Pathophysiology of Pulmonary Hypertension in Sickle Cell Disease
Article Information
Simrat Kaur Batth1*, Kim Bloom2, Kenneth Scott Lloyd3
1Department of Medicine, Punjab Institute of Medical Sciences, Jalandhar, India
2Department of Pulmonary Medicine, Houston Methodist Hospital, Houston, USA
3Department of Pulmonary Medicine, Houston Methodist Hospital, Baylor College of Medicine, Houston, USA
*Corresponding author: Simrat Kaur Batth, Department of Medicine, Punjab Institute of Medical Sciences, Jalandhar, Ranjit Avenue, Amritsar, Punjab, India
Received: 13 May 2021; Accepted: 25 May 2021; Published: 03 June 2021
Citation:
Simrat Kaur Batth, Kim Bloom, Kenneth Scott Lloyd. Pathophysiology of Pulmonary Hypertension in Sickle Cell Disease. Archives of Internal Medicine Research 4 (2021): 149-159.
View / Download Pdf Share at FacebookAbstract
The pathophysiology of pulmonary hypertension (PH) in sickle cell disease (SCD) is multifactorial: hemolysis, hypercoagulability, hypoxemia, oxidative stress, platelet activation, increased adhesiveness, inflammatory cell activation and genetic susceptibility, all contributing in varying degrees to endothelial dysfunction. Intravascular hemolysis is the main pathological process contributing to vasculopathy by releasing toxic red blood cell products that impair endothelial function, cause hypercoagulable state and drive oxidative and inflammatory stress. Hemolysis induced nitric oxide imbalance is one the most important contributors to high pulmonary artery pressures seen in SCD. Multi-faceted, targeted interventions, before irreversible vasculopathy develops, will allow for improved patient outcomes and life expectancy, stressing the need for a better understanding of the multiple pathophysiological mechanisms involved in the development of PH before considering those patients for targeted therapies. Hemolysis is still considered as the main contributor of PH in SCD but the mechanisms by which it causes PH are still not completely known. This review precisely presents the various pathophysiological mechanisms and factors that have been proposed till date to help the reader get an overview.
Keywords
Pulmonary Hypertension, Sickle Cell Disease, Hemolysis, Nitrous Oxide (NO), Hypoxia, Genetics
Pulmonary Hypertension articles; Sickle Cell Disease articles; Hemolysis articles; Nitrous Oxide (NO) articles; Hypoxia articles; Genetics articles
Article Details
1. Introduction
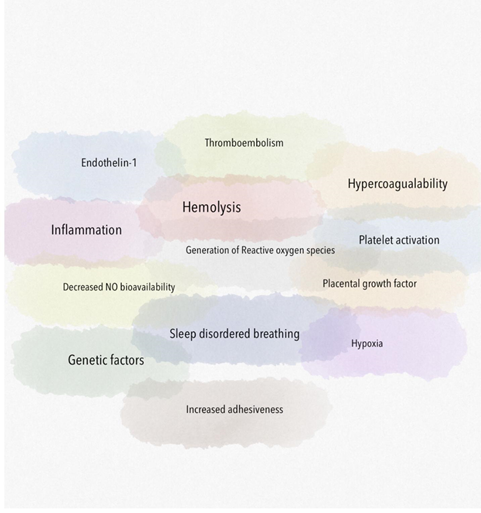
Due to the multifactorial mechanisms of increased pulmonary pressures in SCD (Figure 1), the World Health Organization classification for PH due to SCD considers this in the Group 5 category with other hematologic disorders [1]. It is broadly divided into precapillary (pulmonary arterial), postcapillary (pulmonary venous due to left heart failure) and mixed. This review primarily focuses on precapillary causes of PH. Chronic pre-capillary PH has been identified as a devastating complication of SCD. PH has a major impact on the functional status of patients with SCD. In addition the prognosis of patients with SCD and PH appears to be severely compromised [2]. SCD patients with PH appear to have increased endothelial dysfunction, coagulation activation and inflammation compared with patients without PH [3]. Various pathophysiological processes add to the PH like hemolysis induced decrease in nitric oxide bioavailability, hypercoagulability induced pulmonary thromboembolism, hypoxia, generation of reactive oxygen species, activation of inflammatory cells and platelets.
