Pharmacological Effects of Cannabinoids Extracted from Industrial hemp on Epilepsy
Article Information
Aayushi Patel, Ruba Agili-Shaban, Shrina Patel, Renata Proano, Fatima Riarh, Eman Hasan, Shobha Potlakayala, Sairam Rudrabhatla*
Penn State Harrisburg, Middletown, PA, United States of America
*Corresponding author: Sairam Rudrabhatla, Professor of Biology, Program Chair of Biology & Science, Director for Central Pennsylvania Research and Teaching Laboratory for Biofuels, Penn State Harrisburg, Middletown, PA, United States of America.
Received: 20 April 2022; Accepted: 03 May 2022; Published: 07 June 2022
Citation: Aayushi Patel, Ruba Agili-Shaban, Shrina Patel, Renata Proano, Fatima Riarh, Eman Hasan, Shobha Potlakayala, Sairam Rudrabhatla. Pharmacological Effects of Cannabinoids Extracted from Industrial hemp on Epilepsy. Journal of Pharmacy and Pharmacology Research 6 (2022): 35-48
View / Download Pdf Share at FacebookAbstract
Epilepsy is a disease caused by abnormal brain activity due to disturbed nerve cell activity. It is the fourth most common neurological disorder. Only about 7 out of 10 individuals with epilepsy are successfully treated using anti-epileptic drugs. In the pharmaceutical industry, there has been a growing demand for cannabinoids from Cannabis sativa, commonly known as marijuana, for therapeutic, clinical, and nutraceutical supplements. More recently, the legalization of marijuana for clinical research has allowed to further explore the efficacy in the treatment of several neurological disorders like epilepsy. Unlike opioids, cannabinoids are not psychoactive, making it a potentially more favorable therapeutic drug. Most studies showing the efficacy of Cannabis as a treatment strategy point to the pain management associated with the binding of endocannabinoid G coupled protein receptors CB1 and CB2. Though there are cannabinoid therapeutic drugs like Epidiolex approved by the Food and Drug Administration (FDA), plant-based natural compounds are safer, and effective with no side effects.
Keywords
Cannabinoids, Epilepsy, Endocannabinoid system, CBD, C. Sativa
Cannabinoids articles, Epilepsy articles, Endocannabinoid system articles, CBD articles, C. Sativa articles
Article Details
1. Introduction
Evidence of medicinal Cannabis sativa use dates back to BCE with ancient civilizations using it as therapy for a variety of diseases [1]. There is evidence of delta 9-tetrahydrocannabinol (delta 9-THC) in ashes dating back to 400 AD, providing evidence of medical cannabis use. The plant has more than 100 phytocannabinoids leading to its diverse medicinal and psychotropic uses that have been used for centuries. It was first used in the United States as a patent medicine in the late 19th and early 20th centuries.
The female Cannabis sativa (C. sativa) flower is of therapeutic interest, as it is high in cannabinoids and low (< 0.3%) in tetrahydrocannabinol (THC). Most nutritional supplements commercially available in the market focus on CBD, however there are other cannabinoids such as cannabigerol (CBG), cannab-ichromene (CBC), cannabitriol (CBT), cannabinol (CBN) that have shown promising clinical results in the preliminary studies [2]. Delta 9 THC contains the psychoactive property in which recreational marijuana users seek for the euphoric feeling, known as a “high” [3]. While CBD, CBG, CBC, CBN, and CBT does not have psychoactive properties, it is being studied for its potential in treating symptoms of neurological disorders such as epilepsy.
The FDA has also approved one Cannabis-derived drug, Epidiolex, which is a pharmaceutical-grade CBD [4]. The FDA has also approved three synthetic Cannabis-based products. Out of these three, Marinol and Syndros are pharmaceutical grade Dronabinol, which is an isomer and a synthetic version of THC. The third one is Cesamet, which is pharmaceutical grade nabilone that is similar in chemical structure to THC [5]. It is used primarily to treat nausea and vomiting caused by chemotherapy treatments.
1.1 Endocannabinoid System
CBD exerts its effect via the endocannabinoid (eCB) system in addition to other mechanisms [6]. The eCB system consists of eCB ligands, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), lipids that activate CB1 and CB2 - such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and metabolic enzymes - including N-acyl-phosphatidylethan-olamine (NAPE), NAPE-specific phospholipase D (NAPE-PLD), and fatty acid amide hydrolase (FAAH) among others [7-8]. The eCB system is often found to be altered in patients with neurological diseases or has experienced a traumatic brain injury, and the components of the system became therapeutic targets. The endocannabinoidome is the regulation system of the endocannabinoid system and often becomes the target of therapy for neurological diseases [8].
