Periodontitis Increases Risk of Peripheral Arterial Occlusive Disease in Diabetics Subjects Living in Cameroon
Article Information
Essama Eno Belinga Lawrence1,2,3*, Mbango Ekouta Noel Desiree11,4, Nokam Abena Elvire5, Essam Nloo Alain Patrick6, Bell Ngan Williams6, Abanda Dang Gabriel1 , Ebana Mvogo Come5, Bengondo Messanga Charles5, Lemogoum Daniel1
1Faculty of Medicine and Pharmaceutical Sciences, University of Douala, Douala, Cameroon
2Department of Surgery and Specialties, University of Douala, Douala, Cameroon
3Department of Stomatology, Douala General Hospital, Douala, Cameroon
4Endocrinology Unit, Douala General Hospital, Douala, Cameroon
5Faculty of Medicine and Biomedical Sciences, University of Yaoundé, Yaoundé, Cameroon
6Department of Military Health, Yaoundé, Cameroon
*Corresponding Author: Essama Eno Belinga Lawrence, Department of Stomatology, Surgery and Specialties, Faculty of Medicine and Pharmaceutical Sciences, University of Douala, Douala General Hospital, BP: 4856, Douala, Cameroon
Received: 29 August 2020; Accepted: 09 September 2020; Published: 18 September 2020
Citation: Essama Eno Belinga Lawrence (laubell@yahoo.fr)
View / Download Pdf Share at FacebookAbstract
The aim of our study was to evaluate the relationship between Peripheral Arterial Occlusive Disease (PAOD) and Periodontitis in Diabetics subjects followed in Douala General Hospital, Cameroon.
Materials and Method: We conducted a cross-sectional study from January 1 to March 20, 2020. To be included in the study, subjects had to be either type 1 or type 2 diabetics, at least 18 years of age and benefiting from either inpatient or outpatient followed up in the internal medicine department. Ankle Brachial Index (ABI) were measured for each participant with manual sphygmomanometer and pocket Doppler. Periodontitis was classified based on pocket depth (PD) and clinical attachment (CAL) values according the Centers For Disease Control and Prevention and the American Academy of Periodontology 2007 (CDC/AAP) All statistical analyses were performed using Epi Info7 software. Logistic regression analysis was performed to determine factors associated to PAOD in our sample.
Results: A total of 100 patients were recruited. Mean periodontal parameters such as PD, CAL, number of sites with PD>4mm, number of sites with CAL>3mm were significantly higher in PAOD diabetics subjects compared to those without PAOD. After multivariate logistic regression, the factors remaining associated to PAOD were periodontitis (OR=3,60 [1,12-11,53]) and hypercholesteremia (OR=5,99 [2,04-17,55]).
Conclusion: Management of periodontitis may reduce the risk of having PAOD in diabetic subjects followed at the Douala General Hospital, Cameroon.