2. Hemolysis
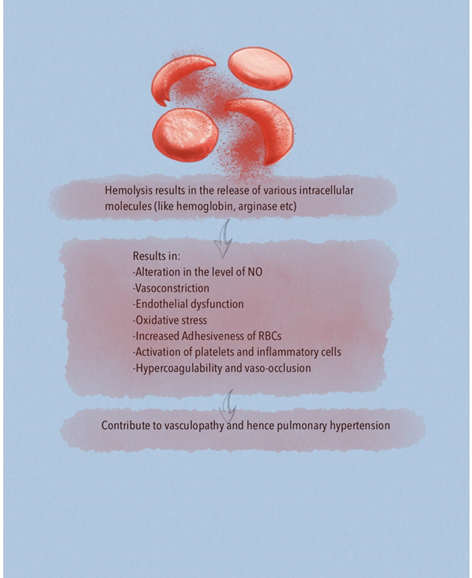
A major risk factor for developing chronic organ injury is hemolytic anemia, which releases red blood cell (RBC) contents into the circulation (Figure 2). There are a number of mechanisms of hemolysis that lead to vasculopathy and are caused by the release of molecules that are typically compartmentalized within the RBC, which shields the endothelium from their pathological effects. Cell free plasma hemoglobin, heme and arginase disrupt endothelial function, drive oxidative and inflammatory stress. With increased studies characterizing the pathogenic properties of these intracellular molecules when released into the plasma, these have now been collectively referred to as erythrocyte danger-associated molecular pattern molecules (eDAMPs), which similar to other intracellular DAMPs, are released during massive tissue injury and can activate innate immunity pathways via the toll-like receptors (TLRs) and the nucleotide binding oligomerization domain (NOD)-like receptors of the inflammasome [4, 5]. Persistent intravascular hemolysis over decades leads to chronic vasculopathy, with some patients developing PH [4].
Placental Growth Factor (PlGF), an angiogenic peptide of the VEGF family, is elaborated from erythroblast cells. Due to increased compensatory erythropoiesis in response to hemolysis of sickle RBCs, there are increased levels of circulating PlGF in SCD individuals which is associated with elevated plasma endothelin-1 (ET-1) levels, a potent vasoconstrictor, and elevated pulmonary artery pressure, reflective of PH [6]. ET-1 is normally induced in endothelial cells in response to hypoxia but PlGF can induce expression of ET-1 independent of hypoxia via activation of hypoxia-inducible factor-1 (HIF-1) [7, 8]. High circulating levels of PlGF have been associated with echocardiographic markers of PH in SCD [7]. Strategies that block PlGF signaling may be therapeutically beneficial. Hemolysis may also contribute to the development of PH in SCD by the release of adenosine deaminase (ADA) and purine nucleoside phosphorylase (PNP) from RBCs likely by abolishing the vasoprotective effects of adenosine, inosine and guanosine that in aggregate may produce vasoprotective and anti-occlusive effects [9]. Peroxisome proliferator activated receptor ?(PPAR?) has been shown to regulate PH and endothelial dysfunction in SCD. Chronic hemolysis in SCD stimulates loss of PPAR? and increased expression of the proliferative mediator, ET-1, which contributes to PH pathogenesis. Targeting PPAR? with existing pharmacological ligands may represent a novel therapeutic approach in SCD [10].
Not only the hemolysis but also the increased RBC stiffness might play a role in PH. RBC stiffness alone might affect pulmonary pulsatile hemodynamics like increasing mean pulmonary artery pressure, pulmonary vascular resistance, and wave reflections [11]. Haptoglobin, a hemoglobin scavenger protein, has protective effects on the pulmonary vasculature within the context of mitigating the consequences of chronic hemoglobin exposure in the progression of PH. Haptoglobin can act as a potential therapeutic to attenuate the progression of PH exacerbation by extracellular hemoglobin exposure [12].