The secondary metabolites of the endocannabinoid system are AEA and 2-AG [7, 9]. AEA, known as the bliss molecule, which results in the feeling of well-being when it binds to presynaptic CB1 or CB2. The levels of AEA and 2-AG present are known as the endocannabinoid tone which is regulated by FAAH and monoacyl-glycerol lipase (MAGL) both of which metabolize these chemical analogs [7, 9]. Endocan-nabinoids inhibit the release of neurotransmitters by inhibiting the influx of intracellular calcium. Usually, endocannabinoids are produced after trauma; they can also be produced by large amounts of nerve cell depolarization. It is produced locally and has a brief activity as it is quickly degraded due to its short half-life [9].
N-acylethanolamines and 2-acylglycerols share the same biosynthetic pathways and enzymes as AEA and 2-AG. They also target transient receptor potential cation channel V1 (TRPV1), peroxisome proliferator-activated nuclear receptor-α (PPARα), peroxisome proliferator-activated nuclear receptor γ (PPARγ), T-type calcium ion channels, G-protein coupled receptor 18 (GPR18), G-protein coupled receptor 55 (GPR55), G-protein coupled receptor 110 (GPR110), and G-coupled protein receptor 119 (GPR119). The precursor to 2-AG is known to target the protein kinase C (PKC), GPR55, and lysophosphatidic acid receptors 1-3 (LPA1-3). The expanded endocannabinoid system also includes other long-chain fatty acid amides - including primary amides, lipoamino acids, and N-acyl-neurotransmitters. However, their targets are not fully known even though distinct biosynthetic pathways exist for lipoamino acids and N-acyl-neurotransmitters [8].
Enzymes of the endocannabinoid system have different substrate selectivity. Fatty acid amide hydrolase (FAAH) is known to break down long-chain N-acylethanolamines, N-acyl taurines, and N-acyl glycines. Fatty acid amide hydrolase 2 (FAAH2) however, prefers to break down oleoylethanolamide (OEA) and linoleoyl ethanolamide (LEA). N-acylethanolamine acid amidohydrolase (NAAA) reacts with and breaks down N-acylethanolamines. Monoacylglycerol lipase has substrate specificity for long-chain 2-acylglycerols, preferring its unsaturated form. 2-acylglycerols are also substrates for alpha and beta hydrolases 6 and 12. Cyclooxygenase 2 (COX 2) and lipoxygenases (LOX) are oxidizing enzymes of the arachidonate cascade. These enzymes recognize polyunsaturated fatty acid chains that contain endocannabinoid congeners. Metabolic products derived from LOX or cytochrome P450 oxygenase (P450) have their specific receptors but can also activate CB1 and CB2 receptors [8].
CB1 and CB2 receptors of the endocannabinoid system are involved in many pathways and are an essential component in many biological processes - including but not limited to neuronal plasticity, pain, anxiety, neuroinflammation, immune function, metabolic regulation, and bone growth. The CB1 receptors are typically found in the brain and neuronal cells. CB2 cells are predominately found in tissue and immune cells leading to the pathways listed previously. These receptors also respond to plant-based ligands [7, 9]. This review highlights the current status of usage of Cannabinoids for the management and/or treatment of Epilepsy.
2. Epilepsy
Around 50 million people globally, including an estimated 3.4 million people within the United States, have epilepsy [10-12]. Epilepsy is a chronic non-communicable neurological disorder characterized by seizures. It may occur because of neurological injuries, deficits, or brain abnormalities. However, most cases are thought to be caused by genetic factors that may play a role in the onset of epilepsy [13]. Seizures occur due to excessive electrical discharges in the neurons; this is known as neuronal hyperexcitability [10]. The resistance of approximately 1/3 of epileptic patients to traditional antiepileptic drugs is known as refractory epilepsy. Refractory epilepsy has been associated with worsened morbidity and mortality, and the issues can range from negative health consequences of uncontrolled seizures to decreased quality of life [14]. This leads to a medicinal demand for an antiepileptic drug that has better efficacy and fewer adverse effects [13, 15].
