberculous mycobacteria (NTM) are endemic [26], most data on the different NTM bacterial species come from India, with only limited information available from neighboring countries. In Bangladesh, NTM were first detected in sputum samples in 2010 [27] and later identified in various clinical specimens between 2013 and 2015 [28]. Subsequent epidemiological investigations into surgical site infections (SSIs) showed that NTM occurrences were relatively rare [29], though some cases confirmed NTM as causative agents of SSIs [30]. Studies have also reported the presence of rapidly growing mycobacteria from extrapulmonary infections in Bangladesh, identified using PCR-restriction fragment length polymorphism (PCR-RFLP) [31]. However, since these studies did not use gene sequencing for precise bacterial species identification, the specific NTM species involved and their clinical roles remain uncertain. This study, therefore, sought to isolate, identify, and phenotypically and genotypically characterize rapidly growing NTM from SSI cases in a tertiary care hospital and to define their antimicrobial susceptibility patterns.
Materials and Methods
Study design and settings
This cross-sectional observational study was conducted over a period of one year, from July 2022 to June 2023, at the Department of Microbiology in collaboration with the Department of Surgery at Green Life Medical College & Hospital, located in Dhaka 1205, Bangladesh.
Study sample criteria
A total of 149 clinical samples, including swabs, pus, and tissue specimens, were collected from patients with chronic SSI admitted to the hospital.
Inclusion criteria
- Patients with surgical wounds that remained unhealed for two weeks.
- Individuals displaying lesions that were clinically suspected to be tuberculosis.
- Cases where surgical site infection (SSI) lesions failed to improve despite standard treatment with antibiotics, anti-tuberculosis medications, or antifungal agents, regardless of age or sex.
Exclusion criteria
- Those with acute postoperative wound infections lasting less than two weeks.
- Individuals who declined to participate in the study.
Sample Collection: Samples were obtained aseptically. Sterile cotton swabs were used to collect material from the deepest part of the wound after cleaning with normal saline. If the wound surface was dry, the swab was first moistened with sterile saline. Pus was aspirated from drainage tubes or deep abscesses and transferred into sterile containers. Before collecting tissue samples, wounds were thoroughly cleansed and debrided using sterile non-bacteriostatic saline and antiseptic (70 % alcohol or chlorhexidine) to expose viable tissue. Biopsy was performed at the advancing margin or base of the wound using sterile instruments, avoiding necrotic or superficial tissue. Biopsy specimens were placed into sterile containers with minimal sterile saline.
Data Collection: Patient information, including age, sex, medical history, clinical observations, and laboratory findings, was systematically collected using a structured questionnaire and recorded on a dedicated data sheet.
Laboratory Procedures
Detection of RGM
All specimens underwent Gram and Ziehl-Neelsen (ZN) staining. Cultures were set up on Blood Agar, MacConkey Agar, and Lowenstein-Jensen (LJ) media and incubated at 37ºC aerobically up to 7 days. Typical bright red colored acid-fast bacilli (AFB), which were shorter, less curved, and not beaded on ZN stain, and rapid growth (≤7 days) with small (1-2mm), white to slightly yellowish in color, dry or smooth glistening pigmented or non-pigmented colonies on LJ and Blood Agar media indicated rapidly growing NTM. GeneXpert MTB/RIF assay excluded M. tuberculosis. Species of RGM were further characterized phenotypically (biochemical assays and antibiotic susceptibility) and genotypically (whole-genome sequencing). Ciprofloxacin and Polymyxin B susceptible RGM were interpreted as M. fortuitum, and resistant RGM were interpreted as M. abscessus [32,33].
Antimicrobial susceptibility testing
Broth microdilution (CLSI M24-A2): Minimum inhibitory concentrations (MICs) for Amikacin, Ciprofloxacin, Meropenem, Clarithromycin, Doxycycline, Linezolid, and Trimethoprim-Sulfamethoxazole were determined in cation-adjusted Mueller–Hinton broth, with Neotetrazolium chloride dye to enhance endpoint reading [34].
