Peritoneal Dialysis in Scleroderma Renal Crisis, A Bright Perspective: Case Report and Literature Review
Article Information
Abdullah Al-Hwiesh1, Khadija M. Alshehabi1, Alawiah Alramadhan1, Muaz M. Abdelgalil1, Fatimah I. Alabdrabalnabi1, Somaya A. Aleissa1, Badran Alhwiesh1, Amani Al-Hwiesh1, Nadia Al-Audah2
1Nephrology Division, Department of Internal Medicine, King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, Saudi Arabia
2Department of Pathology Dammam Central Hospital, Saudi Arabia
*Corresponding Author: Dr. Abdullah Al-Hwiesh, Professor and Consultant of Nephrology, King Fahd Hospital of the University, Al-Khobar, 40246, Saudí Arabia
Received: 21 March 2021; Accepted: 19 April 2021; Published: 26 April 2021
Citation: Abdullah Al-Hwiesh, Khadija M. Alshehabi, Alawiah Alramadhan, Muaz M. Abdelgalil, Fatimah I. Alabdrabalnabi, Somaya A. Aleissa, Badran Alhwiesh, Amani Al-Hwiesh, Nadia Al-Audah. Peritoneal Dialysis in Scleroderma Renal Crisis, A Bright Perspective: Case Report and Literature Review. Archives of Nephrology and Urology 4 (2021): 063-070.
View / Download Pdf Share at FacebookAbstract
Systemic sclerosis is an immune-mediated connective tissue disease characterized by fibrosis of the skin and internal organs. Although systemic sclerosis is uncommon, it has high morbidity and mortality and associated with various complications such as scleroderma renal crisis, pulmonary arterial hypertension and lung fibrosis. Understanding the pathogenesis of systemic sclerosis is essential to allow better management of the disease. Scleroderma renal crisis (SRC) is a serious complication and up to 50% of the patients will progress to End-Stage Renal Disease (ESRD) requiring renal replacement therapy (RRT). Peritoneal dialysis has emerged as an effective modality to treat this condition. Here, we report a unique case of a 34-year-old female who developed ESRD secondary to SRC and remained on peritoneal dialysis for five years with excellent outcomes.
Keywords
Scleroderma, Peritoneal Dialysis, Scleroderma Renal Crisis, End-Stage Renal Disease, Pulmonary Hypertension
Scleroderma articles, Peritoneal Dialysis articles, Scleroderma Renal Crisis articles, End-Stage Renal Disease articles, Pulmonary Hypertension articles
Scleroderma articles Scleroderma Research articles Scleroderma review articles Scleroderma PubMed articles Scleroderma PubMed Central articles Scleroderma 2023 articles Scleroderma 2024 articles Scleroderma Scopus articles Scleroderma impact factor journals Scleroderma Scopus journals Scleroderma PubMed journals Scleroderma medical journals Scleroderma free journals Scleroderma best journals Scleroderma top journals Scleroderma free medical journals Scleroderma famous journals Scleroderma Google Scholar indexed journals Peritoneal Dialysis articles Peritoneal Dialysis Research articles Peritoneal Dialysis review articles Peritoneal Dialysis PubMed articles Peritoneal Dialysis PubMed Central articles Peritoneal Dialysis 2023 articles Peritoneal Dialysis 2024 articles Peritoneal Dialysis Scopus articles Peritoneal Dialysis impact factor journals Peritoneal Dialysis Scopus journals Peritoneal Dialysis PubMed journals Peritoneal Dialysis medical journals Peritoneal Dialysis free journals Peritoneal Dialysis best journals Peritoneal Dialysis top journals Peritoneal Dialysis free medical journals Peritoneal Dialysis famous journals Peritoneal Dialysis Google Scholar indexed journals Scleroderma Renal Crisis articles Scleroderma Renal Crisis Research articles Scleroderma Renal Crisis review articles Scleroderma Renal Crisis PubMed articles Scleroderma Renal Crisis PubMed Central articles Scleroderma Renal Crisis 2023 articles Scleroderma Renal Crisis 2024 articles Scleroderma Renal Crisis Scopus articles Scleroderma Renal Crisis impact factor journals Scleroderma Renal Crisis Scopus journals Scleroderma Renal Crisis PubMed journals Scleroderma Renal Crisis medical journals Scleroderma Renal Crisis free journals Scleroderma Renal Crisis best journals Scleroderma Renal Crisis top journals Scleroderma Renal Crisis free medical journals Scleroderma Renal Crisis famous journals Scleroderma Renal Crisis Google Scholar indexed journals End-Stage Renal Disease articles End-Stage Renal Disease Research articles End-Stage Renal Disease review articles End-Stage Renal Disease PubMed articles End-Stage Renal Disease PubMed Central articles End-Stage Renal Disease 2023 articles End-Stage Renal Disease 2024 articles End-Stage Renal Disease Scopus articles End-Stage Renal Disease impact factor journals End-Stage Renal Disease Scopus journals End-Stage Renal Disease PubMed