2.1 Mechanisms of Action: Cannabinoids
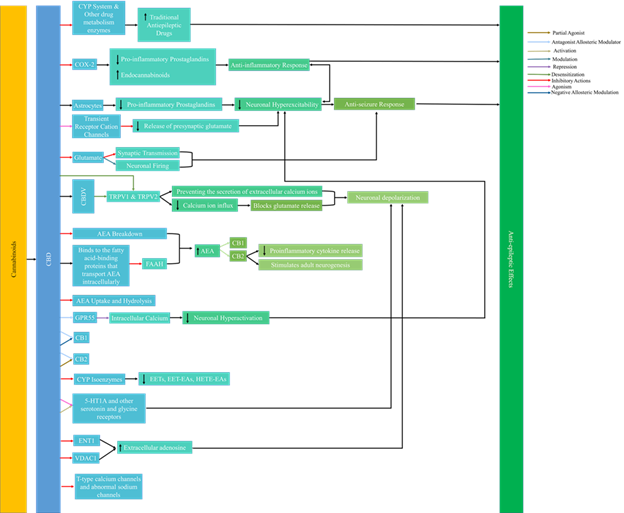
Recent studies have linked epilepsy with neuro inflammation which is a pathophysiological mechanism that is directly related to the onset and severity of seizures. Studies have shown that cannabinoids affect mediators of neuro inflammation including but not limited to the endocannabinoid system and ion channels [13]. Though research has recently expanded to other cannabinoids found in C. sativa, CBD is the most heavily studied for its antiepileptic effects [13, 16]. Figure 1 highlights the mechanism of action of cannabinoids in epilepsy.
There are many mechanisms of action by which cannabinoids can act in epilepsy. Figure 1 summarizes the mechanisms of action by cannabinoids in epilepsy that are currently understood [8, 13, 15, 17-27].
Cannabinoid research shows that cannabinoids target sodium, potassium, and calcium channels among others. The dysfunction of these ion channels including voltage or ion gated channels is what leads to epilepsy. Phytocannabinoids also interact with other diverse targets resulting in antiepileptic effects. It has been concluded throughout many years of studies that defects in ion channels at the axon hillock and their involved proteins are the primary reason for epilepsy [28]. CBD has been proven to treat pain, anxiety, stress, and depression. In addition to this, studies have shown that CBD acts as an antiepileptic agent with efficacy that rivals traditional therapeutic means while having only mild adverse effects on the patients [15]. New research shows multiple possible mechanisms of action for CBD. Specifically, CBD has been reported to be acting on at least 65 potential molecular targets in epileptic patients [17]. Its mechanism of action is proven to be independent of the CB1 receptor.
A study has also suggested that CB2 reduces postsynaptic neuronal hyper excitability [8]. This increases the availability to activate CB1 and CB2 receptors allowing for indirect regulation of the endocannabinoid system [18]. CBD is an antagonistic allosteric modulator of CB1, CB2, and G-protein coupled receptor 55 (GPR55) though it only binds weakly to CB1 and CB2 [16-17, 19, 21]. The activation of GPR55 increases intracellular calcium release which leads to neuronal hyper excitability. CBD’s role as an antagonist to GPR55 leads to the repression of the intracellular calcium directly limiting and decreasing neuronal hyper activation [13].
A chronic epilepsy model showed that GPR55 mediated neurotransmission was potentiated in excitatory neurons and reduced in inhibitory neurons. CBD antagonizes GPR55 at excitatory synapses which inhibits intracellular calcium release. This decreases the excitatory currents decreasing seizure activity. In the same study, CBD blocked GPR55 mediated increase in excitatory postsynaptic current frequency in excitatory neurons but did not do so in inhibitory neurons which showed that CBD’s anticonvulsant effect was due to knocking out GPR55 [8,18,22]. The results also indicated that CBD has a fluid affinity for both allosteric and orthosteric sites at CB1 receptors and acted as an orthosteric ligand at CB2 receptors [16, 19, 23].
This leads to an indirect effect on CB1 and CB2 receptors through up regulation and down regulation of downstream eicosanoids resulting in an anti-seizure effect on the patient [13, 29]. CBD is thought to be able to target abnormal sodium channels and block T-type calcium channels both of which have excitatory functions [8, 17, 21]. It is also believed that the anticonvulsant effect of CBD could be related to its ability to inhibit voltage-gated ion channels [20, 30]. CBD blocks T-type calcium channels similar to traditional antiepileptic drugs. A study found that CBD's ability to regulate calcium and sodium ion influx into the neuron by binding to human T-type voltage-gated channels and by melastatin and vanilloid type transient receptor potential membrane receptors leads to neuronal depolarization leading to an antiepileptic effect [16].