Disc diffusion: Kirby-Bauer method on Mueller-Hinton agar for comparative screening. The antibiotic discs used in this study were Amikacin (30µg), Ciprofloxacin (5 µg), Meropenem (10 µg), Doxycycline (30 µg), Clarithromycin (15 µg), Linezolid (30 µg), and Trimethoprim-Sulfamethoxazole (25 µg), and Polymyxin B (300 µg) obtained from Oxoid, UK.
Genotypic analysis
The RGM isolates were sequenced with Illumina next-generation sequencing (NGS) technology [35]. DNA was extracted (Maxwell RSC), quantified, and prepared for Illumina MiniSeq sequencing. Reads were quality-checked (FastQC), trimmed (fastp), assembled (Unicycler), and species-typed (rbMLST). All RGM isolates were preserved at –20 °C in trypticase soy broth with 15% glycerol for future studies.
Data analysis
Demographic and laboratory data were entered and analyzed using IBM SPSS Version 26 (New York, USA). Descriptive statistics were presented as frequencies (percentages) for categorical data and means (±SD) for continuous data.
Ethical statement
Participation was voluntary, with confidentiality ensured through individual code numbers. Informed consent was obtained after the study's objectives and potential outcomes were explained. The research adhered to the 2013 revised Declaration of Helsinki and its amendments, or comparable ethical standards. Ethical approval was obtained from the local Ethical Review Committee.
Results
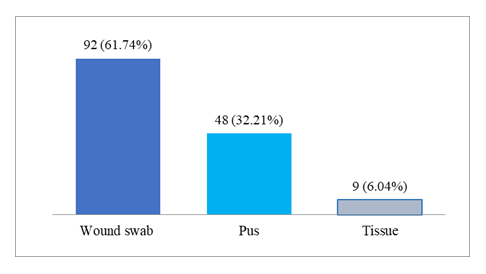
A total of 149 patients with a history of laparoscopic or open surgical procedures presented with chronic surgical site infections characterized by pus discharge, localized pain, and, in some cases, low-grade fever and granulomatous swelling at the scar site. The age of the patients ranged from 22 to 80 years, with a mean age of 51.0 ± 16.74 years, indicating that middle-aged and elderly individuals were commonly affected. Males constituted a slightly higher proportion (54.36%) compared to females (45.63%). The majority of infections (67.79%) followed laparoscopic procedures, while the remaining 32.21% occurred after open surgeries. The onset of symptoms typically occurred between 2 to 5 weeks post-surgery, with the highest proportion (34.22%) developing signs of infection by the third week. Only a small number of patients (2.01%) presented as late as eight weeks postoperatively. Figure 1 illustrates the distribution of sample types collected from patients with SSI. Wound swab samples were the most commonly received, accounting for 61.74% of the total, indicating their routine use in SSI diagnosis. Pus samples comprised 32.21%, reflecting cases with evident purulent discharge, while tissue samples were the least common (6.04%), typically collected during surgical debridement or biopsy.
Table 1: Baseline parameters of the RGM infected patients with SSI (n=149)
|
Baseline parameters |
Categories |
Frequency (n) |
Percent (%) |
|
Age (years) |
Range |
22-80 |
|
|
Mean±SD |
51.0±16.74 |
||
|
Gender |
Male |
81 |
54.36 |
|
Female |
68 |
45.63 |
|
|
Type of surgery |
Laparoscopic |
101 |
67.79 |
|
Open |
48 |
32.21 |
|
|
Duration of onset of infection |
2 weeks |
32 |
21.47 |
|
3 weeks |
51 |
34.22 |
|
|
4 weeks |
39 |
26.17 |
|
|
5 weeks |
24 |
16.11 |
|
|
8 weeks |
3 |
2.01 |
Table 2 presents the distribution of organisms isolated from SSI cases. Out of 149 samples, 84 (56.38%) showed growth of microorganisms. NTM were the most frequently isolated pathogen, accounting for 17.45% of all cases, highlighting their emerging role in chronic SSI. Klebsiella spp. was the second most common organism (15.44%), followed by Staphylococcus aureus (10.06%) and Escherichia coli (7.38%), indicating the presence of both gram-positive and gram-negative pathogens. Less frequently isolated organisms included other Enterobacteriaceae (Citrobacter, Enterobacter spp.) and Mycobacterium tuberculosis (2.01% each), while Enterococcus faecalis, Acinetobacter spp., and Nocardia spp. were identified in only 0.67% of cases each.