journals End-Stage Renal Disease medical journals End-Stage Renal Disease free journals End-Stage Renal Disease best journals End-Stage Renal Disease top journals End-Stage Renal Disease free medical journals End-Stage Renal Disease famous journals End-Stage Renal Disease Google Scholar indexed journals Pulmonary Hypertension articles Pulmonary Hypertension Research articles Pulmonary Hypertension review articles Pulmonary Hypertension PubMed articles Pulmonary Hypertension PubMed Central articles Pulmonary Hypertension 2023 articles Pulmonary Hypertension 2024 articles Pulmonary Hypertension Scopus articles Pulmonary Hypertension impact factor journals Pulmonary Hypertension Scopus journals Pulmonary Hypertension PubMed journals Pulmonary Hypertension medical journals Pulmonary Hypertension free journals Pulmonary Hypertension best journals Pulmonary Hypertension top journals Pulmonary Hypertension free medical journals Pulmonary Hypertension famous journals Pulmonary Hypertension Google Scholar indexed journals renal replacement therapy articles renal replacement therapy Research articles renal replacement therapy review articles renal replacement therapy PubMed articles renal replacement therapy PubMed Central articles renal replacement therapy 2023 articles renal replacement therapy 2024 articles renal replacement therapy Scopus articles renal replacement therapy impact factor journals renal replacement therapy Scopus journals renal replacement therapy PubMed journals renal replacement therapy medical journals renal replacement therapy free journals renal replacement therapy best journals renal replacement therapy top journals renal replacement therapy free medical journals renal replacement therapy famous journals renal replacement therapy Google Scholar indexed journals European Dialysis and Transplant Association articles European Dialysis and Transplant Association Research articles European Dialysis and Transplant Association review articles European Dialysis and Transplant Association PubMed articles European Dialysis and Transplant Association PubMed Central articles European Dialysis and Transplant Association 2023 articles European Dialysis and Transplant Association 2024 articles European Dialysis and Transplant Association Scopus articles European Dialysis and Transplant Association impact factor journals European Dialysis and Transplant Association Scopus journals European Dialysis and Transplant Association PubMed journals European Dialysis and Transplant Association medical journals European Dialysis and Transplant Association free journals European Dialysis and Transplant Association best journals European Dialysis and Transplant Association top journals European Dialysis and Transplant Association free medical journals European Dialysis and Transplant Association famous journals European Dialysis and Transplant Association Google Scholar indexed journals Pulmonary Hypertension articles Pulmonary Hypertension Research articles Pulmonary Hypertension review articles Pulmonary Hypertension PubMed articles Pulmonary Hypertension PubMed Central articles Pulmonary Hypertension 2023 articles Pulmonary Hypertension 2024 articles Pulmonary Hypertension Scopus articles Pulmonary Hypertension impact factor journals Pulmonary Hypertension Scopus journals Pulmonary Hypertension PubMed journals Pulmonary Hypertension medical journals Pulmonary Hypertension free journals Pulmonary Hypertension best journals Pulmonary Hypertension top journals Pulmonary Hypertension free medical journals Pulmonary Hypertension famous journals Pulmonary Hypertension Google Scholar indexed journals continuous ambulatory peritoneal dialysis articles continuous ambulatory peritoneal dialysis Research articles continuous ambulatory peritoneal dialysis review articles continuous ambulatory peritoneal dialysis PubMed articles continuous ambulatory peritoneal dialysis PubMed Central articles continuous ambulatory peritoneal dialysis 2023 articles continuous ambulatory peritoneal dialysis 2024 articles continuous ambulatory peritoneal dialysis Scopus articles continuous ambulatory peritoneal dialysis impact factor journals continuous ambulatory peritoneal dialysis Scopus journals continuous ambulatory peritoneal dialysis PubMed journals continuous ambulatory peritoneal dialysis medical journals continuous ambulatory peritoneal dialysis free journals continuous ambulatory peritoneal dialysis best journals continuous ambulatory peritoneal dialysis top journals continuous ambulatory peritoneal dialysis free medical journals continuous ambulatory peritoneal dialysis famous journals continuous ambulatory peritoneal dialysis Google Scholar indexed journals
