Peri-operative Outcomes of Varying Esophagectomy Approaches in the Treatment of Esophageal Malignancy
Article Information
Ryan T Morse MD1*, Tyler Mouw MD2, Matthew Moreno MD3, Jace T Erwin MD4, Peter DiPasco MD2, Mazin Al-Kasspooles MD2, Andrew Hoover MD5
1Department of Radiation Oncology, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA
2Department of Surgical Oncology, University of Kansas Medical Center, Kansas City, KS, USA
3Department of Plastic Surgery, University of Kansas Medical Center, Kansas City, KS, USA
4University of Kansas School of Medicine, University of Kansas Medical Center, Kansas City, KS, USA
5Department of Radiation Oncology, University of Kansas Medical Center, Kansas City, KS, USA
*Corresponding Author:Ryan Morse MD, Department of Radiation Oncology, University of North Carolina, Chapel Hill, 101 Manning Drive, Chapel Hill, NC 27514, USA
Received: 05 December 2021; Accepted: 13 December 2021; Published: 05 January 2022
Citation: Ryan T Morse, Tyler Mouw, Matthew Moreno, Jace T Erwin, Peter DiPasco, Mazin Al-Kasspooles, Andrew Hoover. Peri-operative Outcomes of Varying Esophagectomy Approaches in the Treatment of Esophageal Malignancy. Journal of Surgery and Research 5 (2022): 10-23.
View / Download Pdf Share at FacebookAbstract
Background
Minimally invasive surgery is becoming widely adopted to decrease surgical morbidity and mortality, however data is still evolving and the optimal approach remains an area of controversy. We compared our unique single-institution experience with transhiatal, transthoracic, and minimally invasive approaches to examine survival and toxicity outcomes among patients treated for esophageal cancer.
Methods
Consecutive patients undergoing esophagectomy for esophageal or gastroesophageal junction (GEJ) cancer at a single institution between 2008 and 2017 were retrospectively reviewed. The patients were stratified by surgical approach. The Kaplan-Meier method was performed using the log-rank test to calculate two-year overall survival (OS) and two-year progression-free survival (PFS).
Results
A total of 198 consecutive patients were identified: 118 transhiatal esophagectomy (THE), 34 Ivor Lewis esophagectomy (ILE), and 46 minimally invasive esophagectomy (MIE) with a median follow-up of 30.0 months (range, 0.5-136.9 months). Most tumors were adenocarcinoma (89.9%) located in the distal esophagus and GEJ (94%). Neoadjuvant chemoradiotherapy was received by 75.8% of patients. Length of hospitalization, readmission rate, perioperative adverse events, reoperation rates, tracheoesophageal fistula, anastomotic leak, anastomotic stenosis, and 30-day mortality were comparable. Two-year overall survival rates for MIE, THE, and ILE were 71.7%, 67.8%, and 58.8%, respectively (p=0.003). Progression-free survival at 2 years for MIE, THE, and ILE were 69.6%, 58.5%, and 35.3%, respectively (p=0.002).
Conclusion
Minimally invasiv
Keywords
Esophagectomy, Esophageal cancer, Minimally Invasive, Transhiatal, Ivor Lewis
Esophagectomy articles; Esophageal cancer articles; Minimally Invasive articles; Transhiatal articles; Ivor Lewis articles
Esophagectomy articles Esophagectomy Research articles Esophagectomy review articles Esophagectomy PubMed articles Esophagectomy PubMed Central articles Esophagectomy 2023 articles Esophagectomy 2024 articles Esophagectomy Scopus articles Esophagectomy impact factor journals Esophagectomy Scopus journals Esophagectomy PubMed journals Esophagectomy medical journals Esophagectomy free journals Esophagectomy best journals Esophagectomy top journals Esophagectomy free medical journals Esophagectomy famous journals Esophagectomy Google Scholar indexed journals Esophageal cancer articles Esophageal cancer Research articles Esophageal cancer review articles Esophageal cancer PubMed articles Esophageal cancer PubMed Central articles Esophageal cancer 2023 articles Esophageal cancer 2024 articles Esophageal cancer Scopus articles Esophageal cancer impact factor journals Esophageal cancer Scopus journals Esophageal cancer PubMed journals Esophageal cancer medical journals Esophageal cancer free journals Esophageal cancer best journals Esophageal cancer top journals Esophageal cancer free medical journals Esophageal cancer famous journals Esophageal cancer Google Scholar indexed journals Minimally Invasive articles Minimally Invasive Research articles Minimally Invasive review articles Minimally Invasive PubMed articles Minimally Invasive PubMed Central articles Minimally Invasive 2023 articles Minimally Invasive 2024 articles Minimally Invasive Scopus articles Minimally Invasive impact factor journals Minimally Invasive Scopus journals Minimally Invasive PubMed journals Minimally Invasive medical journals Minimally Invasive free journals Minimally Invasive best journals Minimally Invasive top journals Minimally Invasive free medical journals Minimally Invasive famous journals Minimally Invasive Google Scholar indexed journals Transhiatal articles Transhiatal Research articles Transhiatal review articles Transhiatal PubMed articles Transhiatal PubMed Central articles Transhiatal 2023 articles Transhiatal 2024 articles Transhiatal Scopus articles Transhiatal impact factor journals Transhiatal Scopus journals Transhiatal PubMed journals Transhiatal medical journals Transhiatal free journals Transhiatal best journals Transhiatal top journals Transhiatal free medical journals Transhiatal famous journals Transhiatal Google Scholar indexed journals Ivor Lewis articles Ivor Lewis Research articles Ivor Lewis review articles Ivor Lewis PubMed articles Ivor Lewis PubMed Central articles Ivor Lewis 2023 articles Ivor Lewis 2024 articles Ivor Lewis Scopus articles Ivor Lewis impact factor journals Ivor Lewis Scopus journals Ivor Lewis PubMed journals Ivor Lewis medical journals Ivor Lewis free journals Ivor Lewis best journals Ivor Lewis top journals Ivor Lewis free medical