Table 2: Presence of various organisms isolated from SSI (n=84)
|
Organism |
Frequency (n) |
Percent (%) |
|
Staphylococcus aureus |
15 |
10.06 |
|
Escherichia coli |
11 |
7.38 |
|
Klebsiella spp. |
23 |
15.44 |
|
Non-tuberculous mycobacteria |
26 |
17.45 |
|
Other Enterobacteriaceae (Citrobacter, Enterobacter) |
3 |
2.01 |
|
Enterococcus faecalis |
1 |
0.67 |
|
Acinetobacter spp. |
1 |
0.67 |
|
Nocardia spp. |
1 |
0.67 |
|
Mycobacterium tuberculosis |
3 |
2.01 |
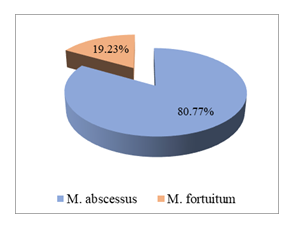
Figure 2 demonstrates that all non-tuberculous mycobacterial isolates from the surgical site infections were RGM. Among these, Mycobacterium abscessus was the predominant species, comprising 80.77% of RGM isolates, while M. fortuitum accounted for the remaining 19.23%.
Table 3 summarizes the phenotypic identification of RGM isolates based on biochemical tests and antibiotic susceptibility patterns using disc diffusion. All 26 isolates tested were negative for citrate utilization and positive for urea hydrolysis, consistent with characteristics of M. fortuitum or M. abscessus. Antibiotic susceptibility testing revealed that only 19.23% of the isolates were sensitive to both Ciprofloxacin and Polymyxin B, showing clear zones of inhibition, while the majority (80.77%) were resistant, exhibiting no inhibition zones.
Table 3: RGM species identification by biochemical tests and antibiotic (Ciprofloxacin and Polymyxin B) sensitivity pattern
|
Biochemical tests |
Interpretation |
Antibiotic disc diffusion |
Interpretation |
||
|
(n=26) |
test (n=26) |
||||
|
Citrate utilization |
Urea hydrolysis |
Ciprofloxacin |
Polymyxin B |
||
|
Negative (100%) |
Positive (100%) |
M. fortuitum |
Sensitive (19.23%) |
Sensitive (19.23%) |
M. fortuitum |
|
or |
|||||
|
M. abscessus |
Resistant (80.77%) |
Resistant (80.77%) |
M. chelonae |
||
|
or |
|||||
|
M. abscessus |
|||||
The species of RGM: M. fortuitum and M. abscessus, were further confirmed by whole-genome sequencing.
Table 4 shows antibiogram results of RGM by MIC broth microdilution and disc diffusion method on Muller-Hinton Agar. In the disc diffusion method, Amikacin produced 14.3 ± 2.46 mm mean zone of inhibition in case of M. abscessus and 19.5 ± 1.91 mm in case of M. fortuitum isolates, when all of them showed susceptibility to Amikacin by broth microdilution. All of the Ciprofloxacin-sensitive isolates of M. fortuitum produced a mean zone of 19.6 ± 2.25 mm by the disc diffusion method. No zone of inhibition was produced by Ciprofloxacin and Meropenem discs in all cases of the M. abscessus isolates. The mean zone diameter produced by all 5 Meropenem-sensitive M. fortuitum isolates by the disc diffusion method was 15 ± 2.12 mm. Mean zone of inhibition around Clarithromycin for M. fortuitum and M. abscessus were 23.80 ± 1.2 mm and 21.65 ± 3.35 mm, respectively. Doxycycline-resistant M. abscessus isolates produced 6.25 ± 1.50 mm, and Doxycycline-sensitive M. fortuitum isolates, 19.25 ± 1.3 mm mean zone diameter. Linezolid was sensitive to M. fortuitum isolates, and M. abscessus isolates produced 20 mm and 13.6 ± 3.02 mm zones of inhibition, respectively. No M. fortuitum isolates and M. abscessus isolates produced any zone of inhibition in the disc diffusion method in the case of Trimethoprim-Sulfamethoxazole, as in accordance with their MIC value, showing resistance.