Keywords
Periodontitis, Peripheral Arterial Occlusive Disease, Ankle Brachial Index, Diabetes, Cameroon
Periodontitis articles, Peripheral Arterial Occlusive Disease articles; Ankle Brachial Index articles; Diabetes articles; Cameroon articles
Periodontitis articles Periodontitis Research articles Periodontitis review articles Periodontitis PubMed articles Periodontitis PubMed Central articles Periodontitis 2023 articles Periodontitis 2024 articles Periodontitis Scopus articles Periodontitis impact factor journals Periodontitis Scopus journals Periodontitis PubMed journals Periodontitis medical journals Periodontitis free journals Periodontitis best journals Periodontitis top journals Periodontitis free medical journals Periodontitis famous journals Periodontitis Google Scholar indexed journals Peripheral Arterial Occlusive Disease articles Peripheral Arterial Occlusive Disease Research articles Peripheral Arterial Occlusive Disease review articles Peripheral Arterial Occlusive Disease PubMed articles Peripheral Arterial Occlusive Disease PubMed Central articles Peripheral Arterial Occlusive Disease 2023 articles Peripheral Arterial Occlusive Disease 2024 articles Peripheral Arterial Occlusive Disease Scopus articles Peripheral Arterial Occlusive Disease impact factor journals Peripheral Arterial Occlusive Disease Scopus journals Peripheral Arterial Occlusive Disease PubMed journals Peripheral Arterial Occlusive Disease medical journals Peripheral Arterial Occlusive Disease free journals Peripheral Arterial Occlusive Disease best journals Peripheral Arterial Occlusive Disease top journals Peripheral Arterial Occlusive Disease free medical journals Peripheral Arterial Occlusive Disease famous journals Peripheral Arterial Occlusive Disease Google Scholar indexed journals Ankle Brachial Index articles Ankle Brachial Index Research articles Ankle Brachial Index review articles Ankle Brachial Index PubMed articles Ankle Brachial Index PubMed Central articles Ankle Brachial Index 2023 articles Ankle Brachial Index 2024 articles Ankle Brachial Index Scopus articles Ankle Brachial Index impact factor journals Ankle Brachial Index Scopus journals Ankle Brachial Index PubMed journals Ankle Brachial Index medical journals Ankle Brachial Index free journals Ankle Brachial Index best journals Ankle Brachial Index top journals Ankle Brachial Index free medical journals Ankle Brachial Index famous journals Ankle Brachial Index Google Scholar indexed journals Diabetes articles Diabetes Research articles Diabetes review articles Diabetes PubMed articles Diabetes PubMed Central articles Diabetes 2023 articles Diabetes 2024 articles Diabetes Scopus articles Diabetes impact factor journals Diabetes Scopus journals Diabetes PubMed journals Diabetes medical journals Diabetes free journals Diabetes best journals Diabetes top journals Diabetes free medical journals Diabetes famous journals Diabetes Google Scholar indexed journals gingival bleeding articles gingival bleeding Research articles gingival bleeding review articles gingival bleeding PubMed articles gingival bleeding PubMed Central articles gingival bleeding 2023 articles gingival bleeding 2024 articles gingival bleeding Scopus articles gingival bleeding impact factor journals gingival bleeding Scopus journals gingival bleeding PubMed journals gingival bleeding medical journals gingival bleeding free journals gingival bleeding best journals gingival bleeding top journals gingival bleeding free medical journals gingival bleeding famous journals gingival bleeding Google Scholar indexed journals periodontal pocket articles periodontal pocket Research articles periodontal pocket review articles periodontal pocket PubMed articles periodontal pocket PubMed Central articles periodontal pocket 2023 articles periodontal pocket 2024 articles periodontal pocket Scopus articles periodontal pocket impact factor journals periodontal pocket Scopus journals periodontal pocket PubMed journals periodontal pocket medical journals periodontal pocket free journals periodontal pocket best journals periodontal pocket top journals periodontal pocket free medical journals periodontal pocket famous journals periodontal pocket Google Scholar indexed journals
Article Details
1. Introduction
Peripheral arterial occlusive disease (PAOD) reflects damage to the arterial network of the lower limbs by the process of atherosclerosis. It affects more than 200 million people worldwide [1]. Diabetes is a potent risk factor for PAOD with major amputation of the lower limbs as main complication [1, 2]. The frequency of PAOD is 22% in black African diabetics [2]. PAOD diagnosed by finding an Ankle Brachial Index (ABI) value less than 0.90 [3]. Periodontitis is an inflammatory disease linked to an imbalance of the oral flora leading to an irreversible destruction of the periodontal attachment system. The prevalence of periodontitis varies from 20 to 50% in the general population [4]. In Cameroon, its frequency is 15% [5]. Etiopathogenic mechanisms of periodontitis are complex and result from the imbalance between bacterial aggression and the host's defenses on one hand, and general, systemic and environmental risk factors on the other [6].
