Pericardial Effusion as a Primary Manifestation of a Lung Adenocarcinoma
Article Information
Antoine Egbe, Eyouab Tadesse, Catrina Ruffino, Khurram Arshad, Aubin Sandio*, Ali Mozaffari, Ahmed Subahi, Mariam Jamil, Donald Tynes, Patrice Delafontaine
Internal Medicine Wayne state University School of Medicine, Detroit, MI, 48201, USA
*Corresponding author:Aubin Sandio, Internal Medicine Wayne state University School of Medicine, Detroit, MI, 48201, USA
Received: 24 January 2024; Accepted: 01February 2024; Published: 16 April 2024
Citation: Antoine Egbe, Eyouab Tadesse, Catrina Ruffino, Khurram Arshad, Aubin Sandio, Ali Mozaffari, Ahmed Subahi, Mariam Jamil, Donald Tynes, Patrice Delafontaine. Pericardial Effusion as a Primary Manifestation of a Lung Adenocarcinoma. Cardiology and Cardiovascular Medicine. 8 (2024): 147-150.
View / Download Pdf Share at FacebookAbstract
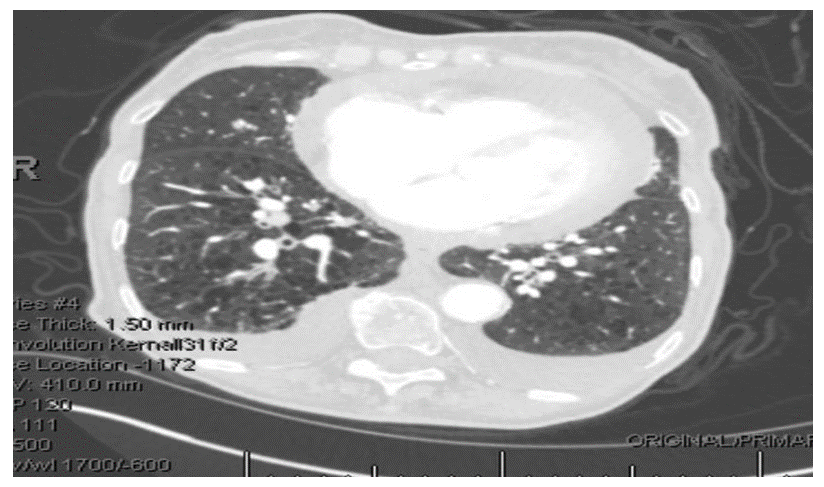
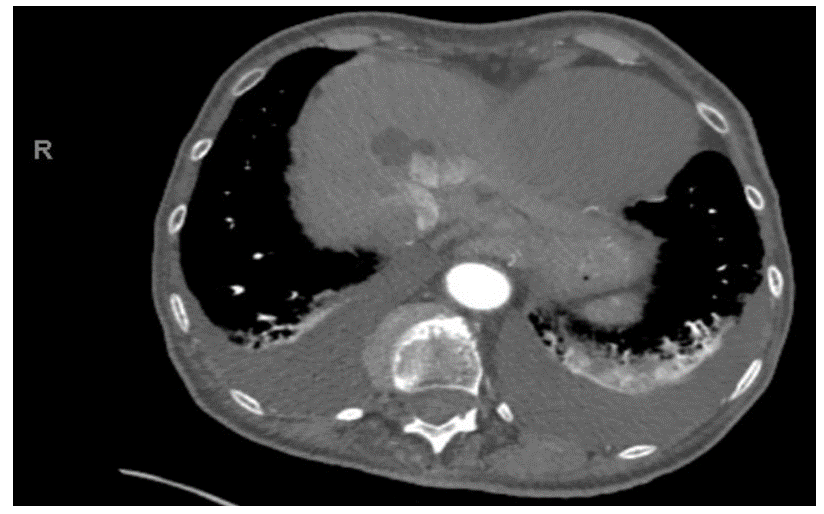
Pericardial effusions are a frequent finding in everyday clinical practice, typicallyresulting from malignancy in developed countries. This presentation is generally a secondarymanifestation of malignancy, rather than the primary manifestation, as in this case of lungadenocarcinoma. An 85-year-old woman on 3L of home oxygen for chronic obstructivepulmonary disease with extensive smoking history presented to the emergency department withshortness of breath, along with a one week history of cough productive of clear sputum, bilaterallower extremity edema, decreased urine output and loss of appetite. Her family also reportedweight loss over the past year. CT(computed tomography) of the chest revealed moderatepericardial effusion, a 1.5cm pulmonary nodule and bilateral pleural effusions. Echocardiogramshowed a large pericardial effusion but no evidence of hemodynamic compromise. Due to thefindings of moderate pericardial effusion, shortness of breath and extensive smokinghistory,1.5cm pulmonary nodule and reports of weight loss, a decision was made to drain thepericardial effusion despite hemodynamic stability. Pericardial fluid cytology returned positive formalignancy with tumor marker PDL-1(programmed death ligand-1), consistent with a non-smallcell lung cancer. After readmission with shortness of breath, the deteriorating patient was placedon comfort care, passing away only 26 days after her initial presentation. Malignancy can causepericardial effusion in various ways (commonly metastasis) with poor prognosis, despite oftenstraightforward management with pericardiocentesis as seen in this patient. In this case,unusually, the primary manifestation of advanced lung adenocarcinoma was pericardial effusionpresenting with shortness of breath without hemodynamic instability. Pericardial fluid cytologyremains the cornerstone of diagnosis, but considering possible false negatives, clearerrecommendations for intrapericardial therapeutic approaches in similar clinical scenarios are needed to address recurrent, malignant pericardial effusions, particularly when it occurs as theprimary manifestation.