Table 4: Antibiogram results of RGM by MIC broth microdilution and disk diffusion method (n=26)
|
Antibiotic |
M. abscessus (n=21) |
M. fortuitum (n=5) |
||||
|
MIC |
Zone of inhibition |
MIC |
Zone of inhibition |
|||
|
Result No. |
Mean diameter in mm ± SD |
Result No. |
Mean diameter in mm ± SD |
|||
|
No |
Yes |
No |
Yes |
|||
|
Amikacin |
(S) 21 |
(S) 5 |
||||
|
(I) 0 |
14.3±2.46 |
(I) 0 |
19.5±1.91 |
|||
|
(R) 0 |
(R) 0 |
|||||
|
Ciprofloxacin |
(S) 0 |
No zone |
(S) 5 |
|||
|
(I) 3 |
(I) 0 |
|||||
|
(R) 18 |
(R) 0 |
19.6±2.25 |
||||
|
Meropenem |
(S) 0 |
(S) 5 |
||||
|
(I) 0 |
No zone |
(I) 0 |
||||
|
(R) 21 |
(R) 0 |
15±2.12 |
||||
|
Clarithromycin |
(S) 13 |
(S) 5 |
||||
|
(I) 0 |
21.65±3.35 |
(I) 0 |
23.80±1.2 |
|||
|
(R) 8 |
(R) 0 |
|||||
|
Doxycycline |
(S) 5 |
(S) 5 |
||||
|
(I) 3 |
6.25±1.50 |
(I) 0 |
19.25±1.3 |
|||
|
(R) 13 |
(R) 0 |
|||||
|
Linezolid |
(S) 16 |
(S) 5 |
||||
|
(I) 5 |
13.6±3.02 |
(I) 0 |
20±0 |
|||
|
(R) 0 |
(R) 0 |
|||||
|
Trimethoprim- Sulfamethoxazole |
(S) 0 |
No zone |
(S) 0 |
No zone |
||
|
(R) 21 |
(R) 5 |
|||||
S=Sensitive, I=Intermediate, R=Resistant
Discussion
In this study, patients ranged in age from 22 to 80 years, with an overall mean age of 51.0 ± 16.74 years, indicating a predominance of middle-aged and older adults. The slight male predominance (54.36% male vs 45.63% female) aligns with broader data suggesting gender-related differences in surgical site infection susceptibilities. Notably, two-thirds of infections (67.79%) occurred following laparoscopic procedures, compared to 32.21% after open surgeries. This finding is particularly striking given the established advantages of minimally invasive approaches, which typically reduce surgical site infections (SSIs) through smaller incisions, faster recovery, and lower inflammatory response. The prevalence of infections post-laparoscopy may indicate gaps in sterilization techniques specific to minimally invasive instrumentation, which—despite their benefits—can harbour pathogens if not meticulously cleaned. The timing of symptom onset further underscores diagnostic challenges: symptoms manifested between 2 to 5 weeks post-operation, most commonly in the third week (34.22%). Moreover, a small subset (2.01%) experienced onset as late as eight weeks post-operatively. This temporal pattern, consistent with other reports of rapidly growing mycobacteria (RGM) infections, which often present insidiously weeks to months after surgery, highlights the need for heightened clinical vigilance during this window, particularly when conventional antimicrobial therapies and routine cultures fail to yield results [13,36].
In this study, the distribution of specimen types collected from patients with surgical site infections (SSI) showed a clear preference for minimally invasive methods. Wound swabs constituted the majority, accounting for approximately 61.74% of all specimens, underscoring their role as the standard initial diagnostic tool in routine clinical practice. Pus samples were the second most frequent (32.21%), reflecting instances of overt purulent discharge requiring more targeted sampling. In contrast, tissue specimens were notably scarce, comprising only around 6.04%, and were generally reserved for situations involving surgical debridement or biopsy, where deeper tissue evaluation was clinically indicated. This pattern of specimen procurement highlights a pragmatic bias towards less invasive collection techniques in SSI diagnostics, while acknowledging the occasional necessity for deeper, more definitive sampling [9, 20]. Among all surgical site infection (SSI) specimens, 84 samples (56.38%) yielded bacterial pathogens. Notably, nontuberculous mycobacteria (NTM) were the most frequently isolated organisms, accounting for 17.45% of culture-positive cases, underscoring their growing significance in chronic SSI presentations. Among the conventional bacterial pathogens, Klebsiella spp. were the second most common (15.44%), followed by Staphylococcus aureus (10.06%) and Escherichia coli (7.38%), reflecting the diverse involvement of both Gram-positive and Gram-negative species.