Article Details
1. Introduction
Scleroderma is a rare chronic connective tissue disease that affects one to two patients per 100 000 in the United States. It involves multiple organs and characterized by immune activation, autoantibody production, fibroblast dysfunction, vasculopathy and excessive collagen deposition in the skin and internal organs [1,2]. Clinically, it is categorized into limited and diffuse subtypes according to the extent of skin involvement. Kidney disease in scleroderma, particularly scleroderma renal crisis (SRC), is associated with increased morbidity and mortality rates. It manifests with acute onset of accelerated hypertension and oliguric renal failure [1]. Despite the encouraging effects of angiotensin-converting enzyme inhibitors (ACEi) therapy in improving the prognosis and decreasing the prevalence, approximately 25% to 50% of patients with scleroderma renal crisis will develop End-Stage Renal Disease (ESRD) [2].
There is a scarcity of data regarding the prevalence of scleroderma renal crises requiring renal replacement therapy (RRT) which could be explained by the infrequency of this condition. A recent retrospective cohort study from the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Registry observed that survival on RRT in patients with scleroderma was worse than for other causes of ESRD [2]. So far and to the best of our knowledge, there are few cases reported in the literature for patients with scleroderma related ESRD treated with peritoneal dialysis (PD). Favorable outcomes with the use of PD were documented after 15 and 18 months [3,4,5,6]. In this context, we report a 34-year-old Saudi lady with diffuse systemic sclerosis complicated by SRC and Pulmonary Hypertension (PAH) who was treated with Automated Peritoneal Dialysis (APD) over five years with stabilization of PAH and residual renal function and with no mechanical or infectious complications or hospital admissions. In our opinion, PD is a very effective therapeutic modality in patients with diffuse systemic sclerosis with SRC and pulmonary hypertension.
2. Case Presentation
A 34-year-old female who was in her usual health state until the age of 15 when she developed widespread arthralgia affecting multiple joints involving bilateral knees, elbows, metacarpophalangeal and metatarsophalangeal joints. This joint involvement was not associated with any signs of inflammation or restriction in mobility. Initially, she was diagnosed with Rheumatoid Arthritis (RA) and treated accordingly. Nevertheless, her disease progressed, and the patient developed skin thickening and hardening that restricted her movement and caused severe deformities of her hands (Figure 1 & 2). Based on further serological markers, she was diagnosed with diffused cutaneous systemic sclerosis. Unfortunately, the patient lost follow up at her local hospital and presented to a different hospital at the age of 29 with a history of exertional dyspnea associated with generalized fatigue and dizziness. She also reported a history of progressive worsening of joint pain and frequent episodes of Raynaud’s phenomena. Upon examination, the patient acquired typical facial features of scleroderma including masked-face secondary to tightening of the skin, beaked nose and microstomia with puckering (Figure 3). Her hands were deformed with areas of bluish discolorations in her extremities as well as widespread skin depigmentation, giving a “salt and pepper” appearance. She was noticed to have accelerated hypertension with bi-basal course crepitation on chest examination. The patient was ill-looking, and an additional workup showed deterioration of her renal function. Therefore, she was admitted with the impression of scleroderma renal crisis (SRC) and management was initiated with angiotensin-converting enzyme inhibitors (ACEi). During hospital stay, further investigations confirmed the presence of gastroesophageal reflux disease (GERD), interstitial lung disease and pulmonary arterial hypertension. Her condition was severe enough that it rendered her unable to walk without assistance. Her renal function, unfortunately, did not recover necessitating starting renal replacement therapy (RRT). Subsequently, the patient was transferred to our center where an urgent two-cuff curled Tenckhoff PD catheter was inserted percutaneously using Al-Hwiesh technique [7]. Tidal automated peritoneal dialysis was started instantly with 10 liters Physioneal 1.36% and 15 liters Physioneal 2.27%. Each fill volume was 1.5 liter that was gradually increased to 2 liters over 7-10 days.