journals Ivor Lewis famous journals Ivor Lewis Google Scholar indexed journals squamous cell carcinoma articles squamous cell carcinoma Research articles squamous cell carcinoma review articles squamous cell carcinoma PubMed articles squamous cell carcinoma PubMed Central articles squamous cell carcinoma 2023 articles squamous cell carcinoma 2024 articles squamous cell carcinoma Scopus articles squamous cell carcinoma impact factor journals squamous cell carcinoma Scopus journals squamous cell carcinoma PubMed journals squamous cell carcinoma medical journals squamous cell carcinoma free journals squamous cell carcinoma best journals squamous cell carcinoma top journals squamous cell carcinoma free medical journals squamous cell carcinoma famous journals squamous cell carcinoma Google Scholar indexed journals Adenocarcinomas articles Adenocarcinomas Research articles Adenocarcinomas review articles Adenocarcinomas PubMed articles Adenocarcinomas PubMed Central articles Adenocarcinomas 2023 articles Adenocarcinomas 2024 articles Adenocarcinomas Scopus articles Adenocarcinomas impact factor journals Adenocarcinomas Scopus journals Adenocarcinomas PubMed journals Adenocarcinomas medical journals Adenocarcinomas free journals Adenocarcinomas best journals Adenocarcinomas top journals Adenocarcinomas free medical journals Adenocarcinomas famous journals Adenocarcinomas Google Scholar indexed journals Minimally invasive esophagectomy articles Minimally invasive esophagectomy Research articles Minimally invasive esophagectomy review articles Minimally invasive esophagectomy PubMed articles Minimally invasive esophagectomy PubMed Central articles Minimally invasive esophagectomy 2023 articles Minimally invasive esophagectomy 2024 articles Minimally invasive esophagectomy Scopus articles Minimally invasive esophagectomy impact factor journals Minimally invasive esophagectomy Scopus journals Minimally invasive esophagectomy PubMed journals Minimally invasive esophagectomy medical journals Minimally invasive esophagectomy free journals Minimally invasive esophagectomy best journals Minimally invasive esophagectomy top journals Minimally invasive esophagectomy free medical journals Minimally invasive esophagectomy famous journals Minimally invasive esophagectomy Google Scholar indexed journals morbidity and mortality articles morbidity and mortality Research articles morbidity and mortality review articles morbidity and mortality PubMed articles morbidity and mortality PubMed Central articles morbidity and mortality 2023 articles morbidity and mortality 2024 articles morbidity and mortality Scopus articles morbidity and mortality impact factor journals morbidity and mortality Scopus journals morbidity and mortality PubMed journals morbidity and mortality medical journals morbidity and mortality free journals morbidity and mortality best journals morbidity and mortality top journals morbidity and mortality free medical journals morbidity and mortality famous journals morbidity and mortality Google Scholar indexed journals
Article Details
1. Introduction
Esophageal cancer has classically presented in patients with chronic alcohol and tobacco use, histologically characterized as squamous cell carcinoma (SCC) arising from the upper to middle esophagus. Increasing in incidence over the last few decades, adenocarcinoma compromises about 70% of cases in North America and Western European countries [1]. Adenocarcinomas typically arise from the distal esophagus or gastroesophageal junction (GEJ) and are thought to be related to chronic reflux, obesity, and Barrett's esophagus [2]. Patients present with unintentional weight loss, progressive dysphagia, melena, and heartburn unresponsive to medical therapy [1]. Additionally, the heavy prevalence of Barrett's esophagus has led to increased detection through screening [3]. Esophageal cancer is a rapidly progressive disease with poor 5-year overall survival rates, despite continued improvements in multi-modality care [4,5]. Esophageal resection remains the mainstay of treatment for locoregionally advanced disease [6]. The current standard of care combines neoadjuvant chemoradiotherapy [7,8] or adjuvant treatment in appropriately selected patients [9]. Considerable variation exists in the surgical technique employed for esophagectomy, The Society of Thoracic Surgeons General Thoracic Surgery Database lists 14 different methods. Surgical options are typically based on patient's medical condition, tumor location, stage, and surgeon preference. The two most commonly used techniques for total esophagectomy include transhiatal esophagectomy (THE) and a transthoracic approach, known as the Ivor Lewis esophagectomy (ILE) [10]. Some evidence suggests a transhiatal approach to have lower postoperative morbidity, however several limitations include lower lymph node retrieval and difficulty in resecting large, mid esophageal and/or paratracheal tumors [10]. A minimally invasive approach to esophagectomy is an alternative to conventional open techniques, aimed at further decreasing overall morbidity and mortality [11-13]. Minimally invasive esophagectomy (MIE) has increased in popularity and is being performed at numerous academic centers [11]. Reports have confirmed this approach to be safe and to have comparable survival outcomes with open techniques, although prospective data is still evolving [14]. The optimal surgical approach for esophageal cancer has thus remained an area of controversy, and prospective trial outcomes do not necessarily translate into real-word outcomes. The complex technique of minimally invasive esophagectomy, cost effectiveness, and role in combined trimodality therapy calls for more comparative studies. We compared our unique single-institution experience with transhiatal, transthoracic, and minimally invasive approaches to examine survival and toxicity outcomes among patients.