Less frequently recovered organisms included other Enterobacteriaceae (e.g., Citrobacter, Enterobacter) and Mycobacterium tuberculosis, each detected in 2.01% of cases. Enterococcus faecalis, Acinetobacter spp., and Nocardia spp. were rare, each representing only 0.67% of isolates. These findings indicate a diverse microbiological profile in SSIs, with a significant proportion attributed to atypical organisms such as NTM, which was also observed in these studies [21,36-38]. Importantly, all NTM isolates in this series were rapidly growing mycobacteria (RGM). Of these, Mycobacterium abscessus dominated, constituting 80.77% of RGM isolates, while M. fortuitum made up the remaining 19.23%. This overwhelmingly high prevalence of M. abscessus has substantial clinical implications: it is increasingly recognized for intrinsic resistance to multiple antimicrobial agents and frequently requires more aggressive, tailored antimicrobial therapy compared to other RGM species [25,39]. In particular, M. abscessus has been implicated in healthcare-associated outbreaks, notably due to its resilience in hospital environments, resistance to common disinfectants, and capacity to form biofilms on medical equipment—factors that complicate eradication. Conversely, M. fortuitum, while less frequent, is still a significant pathogen in post-procedural SSIs with a relatively broader antimicrobial susceptibility profile, often including macrolides, fluoroquinolones, tetracyclines, and carbapenems.
The phenotypic identification of RGM isolates was performed using biochemical tests and disc diffusion-based antibiotic susceptibility testing. All 26 isolates demonstrated negative citrate utilization and positive urea hydrolysis, consistent with traits of M. fortuitum or M. abscessus. In antibiotic susceptibility testing, only 19.23% of isolates showed sensitivity to both Ciprofloxacin and Polymyxin B, indicated by clear zones of inhibition. In contrast, the majority (80.77%) were resistant, showing no inhibition zones around either antibiotic disc. This suggests significant antimicrobial resistance among the isolated RGM species, particularly M. chelonae or M. abscessus, emphasizing the need for careful antibiotic selection in managing infections caused by these organisms [40-42]. The antimicrobial susceptibility profiles of Mycobacterium abscessus (n=21) and Mycobacterium fortuitum (n=5) were assessed using both the broth microdilution and disc diffusion methods. In the broth microdilution method, all isolates of both species were fully sensitive (100%) to Amikacin. M. fortuitum demonstrated uniform sensitivity to Ciprofloxacin, Meropenem, Clarithromycin, Doxycycline, and Linezolid. In contrast, M. abscessus exhibited high resistance rates of 85.71% to Ciprofloxacin, 100% to Meropenem, and 61.90% to Doxycycline. Clarithromycin showed moderate efficacy against M. abscessus, with 61.90% of isolates being sensitive and 38.09% resistant. Linezolid was effective in 76.19% of M. abscessus isolates. Both species showed complete resistance to Trimethoprim-Sulfamethoxazole. These findings highlight the variable susceptibility profiles of RGM species, underscoring the importance of species-level identification and susceptibility testing for effective antimicrobial therapy [40,43-45].