The patient received one gram of vancomycin intravenously prior to the procedure. After hospital discharge, she was followed-up regularly and on a monthly basis without any evidence of mechanical or infectious complications or hospital admissions. Table I represents the trend of the biochemical profile and residual renal function over the years. Table II depicts the ejection fraction and pulmonary pressure at the beginning and after five years.
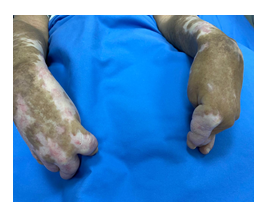
Figure 1:Sclerodactyly with severe flexion contracture.
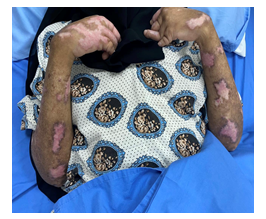
Figure 2: Multiple areas of depigmentation, ulcers and Xerosis.
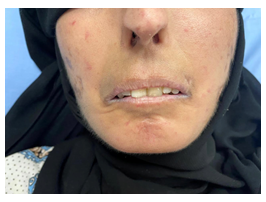
Figure 3: Tight skin, Facial telangiectasis, pinched nose, microstomia with puckering.
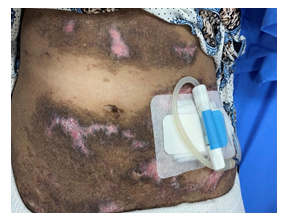
Figure 4: Multiple areas of depigmentation, excoriation and ulceration surrounding the peritoneal catheter.
|
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
|
|
Creatinine |
6.5 |
4.5 |
5.01 |
4.28 |
5.3 |
5.6 |
|
BUN |
53.5 |
42.2 |
44.5 |
38.3 |
49.7 |
51 |
|
Calcium |
8.04 |
8.13 |
8.1 |
7.6 |
8.9 |
9.5 |
|
Phosphorus |
5.32 |
4.3 |
4.7 |
4.7 |
5.3 |
5.2 |
|
Albumin |
2.4 |
2.36 |
2.26 |
2.4 |
2.9 |
3.25 |
|
HB |
9.6 |
11 |
8.4 |
9.9 |
10.4 |
12.25 |
|
RRF |
550 |
1250 |
1050 |
775 |
637.5 |
800 |
BUN: Blood Urea Nitrogen, Hb: Hemoglobin, RRF: Residual Renal Function.
Table 1: The Trend of the Biochemical Profile and Residual Renal Function over the years.
|
March 2016 |
February 2021 |
|
|
Ejection Fraction |
55% |
57% |
|
Pulmonary Pressure |
30-35mmHg |
38mmHg |
Table 2: Echocardiography Findings.