Cannabidivarin (CBDV) also showed anticonvulsant effects with limited neurotoxicity. It is believed to exert these effects independent of the CB1 receptor mechanism [15-16]. Since recent research shows multiple pathways exist in regards to CBD’s antiepileptic activity, a multimodal mechanism of action could exist by which CBD exerts its anticonvulsant properties on epileptic patients. Research is also moving towards studying other phytocannabinoids found in Cannabis sativa as well as studying the whole plant extracts for efficacy in epileptic treatment.
2.2 Pediatric Epilepsy
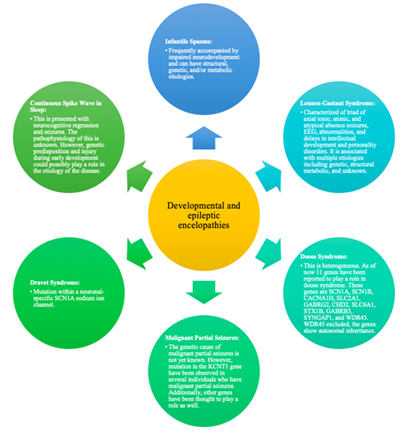
Globally, the prevalence of epilepsy in children is 3.2-5.5 per 1,000 children in developed countries and 3.6-4.4 per 1,000 children in undeveloped countries. Additionally, studies have found that the incidence of epilepsy is the highest during the first year of epilepsy. About 470,000 children in the United States have epilepsy [11-12, 31]. For pediatric epilepsy, there can be different developmental and epileptic encephalopathies which have been summarized in Figure 2.
Figure 2 summarizes different developmental and epileptic encephalopathies that can be present in pediatric epilepsy [10,15-16,32-37].
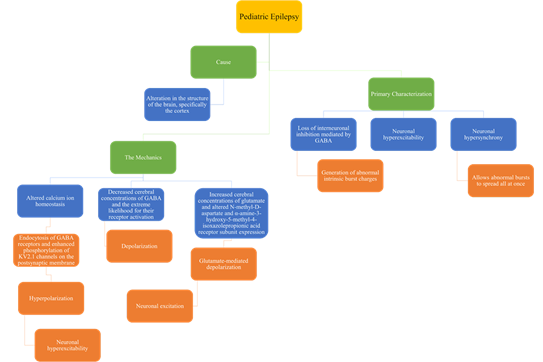
In children, epilepsy has many causes and mechanisms and has many different characterizations as shown in figure 3.
There are many causes, primary characterization, and mechanics of pediatric epilepsy. Figure 3 summarizes all three in relation to pediatric epilepsy [10, 15-16].
In addition to the details discussed in the figure, children are particularly vulnerable to seizures due to the developing brain and neural network. They have a reduced seizure threshold which often leads to neuronal excitation. [16].
2.2.1 Cannabinoids
Multiple animal models of generalized and focal-onset epilepsy have demonstrated and confirmed the anticonvulsant properties of CBD and showed that when administered with other anticonvulsants, its effects become stronger. It is believed that the anticonvulsant effect is not from its metabolites, but from CBD itself based on the correlation of the effect and the concentration of CBD in the brain [15-16].
A review from 2018 detailed that 20mg/kg per day of Epidiolex was more effective than the placebo and 48.5% of the patients experienced a 50% reduction in seizures. The Quality of Life in Childhood Epilepsy scores also improved by 55.8%. Adverse effects in patients were only reported 2.2% of the time and were deemed infrequent [16, 38]. Another review from 2018 analyzed data from four studies of Epidiolex clinical trials in children with treatment-resistant Lennox-Gastaut and Dravet syndromes. The pooled average difference in seizure frequency between the placebo and the treatment group was 19.9% in favor of the treatment group with 37.2% of the treatment group and 21.2% of the placebo group experiencing a 50% reduction in seizure frequency. A study showed that CBD was consistent in reducing major motor seizures by 50% and total seizures by 44% with the most common adverse effects being somnolence and diarrhea [39]. Based on this study, CBD may be an effective long-term treatment option for children and adults with Lennox-Gastuat syndrome or Dravet Syndrome.