In this study, amikacin produced a mean zone of 14.3 ± 2.46 mm and 19.5 ± 1.91 mm in the disc diffusion method for M. abscessus and M. fortuitum, respectively, both of which were susceptible by broth microdilution. For M. abscessus, ciprofloxacin and meropenem failed to produce any zones, whereas M. fortuitum sensitive isolates showed mean zones of approximately 19.6 mm and 15 mm, respectively. Clarithromycin produced comparably large zones in both species: 23.8 ± 1.2 mm (M. fortuitum) and 21.65 ± 3.35 mm (M. abscessus). Doxycycline-sensitive M. fortuitum gave a mean zone of 19.25 ± 1.3 mm, while resistant M. abscessus showed a minimal zone (6.25 ± 1.50 mm). Linezolid produced a 20 mm zone in M. fortuitum, but only 13.6 ± 3.02 mm in M. abscessus. None of the isolates displayed inhibition with trimethoprim–sulfamethoxazole, matching their resistance profiles by the broth microdilution method. The evolving microbiological landscape of chronic SSIs, with a notable emergence of RGM, particularly Mycobacterium abscessus, as significant pathogens. The observed high levels of antimicrobial resistance among RGM isolates highlight the limitations of empirical therapy and emphasize the critical need for species-level identification and individualized susceptibility testing. These findings call for heightened clinical awareness, improved diagnostic strategies, and robust infection control practices to manage and prevent these challenging infections effectively.
Conclusion
This study highlights the significant role of RGM, particularly Mycobacterium abscessus, in chronic SSI, especially following laparoscopic procedures. The infections commonly presented within 2 to 5 weeks postoperatively and were frequently associated with high antimicrobial resistance, notably among M. abscessus isolates. The diverse microbiological spectrum observed emphasizes the importance of accurate identification and susceptibility testing to guide appropriate therapy.
Recommendations
Early suspicion of RGM should be considered in persistent SSIs, particularly when standard antibiotics fail. Incorporating both phenotypic and molecular diagnostic tools can improve detection and species-level identification. Routine antimicrobial susceptibility testing should be performed to guide targeted therapy, with Amikacin and Linezolid showing promise against resistant strains.
Limitations
This study was limited by its single-center design and relatively small sample size, which may not represent broader epidemiological patterns. Future multicenter studies with larger cohorts and expanded molecular analysis are recommended to validate and generalize these findings.
Acknowledgments: The authors gratefully acknowledge the study population and laboratory workforces for their valuable contributions to this study.
Competing Interests: The authors declare that they have no competing interests.
Funding: This research received Special Research Grants from the Ministry of Science and Technology, Bangladesh.
References
- Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clinical Microbiology Reviews. 2014;27(4):727-52.
- Comba IY, Tabaja H, Almeida NE, Fida M, Saleh OA. Bloodstream infections with rapidly growing non-tuberculous mycobacteria. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases. 2021;25: 100288.
- Shah AK, Gambhir RP, Hazra N, Katoch R. Non tuberculous mycobacteria in surgical wounds-a rising cause of concern? Indian Journal of Surgery. 2010;72(3):206-10.
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American Journal of Respiratory and Critical Care Medicine. 2007;175(4):367-416.
- Kannaiyan K, Ragunathan L, Sakthivel S, Sasidar AR, Venkatachalam GK. Surgical site infections due to rapidly growing mycobacteria in puducherry, India. Journal of Clinical and Diagnostic Research. 2015;9(3): DC05.
- Verghese S, Agrawal P, Benjamin S. Mycobacterium chelonae causing chronic wound infection and abdominal incisional hernia. Indian Journal of Pathology and Microbiology. 2014;57(2):335-7.
- Chaudhuri S, Sarkar D, Mukerji R. Diagnosis and management of atypical mycobacterial infection after laparoscopic surgery. Indian Journal of Surgery. 2010;72(6):438-42.
- Regnier S, Cambau E, Meningaud JP, Guihot A, Deforges L, Carbonne A, Bricaire F, Caumes E. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clinical Infectious Diseases. 2009;49(9):1358-64.
- Gonzalez-Santiago TM, Drage LA. Non-tuberculous mycobacteria: skin and soft tissue infections. Dermatologic Clinics. 2015;33(3):563-77.
- Henry MW, Miller AO, Kahn B, Windsor RE, Brause BD. Prosthetic joint infections secondary to rapidly growing mycobacteria: two case reports and a review of the literature. Infectious Diseases. 2016;48(6):453-60.
- Nagata A, Sekiya N, Najima Y, Horiuchi M, Fukushima K, Toya T, Igarashi A, Kobayashi T, Kakihana K, Ohashi K, Doki N. Nontuberculous mycobacterial bloodstream infections after allogeneic hematopoietic stem cell transplantation. International Journal of Infectious Diseases. 2020; 97:131-4.