3. Discussion
Systemic sclerosis is a chronic multisystem disease characterized by widespread vascular dysfunction and progressive fibrosis of the skin and internal organs. The skin is the principal target, but internal organs are also affected. Systemic sclerosis is divided into three subtypes which are limited cutaneous systemic sclerosis, diffuse cutaneous systemic sclerosis and systemic sclerosis sine scleroderma, based on the extent of skin involvement [8]. Systemic sclerosis is relatively rare, with prevalence rates fall between 38 and 341 cases per million persons per year [9]. Renal involvement is common in patients with systemic sclerosis and consists of a spectrum of complications that includes scleroderma renal crisis (SRC), normotensive renal crisis, antineutrophil cytoplasmic antibodies-associated glomerulonephritis, penacillamine-associated renal disease, and reduced renal functional reserves manifested by proteinuria, microalbuminuria, or isolated reduction in glomerular filtration rate [1]. SRC is the most severe sequel which occurs in a minority of patients. SRC is characterized by abrupt onset of moderate to severe hypertension and acute kidney injury (AKI) [1]. Risk factors for renal progression include diffuse cutaneous systemic sclerosis, use of moderate-to high-doses of glucocorticoids and the presence of anti-RNA polymerase III antibodies [10]. Approximately 25 to 50 percent of patients with scleroderma renal crisis will eventually develop ESRD requiring dialysis [2], and the survival rate at 1 and 5 years is 70% and 50% respectively [11]. The five-year survival on hemodialysis is only 20% [12]. Peritoneal Dialysis (PD) is simple, rapid, effective and mimics normal physiology with no hemodynamic disturbances and no requirement for arteriovenous (AV) access that can augment PAH. Therefore, PD may be deemed to be the modality of choice for ESRD due to scleroderma renal crisis. However, other modalities such as hemodialysis and kidney transplantation may be considered as well. In patients with systemic sclerosis, hemodialysis may be associated with vascular access complications and intravascular instability in the early stages of SRC [3,4,13]. On the other hand, renal transplantation is another option, but careful consideration is required regarding the timing of the transplant as renal recovery can occur up to two years following SRC. Although, in general, renal transplantation offers superior survival in SRC patients, graft survival is reduced compared to the general renal transplant population. Possible causes may be attributed to the recurrence of scleroderma or increased risk of SRC secondary to calcineurin inhibitors induced vasoconstriction [13]. Few case reports have evaluated the effectiveness of peritoneal dialysis in prolonging the survival of patients with systemic sclerosis as well as maintaining a reasonable quality of life in such patients. Robson and Oreopoulos studied two scleroderma patients treated with intermittent peritoneal dialysis and found satisfactory control of uremia for up to 10 months [5]. Winfield et al., reported the successful use of continuous ambulatory peritoneal dialysis (CAPD) in two patients followed for 6 and 18 months both of which blood pressure was controlled [6]. Both Copley and Smith and Khaira et al. demonstrated similar satisfactory results in their patients treated with CAPD for 18 and 15 months respectively [3,4]. Our case, on the other hand, illustrates a case of a scleroderma patient who remains on APD for five years with successful control of blood pressure. She is currently off her antihypertensive medications and was kept on spironolactone as an antifibrotic agent with no progression of her devastating disease and reasonable improvement in her quality of life with no single episode of exit-site infection, peritonitis, or hospital admission.
About 50 % with ESRD develop pulmonary hypertension (PAH), and its presence in dialysis patients carries a poor prognosis [14]. The prevalence of PAH in systemic sclerosis is 10-15%, and it is a progressive disease with high morbidity and mortality [15]. The two-year-survival rate is about 60% [16].
The mechanism of PAH in ESRD is multifactorial, including fluid overload with pulmonary vascular remodeling, diastolic dysfunction, increased cardiac output due to an arterio-venous fistula (AVF) or chronic anemia, and chronic inflammation particularly (tumor necrosis factors alpha, endothelial-1, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and interleukin-1). These factors have been shown to induce pulmonary angiogenesis, fibroblast proliferation and apoptosis of cardiac myocytes [17,18]. Moreover, there is mounting evidence indicating that PD has the ability to remove small and middle-size molecules [19,20]. Thus, the removal of these small and middle-weight cytokines by PD is probably another important factor in delaying the progression of PAH in our patient even though she was on APD only three times per week. In addition, PD, more or less, is considered as a normal physiological process with no hemodynamic disturbances and no (AV) access that can augment PAH in dialysis patients, which could explain the stabilization of PAH in our patient over the five years.
In conclusion, to the best of our knowledge, the outcome of patients with diffuse systemic sclerosis complicated by SRC and PAH and treated with APD has not been previously reported in the literature. Here we report a 34-year-old Saudi lady with diffuse systemic sclerosis with SRC and PAH who was treated with APD over five years and demonstrated stabilization of PAH and residual renal function, and with no mechanical or infectious complications or hospital admissions. In our opinion, PD is a highly effective therapeutic modality in patients with diffuse systemic sclerosis with SRC and pulmonary hypertension. Further studies are required to explore the effectiveness of PD in this devastating disease.
Acknowledgment
The authors are pleased to express their great gratitude and appreciation to the peritoneal dialysis nurses at King Fahad University Hospital in Al Khobar for their valuable support during the patient’s course of therapy and follow up.