The diagnosis of periodontitis is made on the basis of certain clinical signs such as clinical attachment loss (CAL), presence of periodontal pocket, gingival bleeding and radiological signs corresponding to bone resorption. The role of diabetes in the pathogenesis of periodontal disease has been highlighted by several studies [7, 8]. Nascimento and al. have shown that diabetes increased the risk of periodontitis in adults (RR =1.86 [1.3-2.8]) [8]; On the other hand, current data suggest that periodontal disease is associated to cardiovascular diseases linked to atherosclerosis such as coronary artery disease, cerebrovascular disease and peripheral arterial disease [9,10]. The link between periodontitis and peripheral artery disease has been the subject of some publications [9-14]. Recent data suggest that patients with PAOD have 1.70 times more risk of developing periodontitis compared to those without PAOD [10]. In addition, the beneficial effect of periodontal therapy on endothelial function has also been described [10, 15]. Because of the high cardiovascular and periodontal risks of the diabetic subject, it seemed relevant to us to assess the link between periodontitis and PAOD in diabetic subjects followed up at the Douala General Hospital.
2. Materials and Method
We conducted a cross-sectional study in the internal medicine department of the Douala General Hospital from January 1 to March 20, 2020. To be included in the study, subjects had to be either type 1 or type 2 diabetics, at least 18 years of age and benefiting from either inpatient or outpatient followed up in the internal medicine department. Diabetic patients who did not have a biological assessment (LDL cholesterol level) dating less than 1 month as well as those who presented any event affecting assessment of ABI, namely; pregnancy, history of vascular surgery, local diseases compromising assessment of ABI, patients with short-term life-threatening alterations in vital function were not included. Likewise, all diabetic patients who presented any event compromising assessment of periodontitis, namely any patient who received periodontal treatment during the last 6 months, and/or under antibiotic therapy, and those with less than 50% of teeth per sextant were excluded from the study.
2.1 Data collected and variables definitions
For each participant, a pre-tested questionnaire was used to collect information related to marital status, age, gender, smoking, medical history of diabetes, hypertension, obesity, LDL cholesterol level and medications. This was done through patients' medical files. The fasting capillary blood glucose test was carried out using glucometers. The weight, height, body mass index (BMI), and ABI were measured by a trained practicing nurse. Periodontal examination was conducted by periodontist.
2.2 ABI assessment
Brachial and ankle pressures were measured for each
participant after an adequate rest of at least 5 minutes in a supine position. A manual sphygmomanometer and pocket Doppler device with a probe to listen to the flow of the brachial, dorsal pedal, and posterior tibial arteries were used. Brachial pressures were measured bilaterally, and were repeated if the difference was > 10 mm Hg between the two arms. Ankle pressures were determined with cuffs placed proximal to the malleoli. The ABI was calculated by dividing the highest systolic ankle pressure (either posterior tibial or dorsal pedal) in each leg by the highest systolic brachial pressure.
2.3 Periodontal examination
A partial mouth periodontal examination was performed on ten index teeth, namely, 17, 16, 11, 26, 27, 37, 36, 31, 46, 47. Periodontal probing was carried out using a WHO periodontal probe at 4 sites per tooth, mesio and disto vestibular, mesio and disto lingual, or mesio and disto-palatine. Periodontal parameters assessed were oral hygiene, pocket depth (PD) and CAL. The values obtained after probing were entered on the survey sheet. The PD measured corresponded to the distance from the gingival margin to the bottom of the sulcus. CAL corresponded to the distance from the cementum enamel junction to the bottom of the sulcus. If an index tooth was absent it was replaced by another tooth belonging to the same sextant. Teeth with significant coronary decay as well as cervical fillings that did not allow the cementum enamel junction to be assessed were not considered. Oral hygiene was evaluated using plaque index scores according to Silness and Löe, 1964.
Score 0: no plaque;
Score 1: thin film of plaque in contact with the marginal gingiva visible only after exploration with the probe;
Score 2: moderate accumulation of plaque in contact
with the marginal gingiva; no plaque in the inter-dental spaces; deposits visible to the naked eye;
Score 3: large accumulation of plaque in contact with the marginal gingiva; presence of plaque in the inter-dental spaces.