2. Materials and Methods
2.1 Patient selection
The medical records of consecutive patients who underwent esophagectomy for histologically proven esophageal or GEJ cancer at a single institution between 2008 and 2017 were retrospectively reviewed. Patients who underwent esophagectomy for reasons other than cancer, including trauma, chronic inflammation, and motility disorders were excluded. Clinical and pathologic staging was completed by the AJCC 7th edition; patients with metastatic disease or recurrence at time of esophagectomy were excluded. All patients, regardless of age, race, tumor location, or neoadjuvant or adjuvant treatment were included in the cohort. Patients were not excluded based on histologic variant. Institution Board Review approval was obtained prior to the collection of all patient information from the electronic medical record.
2.2 Diagnosis and treatment
A complete history and physical exam were performed as part of the pre-treatment evaluation. Patients underwent upper endoscopy with biopsy for initial diagnosis. Routine staging consisted of computed tomography (CT) of the chest, abdomen, and pelvis with oral and IV contrast, positron emission tomography (PET), and endoscopy with ultrasound (EUS). Decisions regarding neoadjuvant or adjuvant chemotherapy and/or radiation therapy were determined by the treating medical oncologist or radiation oncologist, respectively. Esophagectomy approach was determined by the surgical specialist. This institution had two surgical oncologists and four cardiothoracic surgeons trained in esophagectomy approaches who each served as lead surgeons. The patients were retrospectively stratified into three groups including THE, ILE, and MIE. Minimally invasive esophagectomy consisted of three main techniques: (1) combined thoracoscopic and laparoscopic approach (Ivor Lewis); (2) thoracoscopic, laparoscopic, and cervical approach (three-hole); or (3) laparoscopic and cervical approach only (transhiatal). Documented disease characteristics included tumor histology, location, administration of neoadjuvant or adjuvant chemotherapy and/or radiation therapy, pathological staging, lymph node retrieval, and postoperative tumor margins.
2.3 Outcomes and toxicity
For each surgical approach, data collected included operative time, length of hospitalization, readmission rates, post-operative morality, rates of re-operation, and peri-operative adverse events (<90 days following surgery) including renal complications, pulmonary complications, cardiac complications, deep vein thrombosis (DVT)/pulmonary embolism (PE), wound infections, sepsis, and bleed requiring transfusion. Post-operative complications were categorized according to the Clavien-Dindo classification and the Comprehensive Complication Index (CCI) scores for each procedure [15-17]. Gastrointestinal specific complications collected included development of tracheoesophageal fistula (TEF), anastomotic leak, and anastomotic stenosis, with documented intervention, if necessary. Anastomotic leaks deemed high-risk for complications underwent esophagram, endoscopy, or surgical exploration. Clinically diagnosed leaks without overt signs of complication were managed conservatively. Long-term follow-up collected for anastomotic stenosis included number of endoscopy encounters and duration from surgery. As an additional measure of morbidity, timing of oral feeds and duration of artificial nutrition was compared.
2.4 Statistical analyses
Differences between groups were compared according to surgical technique using the one-way analysis of variance (ANOVA) for continuous variables and Pearson’s Chi-square test or Fisher’s exact test, as appropriate, for categorical variables. The primary endpoints were 2-year overall survival (OS) and progression-free survival (PFS). Secondary endpoints were focused on complications of the procedure and the gastrointestinal conduit. All survival time points were evaluated from date of surgery. Patients were analyzed as censored from the date of last clinical contact, if no radiographic or clinical progression was identified during follow-up examinations. The Kaplan-Meier method was performed using the log-rank test to compare OS or PFS between different surgical techniques. All tests were two-tailed, and differences were considered statistically significant at the 0.05 level. Statistical analyses were conducted using IBM SPSS Statistics for Windows (v. 25.0, Armonk, NY).