Studies examining artisanal and CBD-enriched cannabis herbal extract (CHE) oil in epilepsy also showed efficacy in reducing seizures and improving quality of life. However, clinical studies in children have shown certain antiepileptic drugs to be more effective than cannabis products such as stiripentol in Dravet syndrome and rufinamide in Lennox-Gastaut syndrome. A meta-analysis also reported that a larger percentage of children taking CHE with lower daily CBD doses had a reduction in seizure frequency when compared to Epidiolex [16, 40]. Evidence suggests that CBD is effective in lowering the seizure burden in children with Dravet and Lennox-Gastaut syndromes [41].
Studies have reported the side effects of cannabis use to treat epilepsy in children to be 51%, but these effects were considered mild. The secondary effects included sleepiness, fatigue, and gastrointestinal symptoms. The potentially serious effects reported were elevations of liver enzymes, worsening of seizures, and blood dyscrasias. The elevation of liver enzymes was particularly seen in children who took valproic acid simultaneously with CBD. These effects were reported higher in patients taking Epidiolex than those taking CHE. Studies need to be done on weighing the developmental effects of cannabinoids against the neurodevelopmental harm caused by uncontrolled seizures [16]. Studies done on the side effects of CBD in epilepsy treatment have been inconclusive as they showed either no effect, improved, mild side effects, and some more serious adverse side effects. However, the side effect of CBD on the comorbidities of epilepsy is minimal. It is believed that CBD may have antipsychotic and anxiolytic properties, but the cannabinoid’s role in drug metabolism requires careful monitoring of patients for drug-drug interactions to prevent side effects [42].
Observational studies describing Epidiolex and CBD oils with or without THC and limited data on vaporization or smoking of dried cannabis flower buds have reported cannabis to be successful in seizure reduction in sometimes as many as 71% of children. The dosage, formulations, and patient populations were known to vary in these studies [43]. Future research is needed in support of cannabis products as treatments for pediatric epilepsy as well as to define the appropriate dose, frequency, route, and form [44]. However, FDA has approved a pharmaceutical-grade CBD, Epidiolex, for Dravet Syndrome and Lennox-Gastaut Syndrome for anyone older than 2 years old [13,16,45].
2.3 Adult Epilepsy
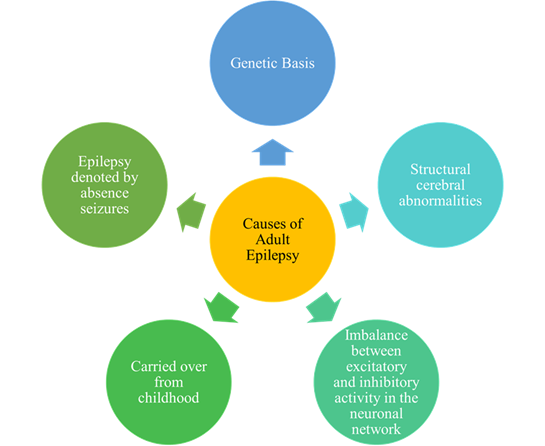
Globally, the prevalence of epilepsy is 4-10 per 1000 people. More diagnoses of epilepsy occur in low and middle-income countries because of the increased presence of endemic conditions; about 80% of people with epilepsy live in low and middle-income countries. Approximately 3 million adults in the United States have epilepsy [11-12, 46]. About ? of epilepsy patients have refractory epilepsy [10, 13, 15]. It is also reported that about ? of newly diagnosed patients will not achieve seizure freedom [47]. There are many different reasons for the occurrence or onset of adult epilepsy as shown in figure 4.
There are many causes as to why adult epilepsy occurs. Figure 4 summarizes these potential causes of adult epilepsy [10, 15, 48].
The pathophysiology of what causes epileptic seizures is not completely known, but it is believed that seizures are primarily caused by abnormal activity in cortical neurons and glial cells and axons in the white matter [48].
2.3.1 Cannabinoids
Most of these studies involved purified CBD products mainly Epidiolex. Additionally, only a few studies involved both CBD and THC. Twenty-three studies reported seizure freedom using cannabis treatment, but another 3 studies reported that no child achieved seizure freedom in their study. Overall, among children with Dravet syndrome who received CBD treatment in one 14 week randomized controlled trial (RCT), 5% became seizure-free in comparison to none in the placebo group while this number was 8% in non-randomized studies (NRS). Fourteen studies looked at seizure frequency. Overall, these studies found that the total monthly seizures were reduced by CBD when compared to the placebo in the RCTs and NRS. Six studies also assessed the impact on the quality of life of the children, but no studies have considered the impact on caregivers. Three 14-week RCTs with children with Dravet or Lennox-Gastaut Syndrome reported no statistically significant effect, but data from NRS suggest an improvement relative to before the treatment but this is said with very low certainty. Fourteen studies also reported changes in sleep. In children with Dravet or Lennox-Gastuat syndrome in three-week RCTs, there was no statistically significant difference reported, but improved sleep was reported in six NRS. Status epilepticus was reported in 11 studies. While in RCTs there was no statistically significant difference, in NRS, 7% of children receiving cannabis treatment-experienced status epilepticus. Out of 1713 children who received cannabis treatment in 10 studies, 26 deaths occurred which includes 7 from unexpected death in epilepsy? However, most of these deaths were reported to be unrelated. Gastrointestinal adverse effects were reported among children receiving treatment with the risk of diarrhea being higher for those who received CBD [49].