In the event of a tooth loss or significant or total coronary destruction, no score was assigned. The final score was the total score divided by the number of sites examined. Periodontitis were classified based on PD and CAL values according the Centers For Disease Control and Prevention and the American Academy of Periodontology 2007 (CDC/AAP) as Mild periodontitis (at least 2 inter proximal sites with CAL ≥ 3mm on different teeth and at least 2 inter proximal sites with PD ≥ 4mm or at least 1 inter proximal site with PD ≥ 5mm); moderate periodontitis (at least 2 inter proximal sites with CAL ≥ 4mm on different teeth or at least 2 inter proximal sites with PD ≥ 5mm on different teeth); Severe periodontitis (at least 2 inter proximal sites with CAL ≥ 6mm on different teeth or at least 2 inter proximal sites with PD ≥ 5mm on different teeth). The variables measured were defined as follows:
- PAOD: ABI < 0.9;
- Hypertension: subjects with diagnostic of hypertension in their medical file and under anti-hypertensive treatment followed in the internal medicine department of Douala General Hospital;
- Diabetes: subjects with diagnostic of diabetes in their medical file and under anti-diabetic treatment followed in the internal medicine department of Douala General Hospital;
- Smoking: was considered as smoker, anyone declaring to smoke at least 1 cigarette per day;
- Alcoholism: was considered as an alcohol consumer, anyone declaring to drink at least one alcoholic drink per day;
- Obesity: BMI ≥ 30 Kg/m²;
- Hypercholesterolemia: was defined on the basis of
lipid-lowering treatments and/or LDL-cholesterol level > 1.5g/l.
2.4 Statistical analysis
All statistical analyses were performed using Epi Info7 software. The Students’ t test was used to compare the means of quantitative variables; the X2 test was used to compare qualitative variables. Logistic regression analysis was performed to determine factors associated to PAOD in our sample. The significance level for all analyses was set at p < 0.05.
2.5 Ethical considerations
The study was conducted in accordance with the Helsinki Declaration and approved by the Faculty of Medicine and Pharmaceutical Sciences of the University of Douala, and registered at the Ethics Committee of the faculty under the number 2164 CEI-Udo/01/2020/T.
3. Results
3.1 General characteristics of the sample
A total of 100 diabetics patients were included in the study, 95% of them were type 2 diabetics. Over 55% were women and the male/female sex ratio was 0.82. A total of 27 patients were diagnosed with PAOD. Regarding our population with PAOD, 17 patients were women (62.97%) and 10 were men (37.03%). Overall, 52 subjects in our sample suffered from PD. Mean periodontal parameter were significantly higher in PAOD diabetics subjects compared to those without PAOD. The general characteristics of the sample are represented in tables 1 and 2, and in figure 1.
3.2 Factors associated with PAOD
After multivariate logistic regression, the factors remaining associated to PAOD were periodontitis (OR=3,60 [1,12-11,53]) and hypocholesteremia (OR=5,99 [2,04-17,55]). The results of analysis of the relationship between PAOD and other subjects’ characteristics are shown at Tables 3 and 4.
|
Variables |
Mean |
Standard deviation |
Minimum |
Maximum |
Median |
|
Age (years) |
60.04 |
10.27 |
30.00 |
84.00 |
60.10 |
|
ABI |
1.04 |
0.17 |
0.53 |
1.42 |
1,10 |
|
Glycaemia (g/l) |
1,68 |
0.84 |
0,70 |
4.75 |
1.36 |
|
LDL-cholesterol (g/l) |
1.09 |
0.25 |
0.40 |
1.75 |
1.2 |
|
Diabetes duration (years) |
7.81 |
6.49 |
1.00 |
30.00 |
8.00 |
ABI: ankle brachial index; LDL: low density lipoprotein
Table 1: General parameters of the study.