3. Results
3.1 Patient and treatment characteristics
A total of 198 consecutive patients receiving esophagectomy for primary esophageal cancer were identified, which included 118 THE, 34 ILE, and 46 MIE patients. Median clinical follow up was 30.0 months (range, 0.46-136.9 months). Patient characteristics separated by esophagectomy approach are shown in table 1. The ILE and THE groups had a higher BMI than MIE, and transhiatal patients had significantly greater pre-operative weight loss compared to the other two groups. Otherwise, patient characteristics were well balanced (p>0.05). Disease characteristics can be found in Table 2. Mean operating time was longer for MIE and ILE compared to THE (525, 466, 376 minutes, respectively, p<0.001). Treatment and pathologic characteristics including histology, tumor location, neoadjuvant treatment, clinical and pathologic stage, and resection status were well balanced between groups (p>0.05). The most common pathology was adenocarcinoma (89.9%) with most tumors located in the distal esophagus and GEJ (94%). Most patients (75.8%) received neoadjuvant chemoradiotherapy, and 17.7% received no chemotherapy or radiation. Otherwise, 4 patients received neoadjuvant chemotherapy alone, 1 patient received neoadjuvant radiation alone, 5 patients received adjuvant chemotherapy in addition to neoadjuvant chemoradiotherapy, 5 patients received adjuvant chemoradiation alone, 2 patients received adjuvant chemotherapy alone, 1 received neoadjuvant radiation with adjuvant chemotherapy. Clinical stage was not significantly different between groups, with comparable rates of patients with advanced-stage II and III cancers (76.5% ILE, 77.9% THE, 76.1% MIE, p=0.431). There were 43 (21.7%) patients with no residual tumor noted on final pathology. Higher amounts of lymph nodes were retrieved with THE, followed by ILE and MIE (17.8, 15.4, and 14.4, respectively, p=0.011).
|
Ivor-Lewis Esophagectomy |
Transhiatal Esophagectomy |
Minimally Invasive Esophagectomy |
P-value |
|
|
Sex |
0.135 |
|||
|
Male |
32 (94.1 %) |
95 (80.5 %) |
40 (87.0 %) |
|
|
Female |
2 (5.9 %) |
23 (19.5 %) |
6 (13.0 %) |
|
|
Age in years (range) |
60.5 (40 -79) |
63.4 (32 -81) |
64.5 (33 -82) |
0.163 |
|
BMI (range) |
28.9 (17.2 -43.0) |
28.8 (15.0 -45.0) |
26.3 (16.8 -42.4) |
0.025 |
|
Smoking History |
23 (67.6 %) |
88 (74.6 %) |
32 (69.6 %) |
0.656 |
|
Weigh Loss of 10% |
11 (32.4 %) |
63 (53.4 %) |
15 (32.6 %) |
0.015 |
|
ASA Class |
0.922 |
|||
|
2 |
2 (5.9 %) |
7 (5.9 %) |
3 (6.5 %) |
|
|
3 |
29 (85.3 %) |
104 (88.1 %) |
39 (84.8 %) |
|
|
4 |
3 (8.8 %) |
7 (5.9 %) |
4 (8.7 %) |
|
|
Diabetes |
8 (23.5 %) |
41 (34.7 %) |
9 (19.6 %) |
0.114 |
|
Cardiac Diseasea |
8 (23.5 %) |
41 (34.7 %) |
13 (28.3 %) |
0.406 |
|
Chronic Kidney Disease |
1 (2.9 %) |
7 (5.9 %) |
3 (6.5 %) |
0.831 |
|
COPD |
8 (23.5 %) |
11 (9.3 %) |
3 (6.5 %) |
0.06 |
COPD chronic obstructive pulmonary disease, ASA American Society of Anesthesiologists, BMI body mass index
aIncluded History of myocardial infarction (MI), coronary artery disease (CAD), and congestive heart failure (CHF).
Table 1: Patient demographics and medical comorbidities.
|
Ivor-Lewis Esophagectomy |
Transhiatal Esophagectomy |
Minimally Invasive Esophagectomy |
P-value |
|
|
Operation Time in minutes (range) |
466 (202 -996) |
376 (187 -1087) |
525 (326 -948) |
<0.001 |
|
Pathology |
0.242 |
|||
|
Squamous Cell Carcinoma |
3 (8.8 %) |
10 (8.5 %) |
5 (10.9 %) |
|
|
Adenocarcinoma |
31 (91.2 %) |
108 (91.5 %) |
39 (84.8 %) |
|
|
GIST |
0 (0.0 %) |
0 (0.0 %) |
2 (4.3 %) |
|
|
Tumor Location |
0.055 |
|||
|
Proximal |
0 (0.0 %) |
1 (0.8 %) |
1 (2.2 %) |
|
|
Middle |
0 (0.0 %) |
6 (5.1 %) |
4 (8.7 %) |
|
|
Distal |
20 (58.8 %) |
45 (38.1 %) |
12 (26.1 %) |
|
|
GE Junction |
14 (41.2 %) |
66 (55.9 %) |
29 (63.0 %) |
|
|
Neoadjuvant Chemotherapy |
23 (67.6 %) |
95 (80.5 %) |
36 (78.3 %) |
0.282 |
|
Neoadjuvant Radiotherapy |
25 (73.5 %) |
92 (78.0 %) |
35 (76.1 %) |
0.858 |
|
Clinical Stage |
0.431 |
|||
|
0 |
0 (0.0 %) |
2 (1.7 %) |
0 (0.0 %) |
|
|
I |
8 (23.5 %) |
24 (20.3 %) |
11 (23.9 %) |
|
|
II |
10 (29.4) %) |
47 (39.8) %) |
23 (50.0 %) |
|
|
III |
16 (47.1 %) |
45 (38.1) %) |
12 (26.1 %) |
|
|
Pathologic Stage |
0.084 |
|||
|
0 |
4 (11.8 %) |
22 (18.6 %) |
17 (37.0 %) |
|
|
I |
8 (23.5 %) |
37 (31.4 %) |
9 (19.6 %) |
|
|
II |
13 (38.2 %) |
31 (26.3 %) |
12 (26.1 %) |
|
|
III |
9 (26.5 %) |
28 (23.7 %) |
8 (17.4 %) |
|
|
Resection Completeness |
0.122 |
|||
|
R0 |
30 (88.2 %) |
112 (94.9 %) |
44 (95.7 %) |
|
|
R1 |
2 (5.9 %) |
6 (5.1 %) |
2 (4.3 %) |
|
|
R2 |
2 (5.9 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
Lymph Nodes Removed (range) |
15.4 (3 - 38) |
17.84 (2 – 49) |
14.41 (0 – 36) |
0.011 |
GIST gastrointestinal stromal tumor
Table 2: Tumor characteristics and surgical outcomes.