Recent clinical studies show that CBD reduces seizure frequency in patients with refractory epilepsies. One of these studies even found that CBD is an effective form of treatment for patients with Lennox-Gastaut syndrome who experience drop seizures [50-51]. Short term analysis deems CBD as a safe drug for humans as it has no psychoactive properties, no changes in clinical and laboratory examination, and has no effect on the electroenceph-alogram (EEG) and electrocardiogram (ECG). CBD also has a long half-life if given intravenously or orally. If administered intravenously, the half-life is 30 hours while given orally, the half-life is 23 hours. This long half-life is helpful in patient-compliance. A study by Devinsky showed that CBD had antiepileptic effects with a few adverse effects that were mild to moderate in severity. Another study showed a 43.9% reduction in drop seizures in the treatment group that was treated with CBD while only seeing a 21.8% reduction in patients in the placebo group. The most serious treatment-related adverse effect was the elevation of liver enzymes, but this effect diminished on its own with the continuation of the treatment [15].
Observational studies describing Epidiolex and CBD oils with or without THC and limited data on vaporization or smoking of dried cannabis flower buds have reported cannabis to be successful in seizure reduction in sometimes as many as 89.5% of adults. The dosage, formulations, and patient populations were known to vary in these studies [43].These studies show that CBD can be an effective form of treatment for epilepsy and severe side effects have not been reported. However, more research is needed in specifically studying the effects of CBD, and in determining the treatment mechanism itself.
3. Conclusions
Cannabis shows promising applications in the treatment of epilepsy. Specifically, CBD is preferred over THC as it is non-psychoactive, and it has multiple targets. These various mechanisms of action, as well as its non-psychoactive characteristic, make this molecule preferred over THC. Epilepsy studies have found CBD to be effective in treating epilepsy, as it lacks psychoactive properties, and has an anti-inflammatory effect, as well the ability to limit seizures. The endocannabinoid system is not yet fully understood and requires further research to comprehend the extent to which cannabis can be used for the treatment of epilepsy.
Abbreviations
2-AG: 2-arachidonoylglycerol
AEA: anandamide
CB1: cannabinoid receptor 1
CB2: cannabinoid receptor 2
CBC: cannabichromene
CBD: cannabidiol
CBDV: cannabidivarin
CBG: cannabigerol
CBN: cannabinol
CBT: cannabitriol
CHE: cannabis herbal extract
COX 2: Cyclooxygenase 2
eCB: endocannabinoid
ECG: electrocardiogram
EEG: electroencephalogram
FAAH: fatty acid amide hydrolase
FAAH2: fatty acid amide hydrolase 2
FDA: Food and Drug Administration
GPR110: G-protein coupled receptor 110
GPR119: G-protein coupled receptor 119
GPR18: G-protein coupled receptor 18
GPR55: G-protein coupled receptor 55
LEA: linoleoyl ethanolamide
LOX: lipoxygenases
LPA1-3: lysophosphatidic acid receptors 1-3
MAGL: monoacyl-glycerol lipase
NAAA: N-acylethanolamine acid amidohydrolase
NAPE: N-acyl-phosphatidylethanolamine
NAPE-PLD: NAPE-specific phospholipase D
NRS: non-randomized studies
OEA: oleoylethanolamide
P450: cytochrome P450 oxygenase
PPARα: peroxisome proliferator-activated nuclear receptor-α
PPARγ: peroxisome proliferator-activated nuclear receptor γ
RCT: randomized controlled trials
THC: tetrahydrocannabinol
TRPV1: transient receptor potential cation channel V1
Acknowledgments
The authors would like to acknowledge the School of Science, Engineering, and Technology at Penn State Harrisburg and PA Options for Wellness.