|
Periodontal Parameters (Mean) |
PAOD |
p |
|
|
Yes (n= 27) |
No (n=73) |
||
|
Mean ± Sd |
Mean ± Sd |
||
|
PI |
0.44 ± 0.80 |
0.48 ± 0.83 |
>0.5 |
|
PD |
3.48 ± 0.71 |
2.54 ± 1.09 |
<0.00001 |
|
CAL |
0.84 ± 0.57 |
0.53 ± 0.69 |
0.042 |
|
Number of sites with PD ≥ 4mm |
13.44 ± 6.48 |
6.52 ± 8.99 |
<0.00001 |
|
Number of sites with PAC ≥ 3mm |
6.81 ± 4.00 |
3.07 ± 4.50 |
<0.00001 |
PAOD: Peripheral Arterial Occlusive Disease; Sd: standard deviation; PI: plaque index; PD: pocket depth; CAL: clinical attachment loss.
Table 2: Periodontal parameters of subjects.
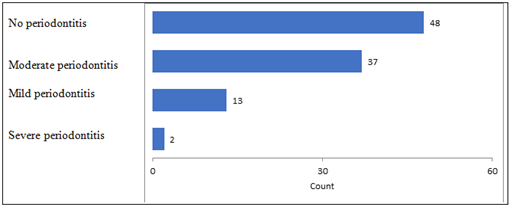
Figure 1: Frequency of periodontitis in the sample.
|
Variables |
PAOD |
OR (CI95%) |
p |
||||
|
Yes |
No |
||||||
|
n |
% |
n |
% |
||||
|
Hypertension |
No |
7 |
25.9 |
28 |
38.4 |
1.77 (0.66-4.74) |
0.251 |
|
Yes |
20 |
74.1 |
45 |
61.6 |
|||
|
Hypercholesterolemia |
No |
11 |
40.7 |
63 |
86.3 |
9.16 (3.31-25.33) |
0.001 |
|
Yes |
16 |
59.3 |
10 |
13.7 |
|||
|
Obesity |
No |
7 |
25.9 |
25 |
34.2 |
1.48 (0.55-3.99) |
0.43 |
|
Yes |
20 |
74.1 |
48 |
65.8 |
|||
|
Smoking |
No |
25 |
92.6 |
66 |
90.4 |
0.75 (0.14-3.87) |
0.736 |
|
Yes |
2 |
7.4 |
7 |
9.6 |
|||
|
Periodontitis |
No |
5 |
18.5 |
43 |
58.9 |
6.30 (2.14-18.51) |
|
|
Yes |
22 |
81.5 |
30 |
41.1 |
|||
PAOD: Peripheral Arterial Occlusive Disease; OR: odd ratio; CI95%: 95% confidence interval.
Table 3: Factors associate to PAOD, univariate analysis.
|
Risk Factor |
OR (CI95%) |
p-value |
|
Hypercholesterolemia |
5.99 (2.04-17.55) |
0.001 |
|
Periodontitis |
3.60 (1.12-11.53) |
0.031 |
OR: odd ratio, CI95%: 95% confidence interval
Table 4: Significant risk factors associate to PAOD, multivariate analysis.
4. Discussion
In this cross-sectional study, we found that diabetic subjects suffering from periodontitis were 3 times at risk of developing PAOD after adjusting for hypercholesterolemia. This study also provides the evidence that mean periodontal parameters of PAOD diabetics such as PD, CAL, sites with PD ≥ 4mm and sites with CAL ≥ 3mm were significantly higher compared to those without PAOD. These results are similar to those reported in the literature regarding the association between periodontitis and PAOD [11-15]. In fact, the systematic review by Kaschwish et al identified 9 studies out of 31 which found a positive association between periodontitis and PAOD [12]. The first published report was made by Mendez et al in a study conducted in 1998 [13]. It reported that the risk of developing PAOD was 2.27 [1.32-3.9] times higher in men with periodontitis at baseline, compared to those without periodontitis or those with mild periodontitis.
Similarly, Lu et al in 2008, showed that periodontitis was significantly associated with PAOD in 172 subjects (OR = 2.3 [1.2–4.2]) [14]. Chen et al in 2008, in Japan observed in a case-control study that periodontitis was significantly associated with PAOD after adjusting for age, gender, diabetes and tobacco (OR = 5.5 [1.6–18.9]) [15]. Calapkorur et al in 2017, found that periodontitis increased the risk of having PAOD by 5.8 [1.5-21.9] after adjusting for age, diabetes, hypertension and BMI. However, in this study the authors did not find a significant association between clinical periodontal parameters and peripheral arterial disease in 60 participants [16].