|
Ivor-Lewis Esophagectomy |
Transhiatal Esophagectomy |
Minimally Invasive Esophagectomy |
P-value |
|
|
Length of hospitalization in days (range) |
15.0 (5 -80) |
15.8 (6 -88) |
17.5 (7 -84) |
0.688 |
|
90-day Readmission rate |
10 (29.4%) |
35 (29.7 %) |
20 (43.5 %) |
0.214 |
|
Wound complicationsa |
4 (11.8 %) |
17 (14.4 %) |
6 (13.0 %) |
1 |
|
Pulmonary complicationsb |
12 (35.3 %) |
51 (43.2 %) |
22 (47.8 %) |
0.532 |
|
Cardiac complicationsc |
0 (0.0 %) |
3 (2.5 %) |
2 (4.3 %) |
0.68 |
|
Renal complicationsd |
9 (26.5 %) |
34 (28.8 %) |
18(39.1 %) |
0.365 |
|
Transfusion required |
8 (23.5 %) |
31 (26.3 %) |
13 (28.3 %) |
0.893 |
|
DVT |
1 (2.9 %) |
9 (7.6 %) |
1 (2.2 %) |
0.377 |
|
Sepsis |
4 (11.8 %) |
19 (16.1 %) |
11 (23.9 %) |
0.322 |
|
Re-operation |
5 (14.7 %) |
11 (9.3 %) |
9 (19.6 %) |
0.181 |
|
Clavien-Dindo grade ≥III |
18 (52.9 %) |
59 (50.0 %) |
26 (56.5 %) |
0.749 |
|
CCI score (range) |
32.6 (0-100) |
35.5 (0 -100) |
36.5(0 -100) |
0.797 |
|
Anastomotic leak |
9 (26.5 %) |
39 (33.1 %) |
14 (30.4 %) |
0.758 |
|
Tracheoesophageal fistula (TEF) |
1 (2.9 %) |
5 (4.2 %) |
3 (6.5 %) |
0.797 |
|
Prolonged tube feeding/TPN (>3 months) |
5 (14.7 %) |
17 (14.4 %) |
15 (32.6 %) |
0.022 |
|
Time to oral feeds |
0.629 |
|||
|
<1 month |
28 (82.4 %) |
92 (78.0 %) |
33 (71.7 %) |
|
|
1 – 3 months |
5 (14.7 %) |
22 (18.6 %) |
12 (26.1 %) |
|
|
4 - 6 months |
1 (2.9 %) |
1 (0.8 %) |
0 (0.0 %) |
|
|
>6 months |
0 (0.0 %) |
3 (2.5 %) |
1 (2.2 %) |
|
|
Anastomotic stenosis |
6 (17.6 %) |
23 (19.5 %) |
13 (28.3 %) |
0.399 |
|
Endoscopy encounters (range) |
1.88 (0 -9) |
2.65 (0 -25) |
3.35 (0 -28) |
0.331 |
DVT deep venous thrombosis, CCI comprehensive complication index, TPN total parenteral nutrition
aIncluded superficial incisional, deep incisional, organ/space, dehiscence.
bIncluded pneumonia, pleural effusion, pulmonary embolism, reintubation, wean failure (>48 hours on vent).
cIncluded cerebrovascular accident, cardiac arrest, myocardial infarction, acute heart failure.
dIncluded acute kidney injury and urinary tract infection.
Table 3: Perioperative adverse events and surgical morbidity.