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
Ethical approval was obtained from Imam Abdulrahman bin Faisal University Review Board of Medical Center, and written consent was taken from the patient.
Author Contributions
Fatimah I. Alabdrabalnabi, Somaya A. Aleissa and Badran AlHwiesh were responsible for data collection. Khadija M. Alshehabi, Alawiah Alramadhan and Muaz M. Abdelgalil performed the literature review and drafted the discussion. Amani Al-Hwiesh, Nadia Al-Audah and Abdullah Al-Hwiesh contributed to writing and reviewing the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Shanmugam VK, Steen VD. Renal disease in scleroderma: an update on evaluation, risk stratification, pathogenesis and management. Current opinion in rheumatology 24 (2012): 669.
- Hruskova Z, Pippias M, Stel VS, Abad-Díez JM, Sánchez MB, et al. Characteristics and outcomes of patients with systemic sclerosis (scleroderma) requiring renal replacement therapy in Europe: results from the ERA-EDTA Registry. American Journal of Kidney Diseases 73 (2019): 184-93.
- Copley JB, Smith BJ. Continuous ambulatory peritoneal dialysis and scleroderma. Nephron 40 (1985): 353-356.
- Khaira A, Rathi OP, Gupta S, Agarwal SK. Continuous ambulatory peritoneal dialysis in a patient with scleroderma. Saudi Journal of Kidney Diseases and Transplantation 20 (2009): 130.
- Robson M, OREOPOULOS O. Dialysis in scleroderma. Annals of internal medicine 88 (1978): 843.
- Winfleld J, Khanna R, Reynolds WJ, Gordon DA, Finkelstein S, et al. Management of End-stage Scleroderma Renal Disease with Continuous Ambulatory Peritoneal Dialysis-Report of two Cases. Peritoneal Dialysis International 2 (1981): 174-177.
- Al-Hwiesh AK Percutaneous peritoneal dialysis catheter insertion by a nephrologist: a new, simple and safe technique. Perit Dial Int 34 (2014): 204-211.
- Denton CP, Khanna D. Systemic sclerosis. The Lancet 390 (2017): 1685-1699.
- Ingegnoli F, Ughi N, Mihai C. Update on the epidemiology, risk factors, and disease outcomes of systemic sclerosis. Best Practice & Research Clinical Rheumatology 32 (2018): 223-240.
- Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, et al. Systemic sclerosis. Nature reviews Disease primers 1 (2015): 1-21.
- Hesselstrand R, Scheja A and Wuttge DM. Scleroderma renal crisis in a Swedish systemic sclerosis cohort: survival, renal outcome, and RNA polymerase III antibodies as a risk factor. Scand J Rheumatol 41 (2012): 39-43.
- Cozzi F, Marson P, Cardarelli S, et al. Prognosis of scleroderma renal crisis: a long-term observational study. Nephrol Dial Transpl 27 (2012): 4398-4403.
- Lynch BM, Stern EP, Ong V, Harber M, Burns A, et al. UK Scleroderma Study Group (UKSSG) guidelines on the diagnosis and management of scleroderma renal crisis. Clinical and experimental rheumatology 34 (2016): 106-109.
- Sise ME, Courtwright AM, Channick RN. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int 84 (2013): 682-692.
- Steen V, Chou M, Shanmugam V, et al. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 134 (2008): 146.
- Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis 62 (2003): 1088.
- Harrison WF, Farber HW, Loscalzo J. Pulmonary arterial hypertension: mechanism of disease. N Engl J Med 351 (2004): 1655-1665.
- Harbaum L, Hennigs JK, Baumann HJ, Luneburg N, Griesch E, Bokemeyer C, et al. N-terminal pro-brain natriuretic peptide is a useful prognostic marker in patients with pre-capillary pulmonary hypertension and renal insufficiency. PLoS One 9 (2014): e94263.
- Zemel D, Imholtz AL, De Waart DR et al. Appearance of tumor necrosis factor-alpha and soluble TNF-receptors I and II in peritoneal effluent of CAPD. Kidney Int 46 (1994): 1422-1430.
- Fincher ME, Campbell HT, Sklar AH et al. Atrial natriuretic peptide (ANP) is removed by peritoneal dialysis in humans. Adv Perit Dial 5 (1989): 16-19.