Keywords
Pericardial effusions; Lung Adenocarcinoma; malignancy; chronic obstructive pulmonary.
Pericardial effusions articles; Lung Adenocarcinoma articles; malignancy articles; chronic obstructive pulmonary articles.
Article Details
Introduction
Pericardial tamponade is the accumulation of excess pericardial fluid in the pericardial cavity thereby leading to decreased cardiac output [1] and decreased cardiac filling. The most common cause of pericardial effusion in the western world is malignancy [2,3,4], while in the developing world, the most common cause of pericardial effusion is tuberculosis [5]. Pericardial effusions usually present as a secondary manifestation of malignancy. However, in this case report, the occurrence of pericardial effusion as a primary manifestation of a lung adenocarcinoma is presented.
Case Presentation
The subject of this case is an 85-year-old woman with hypertension, type 2 diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease on 3L home oxygen, and a history of stroke, right carotid endarterectomy, and heavy tobacco use. She presented to the emergency department with a chief complaint of shortness of breath (SOB). She also has multiple constitutional symptoms including a productive cough with clear sputum, bilateral lowerextremity edema, decreased urine output and loss of appetite. These symptoms have been ongoing for about a week. The patient’s family also reported significant weight loss the past 12 months.
Vital signs on admission included: heart rate of 72 beats per minute, respiratory rate of 22 breaths per minute, blood pressure of 159/79, temperature of 97.5 degrees F. She was put on 2L of oxygen with oxygen saturation ranging between 95% and 98%.
On physical examination, expiratory wheezes and rales were present on auscultation. No jugular venous distension was noted, S1 and S2 were present with no additional heart sounds, and 2+ peripheral edema was appreciated with 1+ pulses noted in the lower extremities. The remainder of the physical exam was unremarkable.
Investigative workup revealed:
|
White Blood Cell Count (billion/L) |
Hemoglobi n (g/dl) |
Hematocrit (%) |
Mean Corpuscular Volume (fl) |
Platelets (billion/L) |
|
14.9 |
13 |
38 |
82 |
212 |
Table 1: Complete blood count
Table 2: Basic Metabolic Panel
|
AST (U/L) |
ALT (U/L) |
ALP (U/L) |
Total Bilirubin (mg/dL) |
Indirect Bilirubin (mg/dL) |
Direct Bilirubin (mg/dL) |
Albumin (g/dL) |
|
53 |
50 |
219 |
0.6 |
0.4 |
0.2 |
4 |
Abbreviations: AST- Aspartate aminotransferase, ALT- Alanine aminotransferase, ALP- Alkaline phosphatase TSH - Thyroid Stimulating Hormone
Table 3: Hepatic Function Tests
|
BNP(pg/mL) |
Troponins(ng/m L) |
TSH(uIU/L) |
Mg(mg/dL) |
Ionized Calcium (mg/dL) |
|
86 |
<0.01 |
0.02 |
1.6 |
4.65 |
Table 4: Miscellaneous Labs
Transthoracic echocardiogram (TTE) revealed a left ventricular ejection fraction of 60%, grade I LV diastolic dysfunction (abnormal relaxation with normal left ventricular filling pressure), no segmental wall motion abnormalities, mild mitral regurgitation, mild to moderate tricuspid regurgitation, right ventricular systolic pressure of approximately 30-35 mmHg and a large pericardial effusion with no evidence of hemodynamic compromise.