3.2 Perioperative outcomes and toxicity
Perioperative outcomes and morbidity are shown in Table 3. Length of hospitalization and readmission rates were comparable among the groups. Overall median time to readmission was 17.0 days (range, 1-90). The most common reasons for readmission were infections and pulmonary complications (n=20), anastomotic leak (n=9), dysphagia (n=6), nausea and abdominal pain (n=6). Greater than 1 readmission within 90 days was seen in 21 total patients, with admission diagnoses comparable to the first readmission. Perioperative adverse events were not significantly different between the groups. Wound, cardiac, and renal complications remained low, while pulmonary complications occurred in 42.9%. A significant portion of patients (52.0% overall) had major complications with a Clavien-Dindo grade ≥ III, correlated with an average CCI score of 35.2, with no difference based on esophagectomy approach. When comparing rates of re-operation in the perioperative period, higher rates occurred in the MIE cohort (19.6%) compared to the ILE (14.7%) and THE (9.3%) patients (p = 0.181). The most common reason for all re-operations was anastomotic failure (n=12). No significant differences between surgical approaches were observed for rates of tracheoesophageal fistula, anastomotic leaks, or anastomotic stenosis. Nor did neoadjuvant chemoradiation predict for these complications. Only 9 patients developed tracheoesophageal fistula. The overall risk of anastomotic leak was 31.3% with treatment as follows: 8 patients required a conduit revision procedure, 15 patients required percutaneous drain placement, 5 patients required endoscopy exploration, and 34 patients were observed clinically. Leak rates with clinical impact (procedural intervention or re-admission) only occurred in 16.7% of patients with no difference between groups (p=0.117). The median time to development of leak was 10 days (range, 2-59). Anastomotic stenosis developed in 42 total patients. Balloon dilations were performed in all 42 patients and stent placement was required in 18 of these patients after failure of dilation for symptomatic dysphagia. One patient refractory to dilations and stents required surgical removal of the stenosis with a subsequent fasciocutaneous deltopectoral flap and split-thickness skin graft as a bridge to colonic interposition. Patients that developed anastomotic leak were significantly more likely to develop stenosis (p=0.003). Time to first stenosis occurred at a median of 3.7 months (range, 1.2- 63.8). The median endoscopy encounters for patients that developed stenosis was 7.5 (range, 1-28). Endoscopy duration for patients developing stenosis was as follows: <1-month (1 patient), 1-3 months (5 patients), 4-6 months (7 patients), 7-12 months (5 patients), 1-3 years (15 patients), >3 years (9 patients).
3.3 Survival endpoints
With a medium survival follow-up of 34.3 months (range, 0.46-136.9 months), 86 patients were alive at the time of this analysis yielding an overall survival rate for the entire esophagectomy cohort of 43.4%. The in-hospital mortality rate (n=6) for ILE, THE, and MIE was 0.0%, 4.2%, and 2.2%, respectively (p=0.724). No patients died intra-operatively and the causes of death were multi-organ failure (n=4) and cardiac arrest (n=2). Mortality 30 days from surgery was 0.0%, 0.8%, and 2.2% (p=0.646) and 90 days from surgery was 0.0%, 5.9%, and 8.7% (p=0.278) between ILE, THE, and MIE, respectively. Kaplan-Meier curves comparing OS and PFS between the cohorts can be seen in figure 1 and figure 2, respectively. A significant survival advantage was shown in MIE and THE patients compared to ILE with an estimated 2-year OS of 71.7%, 67.8%, and 58.8%, respectively (p=0.003). The median OS time was as follows: ILE 27.6 months, THE 42.4 months, and MIE 82.7 months. A similar advantage was seen in PFS at 2 years for MIE and THE compared to ILE with 2-year PFS of 69.6%, 58.5%, and 35.3%, respectively (p=0.002). The median PFS time was as follows: ILE 14.3 months, THE 33.4 months, and MIE 53.0 months. On sub-group analysis of advanced stage (stage II and III) disease, outcomes remained favorable for MIE and THE patients for overall-survival (2-year OS 65.7% MIE, 66.3% THE, 53.8% ILE, p=0.026) and progression-free survival (2-year PFS 62.8% MIE, 54.3% THE, 30.7% ILE, p=0.018).
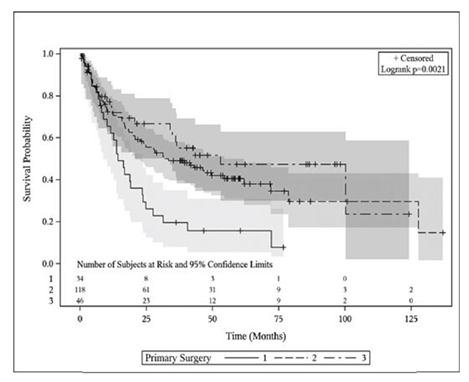
Figure 1: Overall survival plot comparing ILE (1), THE (2) and MIE (3).

Figure 2: Progression-free survival plot comparing ILE (1), THE (2) and MIE (3).