Author Disclosure Statement
There are no competing interests.
Funding Statement
There are no funding sources.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this review.
References
- Pisanti S & Bifulco M. Medical cannabis: A plurimillennial history of an evergreen. Journal of Cellular Physiology, 234 (2018): 8342-8351.
- Bielawiec P, Harasim-Symbor E & Chabowski A. Phytocannabinoids: useful drugs for the treatment of obesity? Special focus on Cannabidiol. Frontiers in endocrinology 11 (2020): 114.
- Bilodeau SE, Wu B-S, Rufyikiri A-S, MacPherson S & Lefsrud M. An update on plant photobiology and implications for cannabis Frontiers Plant Science. 10 (2019): 296.
- Goerl B, Watkins S, Metcalf C, Smith M & Beenhakker M. Cannabidiolic acid exhibits entourage-like improvements of anticonvulsant activity in an acute rat model of seizures. Epilepsy Research169 (2021): 106525.
- Food and Drug Administration. FDA and cannabis: Research and drug approval process (2020, January).
- Houston JT, Nenert R, Allendorfer JB, Bebin EM, Gaston TE, Goodman AM, et al. White matter integrity after cannabidiol administration for treatment resistant epilepsy. Epilepsy research 172 (2021): 106603.
- Zou M, Li D, Li L, Wu L & Sun C. Role of the endocannabinoid system in neurological disorders. International Journal of Developmental Neuroscience 76 (2019): 95-102.
- Cristino L, Bisogno T & Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature Reviews Neurology 16 (2020): 9-29.
- Silver RJ. The endocannabinoid system of animals. Animals 9 (2019): 686.
- World Health Organization. Neurological disorders: Public health challenges. Switzerland: World Health Organization (2006).
- Center for Disease Control and Prevention. Epilepsy: Data and statistics (2020, January).
- Center for Disease Control and Prevention. Epilepsy: Fast facts (2020).
- Kwan Cheung KA, Peiris H, Wallace G, Holland O & Mitchell MD. The interplay between the endocannabinoid system, epilepsy, and cannabinoids. International Journal of Molecular Sciences 20 (2019): 6079.
- Savage TE, Sourbron J, Bruno PL, Skirvin LA, Wolper ES, Anagnos CJ, et al. Efficacy of cannabidiol in subjects with refractory epilepsy relative to concomitant use of clobazam. Epilepsy research 160 (2020): 106263.
- Zaheer S, Kumar D, Khan MT, Guyanwani PR & Kiran F. Epilepsy and cannabis: A literature review. Cureus 10 (2018): e3278.
- Huntsman RJ, Tang-Wai R & Shackelford AE. Cannabis for pediatric epilepsy. Journal of Clinical Neurophysiology 37 (2020): 2-8.
- Farrell JS & Soltesz I. Plants come to mind: Phytocannabinoids, endocannabinoids and the control of seizures. Addiction 114 (2018).
- Gaston TE & Szaflarski JP. Cannabis for the treatment of epilepsy: An update. Current Neurology and Neuroscience Reports 18 (2018):
- Tham M, Yilmaz O, Alaverdashvili M, Kelley MEM, Denovan-Wright EM, Lapraririe RB. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and the type 2 cannabinoid receptors. British Journal of Pharmacology 176 (2018).
- Billakota S, Devinsky O & Marsh E. Cannabinoid therapy in epilepsy. Current Opinion in Neurology 32 (2019): 220-226.
- Dale T, Downs J, Olson H, Bergin AM, Smith S & Leonard H. Cannabis for refractory epilepsy in children: A review focusing on CDKL5 Deficiency Disorder. Epilepsy Research 151 (2019): 31-39.
- Nichol K, Stott C, Jones N, Gray RA, Bazelot M, Whalley BJ. The proposed multimodal mechanism of action of cannabidiol (CBD) in epilepsy: Modulation of intracellular calcium and adenosine-mediated signaling. Neurology 92 (2019): P5.5-007.
- Rodrigues RS, Lourenco DM, Paulo SL, Mateus JM, Ferreira MF, Mouro FM, et al. Cannabinoid actions on neural stem cells: Implications for pathophysiology. Molecules 24 (2019): 1350.
- Watkins AR. Cannabinoid interactions with ion channels and receptors. Channels 13 (2019): 162-167.
- Xu S, Sun Q, Fan J, Jiang Y, Yang W, Yifeng C et al. Role of astrocytes in post-traumatic epilepsy. Frontiers in Neurology, 10 (2019): 1149.