Due to the finding of a moderate pericardial effusion, the patient’s SOB and extensive smoking history, CT revealing a 1.5cm pulmonary nodule and reports of weight loss, a decision was made to drain the pericardial effusion even though she was not hemodynamically unstable and there were no echographic/clinical signs of a pericardial tamponade. Pericardiocentesis was performed on day 2 of hospitalization and a total of 400ml of hemorrhagic fluid was recovered. The pericardial fluid was sent for cytology. Subsequently, other investigative workups were ordered (listed below in Table 5) which could help in identifying the etiology of the hemorrhagic pericardial effusion.
|
ANA |
RF |
CRP |
ESR |
Anti- CCP |
C3 |
C4 |
SSB |
SSA/SS B |
|
Positive 1:320 |
<15 |
10.5 |
2 |
91 |
24 |
4.0AU/ml |
5Au/ml |
Abbreviations: ANA - Antineutrophil antibody, SSA/SSB- Sjogren syndrome antibody, CRP- C reactive protein, ESR- Erythrocyte sedimentation rate, C3/C4– Complement 3/Complement 4, RF– Rheumatoid Factor, CCP – Cyclic Citrulinated peptide
Table 5: Additional Workup
A drainage catheter was left in place after the pericardiocentesis. The drain output was less than 10cc for about 3 consecutive days, so the drain was removed. The patient remained hemodynamically stable and needed only 2L of oxygen by nasal cannula from a respiratory standpoint. A repeat TTE did not show any pericardial effusion. She was discharged from the hospital after 10 days of hospitalization while still awaiting the results of the pericardial fluid cytology.
Her pericardial fluid cytology returned positive for malignancy and for the tumor marker PDL-1(programmed death ligand-1), consistent with a non-small cell lung cancer.
She returned to the hospital about a week later with shortness of breath necessitating oxygen supplementation with 6L by nasal cannula. TTE(transthoracic echocardiogram) showed a moderate pericardial effusion and the patient subsequently had a subxiphoid pericardial window, bronchoscopy and a right chest tube insertion. During this hospitalization, her respiratory status deteriorated and she necessitated 100% FiO2(Fraction of inspired oxygen) while on average volume assured pressure support. Due to the recent diagnosis of metastatic adenocarcinoma and her overall poor prognosis, her family decided to pursue comfort care. Life prolonging treatments were withdrawn and the patient passed away a couple of hours later.
Discussion
This is the case of an 85 year old woman who was found to have lung adenocarcinoma only after she presented to the hospital with SOB and was discovered to have a moderate pericardial effusion.
Pericardial effusions in the western world are commonly idiopathic, but can be malignant in nature, affecting about 10% of cancer patients, one third of which will be fatal [2]. Per previous case series and meta-analysis, these malignancies can include lung cancers, breast cancers and lymphoma/leukemia [4]. In the case of our patient, it happened to be a lung adenocarcinoma causing her pericardial effusion.
Metastatic malignancies are the most likely culprits rather than primary malignancies. However, the mechanism of formation of a pericardial effusion in a patient with a malignancy can be a product of metastasis to the pericardium [5], obstruction of the lymphatic drainage, chemotherapeutic drugs [6] or radiation therapy [2]. The ability to differentiate between these various causes rest on the results of the pericardial fluid analysis. Pericardial fluid cytology has been shown to have a sensitivity of about 95% and a specificity of about 100% in the diagnosis of malignant pericardial effusions. [7,8] Other studies like Kyriaki et al [9] showed a sensitivity of 82.76%, while Dragoescu et al [10] reported a false negative rate of 14.7%. Although pericardial fluid cytology remains a major cornerstone for the diagnosis of pericardial metastasis, false negative results may occur [10,11], meaning a one time negative pericardial fluid cytology sample does not equate to a definitive non-malignant pericardial effusion.
Malignant pericardial effusions can present in a variety of ways. In our patient, the chief complaint was only shortness of breath. However, depending on the acuity or the chronicity of the effusion build up, some patients can present with manifestations of a cardiac tamponade including tachycardia, hypotension, pulsus paradoxus, jugular venous distension, and muffled heart sounds [12].
The management of malignant pericardial effusion with echocardiographic signs of cardiac tamponade is quite straightforward. Emergent pericardiocentesis is the absolute treatment strategy in these patients [13]. However, in patients with pericardial effusions with no signs of hemodynamic instability, a decision has to be made regarding conservative management versus decreasing the risk of the effusion leading to a pericardial tamponade. The current questions for which recommendations are needed from cardiology boards are: When do we intervene in patients with moderate to large pericardial effusions? When are intrapericardial therapies like cisplatin needed [14]? When are invasive diagnostic techniques like a pericardial window and biopsy indicated?