- Umrao J, Singh D, Zia A, Saxena S, Sarsaiya S, Singh S, Khatoon J, Dhole TN. Prevalence and species spectrum of both pulmonary and extrapulmonary nontuberculous mycobacteria isolates at a tertiary care center. The International Journal of Mycobacteriology. 2016;5(3):288-93.
- Maurya AK, Nag VL, Kant S, Kushwaha RA, Kumar M, Singh AK, Dhole TN. Prevalence of nontuberculous mycobacteria among extrapulmonary tuberculosis cases in tertiary care centers in Northern India. BioMed Research International. 2015;2015(1):465403.
- Riegler AN, Leal Jr SM. Diagnosing ocular infections in the clinical microbiology laboratory. Clinical Microbiology Newsletter. 2024; 46:11-21.
- Yanagihara T, Kawamura T, Minagi K, Sekine Y, Sugai K, Ichimura H, Sato Y. Successful long-term management for postoperative sternal infection with multiple disseminated lymphadenitis caused by Mycobacterium abscessus. Surgical Case Reports. 2023;9(1):146.
- Nguyen DC, Lisgaris M, Vasireddy S, Wallace RJ, Perez F, Rhoads DD. A Novel Rapidly Growing Mycobacteria (RGM) Species Causing Soft Tissue and Orthopedic Hardware Infection after Trauma. In Open Forum Infectious Diseases. 2019;6(Suppl 2): S492.
- Bhalla GS, Grover N, Singh G, Kumar M, Bhatt P, Sarao MS, Mishra D. Prevalence of non tuberculous mycobacterial infection in surgical site infections and their antibiotic susceptibility profile. Medical Journal Armed Forces India. 2021;77(3):343-8.
- Yadav RP, Baskota B, Ranjitkar RR, Dahal S. Surgical site infections due to non-tuberculous mycobacteria. Journal of the Nepal Medical Association. 2018;56(211):696.
- Kalita JB, Rahman H, Baruah KC. Delayed post-operative wound infections due to non-tuberculous Mycobacterium. Indian Journal of Medical Research. 2005;122(6):535.
- Desai AN, Hurtado R. Non-tuberculous mycobacterial infections. JAMA. 2021;325(15):1574.
- Samaranayake WA, Kesson AM, Karpelowsky JS, Outhred AC, Marais BJ. Port-site infection due to nontuberculous mycobacteria following laparoscopic surgery. The International Journal of Mycobacteriology. 2020;9(3):231-8.
- Choudhary A, Gopalakrishnan R, Senthur Nambi P, Thirunarayan MA, Ramasubramanian V, Sridharan S. Surgical Site Infections Caused by Rapidly Growing Nontuberculous Mycobacteria: an Under-Recognized and Misdiagnosed Entity. Indian Journal of Surgery. 2021;83(2):418-23.
- Yagnik VD. Port-site infections due to non-tuberculous mycobacteria (atypical mycobacteria) in laparoscopic surgery. Internet Journal of Medical Update. 2017;12(2):1-3.
- Brown-Elliott BA, Nash KA, Wallace Jr RJ. Antimicrobial susceptibility testing, drug resistance mechanisms and therapy of infections with non-tuberculous mycobacteria. Clinical Microbiology Reviews. 2012;25(3):545-82.
- Yang SC, Hsueh PR, Lai HC, Teng LJ, Huang LM, Chen JM, Wang SK, Shie DC, Ho SW, Luh KT. High prevalence of antimicrobial resistance in rapidly growing mycobacteria in Taiwan. Antimicrobial Agents and Chemotherapy. 2003;47(6):1958-62.
- Shrivastava K, Kumar C, Singh A, Narang A, Giri A, Sharma NK, et al. An overview of pulmonary infections due to rapidly growing mycobacteria in South Asia and impressions from a subtropical region. Int J Mycobacteriol 2020; 9:62–70.
- Hasan MS, Hossain MA, Paul SK, Nasreen SA, Ahmed S, Haque N, et al. Distribution and pattern of anti-tubercular drug resistance in patients with pulmonary tuberculosis in Mymensingh region of Bangladesh. Mymensingh Med J 2022; 31 :1102–7.