4. Discussion
We report our experience with varying approaches to esophageal resection from a large esophagectomy database at a single academic medical center. This article provides supportive evidence that minimally invasive esophagectomy results in comparable survival outcomes relative to a transhiatal approach. This study found perioperative complications between esophagectomy approaches to be similar, proving that minimally invasive esophagectomy can safely be performed and should continue to be studied. Regardless of the technique, a significant number of patients remain at risk for major complications that can significantly prolong hospitalization and impact patient morbidity and mortality. The high major complication and readmission rates in this study confirm the importance of aggressive symptom management and close surgical follow-up. As minimally invasive surgical techniques continue to evolve, maintaining favorable disease specific outcomes remains paramount. Minimally invasive esophagectomy was first introduced 20 years ago and has increasingly been the procedure of choice at many institutions, however a consistent survival advantage has not been demonstrated and data collection from clinical trials is ongoing [18-21]. The first randomized controlled trial to compare MIE versus open techniques showed a substantial benefit in reducing postoperative complications, but no differences were found in 3-year disease-free and overall survival [12]. Randomized data by Mariette et al. demonstrated an improved 3-year survival (67% versus 55%) for MIE versus open esophagectomy, closely aligning with MIE results from our study at 3-years (67.4%) [13]. A randomized control trial by the Dutch compared robotic assisted thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy and showed no difference in survival outcomes at a median follow-up of 40 months [22]. Guo et al. recently published a meta-analysis comparing combined thoracoscopic-laparoscopic esophagectomy with open esophagectomy and found comparable survival rates at 5 years [23]. Results from this study, along with previously reported literature, indicates that minimally invasive esophagectomy is a surgical technique that may have comparable survival outcomes to open approaches and results of ongoing trials will be highly anticipated. A minimally invasive approach to esophagectomy has several theoretical advantages to improving potential complications and perioperative mortality by limiting operative trauma that results from open procedures. Several authors have reported decreased pulmonary complications, wound complications, blood loss, postoperative pain, and length of hospital stay with MIE [11,24,25]. Interestingly, our findings did not corroborate these reported advantages from the literature. Pulmonary complications were amongst the most common in our series, occurring in 43% of our study population, and were comparable across surgical groups. Hospitalization length, wound complications, and blood loss were also similar between groups in our study without an advantage seen in the MIE cohort. A clear cause for the discrepancies in relative complication rates with MIE between our study and others is uncertain. As evidenced by Markar et al. outcomes of surgical techniques tested in randomized trials do not always translate to real-world practice [26]. The Dutch Upper GI Cancer Audit (DUCA) was designed to externally validate results from the TIME trial. When comparing over 2,600 MIE to over 1,900 open esophagectomies, authors found a significant increase in overall complications, pulmonary complications, re-operation rate, and length of hospitalization in the MIE cohort [26]. The authors concluded the need for complex surgical innovations to be reproducible in a standardized fashion before implementation in real-world practice. Surgical expertise and peri-operative care may also be a factor in the variable toxicity outcomes observed in different studies. Esophagectomy for esophageal cancer is a complex oncologic technique that results in lower perioperative morbidity and mortality when performed in high-volume hospitals by experienced surgeons [27-29]. Among all complex cancer resections that have been studied, the association between volume and outcome for esophagectomy has remained one of the strongest [28,29]. These outcomes have also translated into long-term survival advantages. When comparing high-volume to low-volume surgical centers, a healthcare-services-linked database has shown an absolute survival difference of 17% at 5 years. This difference in 5-year survival remained the highest among all cancer resections surveyed [30]. One potential contributing factor that is not well represented in the current literature involves the types and quality of pre-operative cancer therapies that patients receive and their associated effects on surgical morbidity. Treatment for esophageal cancer commonly involves upfront chemotherapy and radiation treatments prior to esophagectomy. Each treatment modality imparts its own unique set of toxicities and when combined can lead to significant deficits in patient quality of life, nutritional status, organ function, and overall performance status in the weeks and months leading up to surgery. Radiation remains a critical component of therapy for many esophageal cancer patients which can lead to both acute and chronic local toxicity which directly impacts risk of surgical complications. Radiation delivers dose locally to the skin, heart, lungs, and esophagus that can predispose the patient to post-operative cardiac, pulmonary, wound, and esophageal complications such as anastomotic leak and stricture. While few surgical centers are capable of complex procedures, many patients can receive chemotherapy and radiation in closer proximity to their primary residence for convenience of daily treatments that can last several weeks. As a form of non-operative local treatment, technique and skill with radiation are required to decrease toxicity while maintaining favorable survival. Academic institutions commonly have dedicated gastrointestinal radiation oncology physicians, as well as dosimetry and physics personnel that experience higher volume esophageal cancers. Several studies have researched dosimetric variables associated with complications, but none have looked at the importance of radiation delivery at high-volume cancer centers [31,32]. Given the complexity of these surgeries and discrepancies in reported complication rates reported across studies, our findings demonstrate the need for ongoing study of possible contributing factors and the relative importance of each. There are several limitations of our work, largely by this study being retrospective in nature. Beyond this consideration, another limitation is performing a single-center review. Our results would have benefited from analyzing multiple institutions to account for geographic differences, patient demographics, sample size, and surgeon experience. The surgical preference at this institution favors the transhiatal approach based on training and years of experience and limits the sample size of other approaches. By virtue of being one of the only institutions performing the esophagectomy procedure in the region of this academic hospital, many patients travel great distances for their operation. As a result, attaining long-term follow-up in certain patients proves to be difficult. Medically fit patients with qualifying tumor characteristics should be offered standard of care chemotherapy and radiotherapy, however many patients in this study received neoadjuvant treatment at non-academic institutions and thus could not have been controlled in this study. In similar regards, specific details relating to chemotherapy and radiotherapy were not able to be obtained, including type of chemotherapy, total cycles received, total dose of radiation, and whether prolonged radiation treatment delays occurred, as these all lower survival benefits.