- GLRA1 gene: Glycine receptor alpha 1 (2020, January).
- Petrucci AN, Joyal KG, Purnell BS & Buchanan GF. Serotonin and sudden unexpected death in epilepsy. Experimental Neurology 325 (2020): 113145.
- Lerche H, Shah M, Beck H, Noebels J, Johnston D & Vincent A. Ion channels in genetic and acquired forms of epilepsy. The Journal of physiology, 591 (2013): 753–764.
- Arnold WR, Weigle AT, Das A. Cross-talk of cannabinoid and endocannabinoid metabolism is mediated via human cardiac CYP2J2. Journal of Inorganic Biochemistry 184 (2018): 88-99.
- Asth L, Iglesias LP, De Oliveria AC, Moraes MFD & Moreira FA. Exploiting cannabinoid and vanilloid mechanisms for epilepsy treatment. Epilepsy and Behavior (2019). 106832.
- Camfield P & Camfield C. Incidence, prevalence, and aetiology of seizures and epilepsy in children. Epileptic Disorders 17 (2015): 117-123.
- Berg AT & Scheffer IE. New concepts in classification of the epilepsies: entering the 21st century. Epilepsia 52 (2011): 1058-1062.
- Barcia G, Fleming MR, Deligniere A, Gazula VR, Brown MR, Langouet M, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nature genetics 44 (2012): 1255-1259.
- Hancock EC & Cross JH. Treatment of lennox-gastaut syndrome. Cochrane Database of Systematic Reviews (2013).
- Singhal NS & Sullivan JE. Continuous spike-wave during slow wave sleep and related conditions. International Scholarly Research Notices (2014).
- Calvo A, Buompadre MC, Gallo A, Gutiérrez R, Valenzuela GR & Caraballo R. Electroclinical pattern in the transition from West to Lennox-Gastaut syndrome. Epilepsy Research, 167 (2020): 106446.
- Hinokuma N, Nakashima M, Asai H, Nakamura K, Akaboshi S, Fukuoka M, et al. Clinical and genetic characteristics of patients with Doose syndrome. Epilepsia open 5 (2020): 442-450.
- Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Neilsen S, et al. Evidence for cannabis and cannabinoids for epilepsy: A systematic review for controlled and observational evidence. Journal of Neurology, Neurosurgery & Psychiatry 89 (2018): 741-753.
- Laux LC, Bebin EM, Checketts D, Chez M, Flamini R, Marsh ED, et al. CBD EAP study group. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy research 154 (2019): 13-20.
- Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Giovane CD, et al. Efficacy and safety of cannabidiol in epilepsy: A systematic review and meta-analysis. Drugs, 78 (2018): 1791-1804.
- Elliot J, DeJean D Clifford T, Coyle D, Potter BK, Skidmore B,... & Wells GA. Cannabis-based products for pediatric epilepsy: A systematic review. Epilepsia 60 (2018).
- Yao I, Stein ES & Maggio N. Cannabinoids, hippocampal excitability, and efficacy for the treatment of epilepsy. Pharmacology & Therapeutics 202 (2019): 32-39.
- Inglet S, Winter B, Yost SE, Entringer S, Lian A, Biksacky M, et al. Clinical data for the use of cannabis-based treatments: A comprehensive review of the literature. Annals of Pharmacotherapy 54 (2020): 1109-1143.
- Markle M & Nativio DG. Medical marijuana in the pediatric population with epilepsy - What you should know. Journal of Pediatric Health Care 33 (2019): 626-632.
- Samanta D. Cannabidiol: A review of clinical efficacy and safety in epilepsy. Pediatric Neurology 96 (2019): 24-29.
- World Health Organization (2019).
- Bosak M & Stowik A. Use of complementary and alternative medicine among adults with epilepsy in a university epilepsy clinic in Poland. Epilepsy & Behavior 98 (2019): 40-44.
- Thijs RD, Suges R, O’Brien TJ & Sander JW. Epilepsy in adults. The Lancet 393 (2019): 689-701.
- Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B et al. Cannabis-based products for pediatric epilepsy: an updated systematic review. Seizure 75 (2020): 18-22.
- Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomized, double-blind, placebo-controlled phase 3 trial. The Lancet 391 (2018): 1085-1096.
- Premoli M, Aria F, Bonini SA, Maccarinelli G, Gianoncelli A, Pina SD. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sciences 224 (2019): 120-127.