Yang et al in 2018, via a meta-analysis on 4,307 participants showed a significantly increased risk of PD in the group of patients with PAOD compared to patients who were free from PAOD (RR = 1.70 [1.25-2.29]) [11]. The pathophysiological mechanisms between PAOD and periodontitis are complex and four of them have been resumed in the systematic review of Kaschwich in 2019, to explain this association [12, 18-22]: (1) oral bacteremia is thought to be responsible for the penetration of microorganisms into the bloodstream. origin of damage to the arterial wall [18, 19]; (2) periodontal inflammation is thought to be responsible for the release of pro-inflammatory mediators at the systemic level causing an acute phase reaction, which is pro-atherogenic [20, 21]; (3) the specific components of the pathogens would trigger an immune response from the host, thus promoting autoimmunity [22]; and finally, (4) the specific bacterial toxins produced by oral pathogenic bacteria would have pro-atherogenic effects. Recent works support the fact that periodontal disease and occlusive peripheral artery disease share at least one important predisposing genetic risk haplotype located on chromosome 9p21.3 in a locus called ANRIL/CDKN2B-AS1 [22].
In our study, the global frequency of periodontitis in diabetics patients was 52% and 81% for PAOD diabetics. This figure is higher than that described in previous works on diabetic populations in Cameroon, which had under 12% [23]. This could be explained by the fact that our current sample consisted of 100 diabetic patients versus 40 subjects in the previous study. Likewise, the frequency of severe periodontitis in our global population was low (0.38%), and 71% subjects had moderate periodontitis. Our results differ from those reported by Khumaedi et al in 2018, who found respectively 16.5% of moderate periodontitis, and 75.3% of severe forms in 97 diabetic patients in Indonesia [24]. According to the Global Burden of Disease study published in 2010, severe periodontitis was the sixth most common medical condition among the 291 diseases [25]. Its prevalence is estimated at 11.2% and severe periodontitis has been associated with the occurrence of ischemic cardiovascular events [9, 25].
In this work, hypercholesterolemia increased by 6 times the risk of developing PAOD. Our results corroborate with those in recent literature. In fact, age, smoking, diabetes, obesity, hypercholesterolemia and hypertension are known risk factors for PAOD reported in the literature [1]. Partial mouth periodontal examination could constitute one of the limitations of this study due to the small number of sites examined. Indeed, the periodontal examination in the full mouth constitutes the reference for determining the periodontal state of an individual. Although a thorough examination is possible in the clinical setting, it requires more time and resources during an epidemiological investigation [26]. The 10 index teeth specified in partial registration were identified as the best, in the worst periodontal condition estimate of the mouth. Likewise, the lack of doppler ultrasound use in our study would have contributed to the underestimation of the ABI measurement in the presence of mediacalcosis [1, 3].
5. Conclusion
Periodontitis increased 3 times more risk of developing PAOD in diabetics subjects living in Cameroon after adjusting for hypercholesterolemia . Management of periodontitis may reduce the risk of having PAOD in diabetic subjects followed at the Douala General Hospital, Cameroon.
References
- Aboyans V, Sevestre MA, Désormais I, et al. Epidemiology of arteriopathy of the lower limbs [Epidemiology of lower extremity artery disease]. Presse Med 47 (2018): 38-46.
- Konin C, Essam N’loo AS, Adoubi A, et al. Peripheral arterial disease of the lower limbs in African diabetic patients: ultrasonography and determining factors. J Mal Vasc 39 (2014): 373-381.
- Desormais I, Prudhomme S, Chauvet R, et al. Index de pression systolique : intérêts et limites. Revue Francophone de Cicatrisation 1 (2017): 10-14.
- Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988-1994 and 1999-2004. Vital Health Stat 248 (2007): 1-92.
- Essama Eno Belinga L, Bell Ngan W, Lemougoum D, et al. Association Between Periodontal Diseases And Cardiovascular Diseases In Cameroon. JPHIA (2018).
- Page RC, Offenbacher S, Schroeder HE, et al. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000 14 (1997): 216-248.
- Khader YS, Daoud AS, El-Qaderi SS, et al. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications 20 (2006): 59-68.
- Nascimento GG, Leite FRM, Vestergaard P, et al. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol 55 (2018): 653-667.
- Sanz M, Marco Del Castillo A, Jepsen S, et al. Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol 47 (2020): 268-288.
- Schmitt A, Carra MC, Boutourye P, et al. Parodontite et rigidité artérielle: revue systématique et méta-analyse. Journal de Parodontologie et d’implantologie Orale 36 (2016).
- Yang S, Zhao LS, Cai C, et al. Association between periodontitis and peripheral artery disease: A systematic review and meta-analysis. BMC Cardiovasc Disord 18 (2018): 141.
- Kaschwich M, Behrendt CA, Heydecke G, et al. The Association of Periodontitis and Peripheral Arterial Occlusive Disease-A Systematic Review. Int J Mol Sci 20 (2019): 2936.
- Mendez MV, Scott T, LaMorte W, et al. An association between periodontal disease and peripheral vascular disease. Am J Surg 176 (1998): 153-157.
- Lu B, Parker D, Eaton CB. Relationship of periodontal attachment loss to peripheral vascular disease: an analysis of NHANES 1999-2002 data. Atherosclerosis 200 (2008): 199-205.
- Chen Y-W, Umeda M, Nagasawa T, et al. Periodontitis May Increase the Risk of Peripheral Arterial Disease. Eur. J. Vasc. Endovasc. Surg 35 (2008): 153-158.
- Çalapkorur MU, Alkan BA, Tasdemir Z, et al. Association of peripheral arterial disease with periodontal disease: Analysis of inflammatory cytokines and an acute phase protein in gingival crevicular fluid and serum. J Periodontal Res 52 (2017): 532-539.
- D’Aiuto F, Orlandi M, Gunsolley J. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J periodontol. Avr 84 (2013): S85-S105.
- Armingohar Z, Jørgensen JJ, Kristoffersen AK, et al. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol 6 (2014).
- Figuero E, Lindahl C, Marín MJ, et al. Quantification of periodontal pathogens in vascular, blood, and subgingival samples from patients with peripheral arterial disease or abdominal aortic aneurysms. J Periodontol 85 (2014): 1182-1193.
- Nishida E, Aino M, Kobayashi SI, et al. Serum Amyloid A Promotes E-Selectin Expression via Toll-Like Receptor 2 in Human Aortic Endothelial Cells. Mediators Inflamm 2016 (2016): 7150509.
- Choi JI, Chung SW, Kang HS, et al. Establishment of Porphyromonas gingivalis heat-shock-protein-specific T-cell lines from atherosclerosis patients. J Dent Res 81 (2002): 344-348.
- Aarabi G, Zeller T, Heydecke G, et al. Roles of the Chr.9p21.3 ANRIL Locus in Regulating Inflammation and Implications for Anti-Inflammatory Drug Target Identification. Front Cardiovasc Med 5 (2018): 47.
- Essama E B L, Bell N W, Kouotou Mouliom JS, et al. Evaluation de la Santé buccodentaire des patients diabétiques camerounais. Health Sci. Dis 14 (2013).
- Khumaedi AI, Purnamasari D, Wijaya IP, et al. Association of Periodontitis and Arterial Stiffness in Type 2 Diabetic Patients. Acta Med Indones 50 (2018): 320-327.
- Kassebaum NJ, Bernabe E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res 93 (2014): 1045-1053.
- Tran DT, Gay I, Du XL, et al. Assessment of partial-mouth periodontal examination protocols for periodontitis surveillance. J Clin Periodontol 41 (2014): 846-852.