In patients in whom pericardiocentesis is needed, the surgical approach via a subxiphoid incision is the preferred method for pericardial fluid drainage and it also allows for the possibility of pericardial tissue biopsy [15].
Unsurprisingly, the prognosis associated with malignant pleural effusions is abysmal. This is expected because the presence of malignant cells in the pericardium usually implies these patients have Stage IV cancers, considering the cancer has spread to neighboring organs. In this patient, from her first hospitalization to her death was about 26 days. Cullinane et al. [16] showed a similar median survival rate of about 3.2 months. In this study by Cullinane et al, a preoperative diagnosis of non-small cell carcinoma, the presence of a pleural effusion and a positive pathological finding correlated negatively with survival. Unfortunately, this patient had all three of these indices.
Conclusion
Pericardial effusions are predominantly idiopathic in origin but malignancy remains a strong possibility to be considered in these cases. Recommendations regarding when to intervene in patients with moderate pericardial effusions, without signs of cardiac tamponade or hemodynamic instability remain unclear. Hence, it is important to put the entire clinical picture into context when making the decision of whether pericardiocentesis is needed or not.
Pericardial fluid cytology has a high sensitivity for the diagnosis of malignant pleural effusions, in fact, it has a greater sensitivity than pericardial histology. In the future, clear recommendations are also needed regarding the use of intrapericardial therapies like cisplatin and the utilization of surgical methods to prevent the recurrence of malignant pericardial effusions.
References
- Appleton C, Gillam L, Koulogiannis K. Cardiac Tamponade. Cardiol Clin 35(2017):525-537.
- Honasoge AP, Dubbs SB. Rapid Fire: Pericardial Effusion and Tamponade. Emerg Med Clin North Am 36(2018):557-565.
- Ancion A, Robinet S, Lancellotti P. La tamponnade cardiaque [Cardiac tamponade]. Rev Med Liege 73(2018):277-282.
- Forauer AR, Dasika NL, Gemmete JJ, et al. Pericardial tamponade complicating central venous interventions. J Vasc Interv Radiol 14(2003):255-259.
- Chahine J, Shekhar S, Mahalwar G, et al. Pericardial Involvement in Cancer. Am J Cardiol 145 (2021):151-159.
- Kalogeraki A, Lazopoulos G, Papadakis GZ, et al. Cytology of Pericardial Effusion due to Malignancy. Rom J Intern Med 54(2016):179-183. Erratum in: Rom J Intern Med 55(2017):122-125. Papadakis M, Moustou E, Datseri G, Tzardi M [removed]. PMID: 27658166.
- Meyers, D. G., Meyers, R. E., Prendergast, T. W. The usefulness of diagnostic tests on pericardial fluid. Chest 111(1997):1213-1221.
- Saab J, Hoda RS, Narula N, et al. Diagnostic yield of cytopathology in evaluating pericardial effusions: Clinicopathologic analysis of 419 specimens. Cancer Cytopathol 125(2017):128-137.
- Savvidou K, Dimitrakopoulou A, Kafasi N, et al. Diagnostic role of cytology in serous effusions of patients with hematologic malignancies. Diagn Cytopathol 47(2019):404-411.
- Dragoescu EA, Liu L. Pericardial fluid cytology: an analysis of 128 specimens over a 6-year period. Cancer Cytopathol 121(2013):242-251.
- Strobbe A, Adriaenssens T, Bennett J, et al. Etiology and Long-Term Outcome of Patients Undergoing Pericardiocentesis. J Am Heart Assoc 6(2017):e007598.
- Vakamudi S, Ho N, Cremer PC. Pericardial Effusions: Causes, Diagnosis, and Management. Prog Cardiovasc Dis 59(2017):380-388.
- Lazaros G, Vlachopoulos C, Lazarou E, et al. New Approaches to Management of Pericardial Effusions. Curr Cardiol Rep 23(2021):106.
- Maisch B, Ristic AD, Pankuweit S, et al. Neoplastic pericardial effusion. Efficacy and safety of intrapericardial treatment with cisplatin. Eur Heart J 23(2002):1625-1631.
- Adler Y, Charron P, Imazio M, et al; ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 36(2015):2921-2964.
- Cullinane CA, Paz IB, Smith D, et al. Prognostic factors in the surgical management of pericardial effusion in the patient with concurrent malignancy. Chest 125(2004):1328-1334.