- Rahman MM, Rahim R, Nasrin F, Rasel AH, Khaled A, Nasir TA, et al. Detection of nontuberculous mycobacterium by real time PCR from variety of clinical specimens. Pulse 2017; 9 :15–21.
- Hasan N, Hasan A, Rahim R, Zafar SMA, Rahman M. Detection of non-tuberculous Mycobacteria by PCR from non-healing Surgical site Infections. Pulse 2024; 15:22–7.
- Halder K, Tanni NN, Kabir RB, Nesa M, Rahman MF, Zaman R, et al. Postoperative wound infection by nontuberculous mycobacteria; case series in Dhaka Medical College Hospital of Bangladesh. Clin Case Rep 2023; 11: e8264.
- Barai L, Saha MR, Rahman T, Sukanya M, Ferdous J, Khanduker A, et al. Pattern of rapidly growing mycobacteria (RGM) species isolated from clinical samples: a 10-year retrospective study in a tertiary care hospital of Bangladesh. Indian J Med Microbiol 2025; 53 :100756.
- Bhalla K, Nehra D, Nanda S, Verma R, Gupta A, Mehra S. Prevalence of bronchial asthma and its associated risk factors in school-going adolescents in Tier-III North Indian City. J Family Med Prim Care. 2018;7(6):1452–1457.
- Clinical and Laboratory Standards Institute (CLSI). Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline—Third Edition. CLSI document EP28-A3cS (R2018). Wayne, PA: CLSI; 2018.
- Brown-Elliott BA, Nash KA, Wallace RJ Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev. 2012;25(3):545–582.
- Lipworth B, Kuo CRW, Jabbal S. Current appraisal of single inhaler triple therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13: 3003–3009.
- Bartralot R, García-Patos V, Sitjas D, Rodríguez-Cano L, Mollet J, Martín-Casabona N, Coll P, Castells A, Pujol RM. Clinical patterns of cutaneous non-tuberculous mycobacterial infections. British Journal of Dermatology. 2005;152(4):727-34.
- Negi V, Pal S, Juyal D, Sharma MK, Sharma N. Bacteriological profile of surgical site infections and their antibiogram: A study from resource constrained rural setting of Uttarakhand state, India. Journal of Clinical and Diagnostic Research. 2015;9(10): DC17.
- Sharma P, Singh R, Gupta A, Verma S. Microbial profile of surgical site infections in a tertiary care hospital: emergence of atypical pathogens. Indian Journal of Medical Microbiology. 2022;40(1):45-53.
- Eisner R, Lippmann N, Josten C, Rodloff AC, Behrendt D. Development of the bacterial spectrum and antimicrobial resistance in surgical site infections of trauma patients. Surgical Infections. 2020;21(8):684-93.
- Shen Y, Wang X, Jin J, Wu J, Zhang X, Chen J, Zhang W. In vitro susceptibility of Mycobacterium abscessus and Mycobacterium fortuitum isolates to 30 antibiotics. BioMed Research International. 2018;2018(1):4902941.
- Jayasingam SD, Zin T, Ngeow YF. Antibiotic resistance in Mycobacterium Abscessus and Mycobacterium Fortuitum isolates from Malaysian patients. The International Journal of Mycobacteriology. 2017;6(4):387-90.
- Hatakeyama S, Ohama Y, Okazaki M, Nukui Y, Moriya K. Antimicrobial susceptibility testing of rapidly growing mycobacteria isolated in Japan. BMC Infectious Diseases. 2017;17(1):197.
- Kumar C, Shrivastava K, Singh A, Chauhan V, Varma-Basil M. Skin and soft-tissue infections due to rapidly growing mycobacteria: An overview. The International Journal of Mycobacteriology. 2021;10(3):293-300.
- Tsuchiya K, Hayashi N, Ohji G, Terashi H, Sakakibara S. Polypropylene Mesh Infection From Surgical Site Infections Caused by Mycobacterium fortuitum. Cureus. 2024;16(5).
- Sasmal PK, Mishra TS, Rath S, Meher S, Mohapatra D. Port site infection in laparoscopic surgery: A review of its management. World Journal of Clinical Cases. 2015;3(10):864.