5. Conclusion
In summary, this study adds to the growing literature that minimally invasive esophagectomy results in comparable survival outcomes to the transhiatal approach and suggests inferior outcomes with the Ivor Lewis approach. The perioperative morbidity and mortality are markedly comparable, which are of utmost importance when considering this patient population that typically endures significant toxicity from multimodality treatment. The minimally invasive surgical approach should continue to be explored with definitive large-scale outcomes data.
Acknowledgments
Ying Cao for assistance with graph development and statistical analysis. Elliot Konrade and Randi Ryan for assistance with data management. Kishan Moonesinghe and Holly Zink for their assistance with publication and institutional review.
Declarations
Funding
No specific funding sources were utilized in the publication of this manuscript.
Conflicts of interest
All authors have no conflicts of interest to disclose.
Availability of data and material
All data is stored separately in a data repository.
Authors’ contributions
The authors have fulfilled all the criteria in the definition of authorship.
Ethics approval
Institution Board Review approval was obtained prior to the collection of all patient information from the electronic medical record.
References
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 371 (2014): 2499-509.
- Lundell LR. Etiology and risk factors for esophageal carcinoma. Dig Dis 28 (2010): 641-644.
- Hamel C, Ahmadzai N, Beck A, et al. Screening for esophageal adenocarcinoma and precancerous conditions (dysplasia and Barrett's esophagus) in patients with chronic gastroesophageal reflux disease with or without other risk factors: two systematic reviews and one overview of reviews to inform a guideline of the Canadian Task Force on Preventive Health Care (CTFPHC). Systematic reviews 9 (2020): 20.
- Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 119 (2013): 1149-58.
- Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol 31 (2016): 1141-1146.
- Ilson DH. Adenocarcinoma of the esophagus: controversies and consensus. Chinese clinical oncology 6 (2017): 52.
- Van Hagen P, Hulshof MC, Van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366 (2012): 2074-2084.
- Shapiro J, Van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16 (2015): 1090-1098.
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345 (2001): 725-730.
- Hulscher JB, Van Sandick JW, De Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347 (2002): 1662-1669.
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally invasive versus open esophagectomy for esophageal cancer: A comparison of early surgical outcomes from the society of thoracic surgeons national database. Ann Thorac Surg 101 (2016): 1281-1288.
- Biere SS, Van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 379 (2012): 1887-1892.
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med 380 (2019): 152-162.
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 261 (2015): 702-707.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240 (2004): 205-213.
- Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258 (2013): 1-7.
- Katayama H, Kurokawa Y, Nakamura K, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 46 (2016): 668-685.
- Meredith KL, Maramara T, Blinn P, et al. Comparative perioperative outcomes by esophagectomy surgical technique. J Gastrointest Surg 13 (2019): 12-25.
- Metcalfe C, Avery K, Berrisford R, et al. Comparing open and minimally invasive surgical procedures for oesophagectomy in the treatment of cancer: the ROMIO (Randomised Oesophagectomy: Minimally Invasive or Open) feasibility study and pilot trial. Health Technol Assess 20 (2016): 1-68.
- Van Workum F, Bouwense SA, Luyer MD, et al. Intrathoracic versus cervical anastomosis after minimally invasive esophagectomy for esophageal cancer: study protocol of the ICAN randomized controlled trial. Trials 17 (2016): 505.
- Kataoka K, Takeuchi H, Mizusawa J, et al. A randomized Phase III trial of thoracoscopic versus open esophagectomy for thoracic esophageal cancer: Japan clinical oncology group study JCOG1409. Jpn J Clin Oncol 46 (2016): 174-177.
- Van der Sluis PC, Van der Horst S, May AM, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: A Randomized Controlled Trial. Ann Surg 269 (2019): 621-630.
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 30 (2016): 3873-3881.
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 24 (2010): 1621-1629.
- Espinoza-Mercado F, Imai TA, Borgella JD, et al. Does the approach matter? Comparing survival in robotic, minimally invasive, and open esophagectomies. Ann Thorac Surg 107 (2019): 378-385.
- Markar SR, Ni M, Gisbertz SS, et al. Implementation of minimally invasive esophagectomy from a randomized controlled trial setting to national practice. J Clin Oncol 12 (2020): 24-28.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 346 (2002): 1128-1137.
- Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 280 (1998): 1747-1751.
- Killeen SD, O'Sullivan MJ, Coffey JC, et al. Provider volume and outcomes for oncological procedures. Br J Surg 92 (2005): 389-402.
- Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg 245 (2007): 777-783.
- Koëter M, Van der Sangen MJ, Hurkmans CW, et al. Radiation dose does not influence anastomotic complications in patients with esophageal cancer treated with neoadjuvant chemoradiation and transhiatal esophagectomy. Radiat Oncol 10 (2015): 59.
- Vande Walle C, Ceelen WP, Boterberg T, et al. Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys 82 (2012): e513-519.