3. Alteration of the Balance of Nitric Oxide (NO)
Hemoglobin is a potent NO scavenger. In addition to plasma hemoglobin, the RBCs contain significant concentrations of the enzyme arginase 1, which can metabolize L-arginine to ornithine. This reduces L-arginine availability, which is required for de novo NO synthesis by endothelial NO synthase (eNOS) enzyme [4, 13, 14]. Lower arginine:ornithine ratios are associated with increased severity of PH and mortality [5]. When ornithine production by arginase is favored, downstream production of polyamines and proline is amplified which contribute to smooth muscle cell proliferation as well as peribronchial and perivascular collagen deposition. Ultimately, controlled trials are needed to elucidate the role of arginine therapy for PH [5]. Arginase inhibition improves vascular endothelial NO bioavailability, decreases reactive oxygen species (ROS) production and enhances endothelial-dependent vasodilation. Thus arginase is a potential novel therapeutic target in SCD vasculopathy [15]. Under physiological conditions, NO activates soluble guanylate cyclase (sGC) by binding to the ferrous (Fe2+) heme active site in the beta subunit of the sGC, which stimulates the enzyme to convert GTP to cGMP and promotes downstream vascular smooth muscle relaxation through protein kinase G. It is known that ROS can oxidize sGC heme iron from its ferrous to ferric (Fe3+) form. The consequence is a weak affinity for NO that also destabilizes the binding of the heme group and leads to heme-free sGC, which is insensitive to NO leading to NO resistance. The class of drug referred to as an sGC activator can bind to the oxidized Fe3+ heme and heme-free sGC, activating sGC even in the absence of NO [16].
Prior studies show that cytochrome b5 reductase 3 (CYB5R3), known as methemoglobin reductase in erythrocytes, functions in vascular smooth muscle cell as an sGC heme iron reductase critical for reducing and sensitizing sGC to NO and generating cGMP for vasodilation. PH in SCD can exist in mice by 5 weeks of age when smooth muscle cell CYB5R3 protein is deficient [17]. Hemolysis linked accumulation of asymmetric dimethylarginine (ADMA), an endogenous NOS inhibitor, in SCD is a risk factor for vasculopathy and PH in the SCD population [18]. Also, ADMA normally produced in the body is hydrolyzed by dimethylarginine dimethylaminohydrolase (DDAH). Homocysteine can inhibit DDAH activity. This may represent a mechanism whereby hyperhomo-cysteinemia, a known risk factor for vascular disease and thrombosis, leads to elevated plasma ADMA levels and decreased NO production [14]. A study by Almeida, et al. challenged the hypothesis that a NO deficit contributes to SCD pathobiology. According to the study, there is no conclusive evidence that NO bioavailability is decreased in SCD which might be the reason why the results of clinical trials of therapeutic interventions aimed at increasing NO bioavailability (arginine administration, inhaled NO or administration of the phosphodiesterase-5 inhibitor) have failed to provide reproducible clinical improvements in cardiovascular and pulmonary performances and SCD-related pain [19]. But more studies need to be done to prove the conclusion from this preclinical study.
4. Increase Adhesiveness
Adhesive phenotype of sickle RBCs is related to decreased NO bioavailability, which is a consequence of hyperhemolysis. NO donor reduce the adhesion of sickle RBCs to endothelial cells and cause a significant reduction in the adhesion of SCD neutrophils to the extracellular matrix component fibronectin and the adhesion molecule Intercellular Adhesion Molecule 1 (ICAM-1). NO also has an inhibitory effect on endothelial activation, resulting in downregulation of adhesion molecules such as Vascular Cell Adhesion Molecule 1 (VCAM-1), ICAM-1, and E-selectin in response to cytokines [5, 20]. Targeting various adhesion molecules like selectin family may prove beneficial [20]. Importantly, increased soluble adhesion molecule expression correlated with severity of PH, a clinical manifestation of endothelial dysfunction [21]. Phosphatidylserine, expressed on the cell surface of sickled erythrocytes, also promotes adhesion of sickled cells to the vascular endothelium [5].
5. Generation of ROS
Intravascular hemolysis is one of the mechanisms by which ROS are generated in SCD. Cell free hemoglobin can redox cycle and undergo fenton and peroxidase reactions to form ROS which causes endothelial dysfunction and activates downstream oxidases, such as xanthine oxidase and nicotinamide adenine dinucleotide phosphate (NADPH) oxidases that further promote vascular oxidative stress [4]. Superoxide dismutases (SODs) convert the ROS superoxide to hydrogen peroxide, a less toxic free radical which is further transformed to water by catalase or glutathione peroxidase. Patients with higher tricuspid regurgitant velocity (TRV) had significantly reduced SOD expression in comparison to patients with lower TRV values [22]. In terms of pathophysiology, it provides a link between increased oxidative stress and SCD PH. It also provides the basis of using SOD as a biomarker for PH in SCD [22]. Overproduction of ROS, such as superoxide, by both enzymatic (xanthine oxidase, NADPH oxidase, uncoupled eNOS) and nonenzymatic pathways (Fenton chemistry), promotes intravascular oxidant stress [5].
Additionally, the glutathione pathway is important in modulating oxidative stress, and glutathione metabolism in SCD is impaired. Glutamine is metabolized to glutamate that in turn is essential to glutathione production. However, erythrocyte glutamine concentrations are abnormally low in SCD, particularly those with an elevated TRV at risk for PH. Importantly, glutamine is also a precursor in the production of arginine and NADPH. NADPH is an essential cofactor for glutathione recycling and normal NO synthase function [23]. It is through these mechanisms that glutathione and glutamine potentially contribute to the pathogenesis of PH of SCD [23]. Therefore, treatment of PH with glutamine, arginine and antioxidants and their utility as a biomarker can be considered worth studying. Plasma protein thrombospondin-1 (TSP1) dependent activation of CD47 in SCD contributes to evolution of PH by promoting generation of ROS. TSP1 may promote vascular pathology by inhibiting the vasodilatory, antiadhesive, and homeostatic effects of the NO and vascular endothelial growth factor signaling pathways in the vasculature, thereby adversely affecting regulation of tissue perfusion and vascular tone and inciting inflammation. Therapeutic disruption of the TSP1-CD47 ligand-receptor interaction can both prevent and reverse PH [24].
6. Activation of Platelets
Cell free hemoglobin can trigger platelet activation, an effect that may be mediated by the consumption of NO, a key inhibitor of platelet activation [25]. RBCs contain high levels of adenosine diphosphate (ADP), which can activate platelets via the P2Y receptors. Plasma free hemoglobin can cause extravascular macrophage and neutrophil localization within the lung parenchyma, activate the endothelium and increase proinflammatory effects. VCAM-1 levels have been shown to correlate with both the rate of intravascular hemolysis and with in vivo measures of endothelial dysfunction [4]. Platelet activation was also increased in patients with SCD with elevated echocardiography- measured tricuspid regurgitation jet velocities (TRJV) compared with patients without SCD [20]. Thus, strategies that curtail platelet activity could be important in preventing vaso-occlusion.
7. Activation of Inflammatory Cells
Heme has been found to activate the TLR4 receptor and downstream NF?B-mediated inflammation. Heme has also been shown to increase ROS and induce neutrophils to release DNA neutrophil extracellular traps (NETs). Interestingly, a high rate of hemolysis is also associated with a high rate of RBC production. New RBCs release their nuclei and present significant DNA for metabolism, leading to increasing concentrations of uric acid. Uric acid crystals can bind to the NOD-like receptors to potentially activate the inflammasome and increase IL-1 production. ATP released during hemolysis will also activate this pathway. Hemolysis-dependent elaboration of heme, ATP, and uric acid will all drive sterile inflammation [4]. Although many cell types contribute to pulmonary vascular inflammation, including endothelial, smooth muscle, and fibroblasts, emerging evidence suggests that circulating macrophages recruited to the lung may be potent contributors to the pathophysiology of PH. A study done on a mouse model suggested the critical role of macrophages in the exacerbation of SCD PH indicating a potentially novel maladaptive immune response to concomitant bouts of cell free haemoglobin and hypoxia exposure [26]. Lower levels of Apo-A1 are related to dysregulation of the ubiquitin-proteasome pathway (UPP), the major intracellular protein turnover pathway which regulates central mediators of proliferation, inflammation, and apoptosis. SCD patients with lower-than-median Apo-A1 levels showed deficient vasodilatory responses to the endothelial agonist acetylcholine [27]. Fas/FasL interactions besides inducing apoptosis may be related to augmentation of inflammatory response and cause massive migration of macrophages in vivo, indicating that Fas and Fas ligand act also as proinflammatory proteins. Fas/sFasL ratios are elevated in patients with PH, nephropathy and those who had a history of frequent sickling crisis or high serum ferritin. Elevated sFas/sFasL ratio would help in early crisis prediction and identification of patients at risk of PH [28].
8. Hypercoagulable State and Chronic Thromboembolism
Every aspect of Virchow’s triad-increased coagulability, endothelial dysfunction, and impaired blood flow—is present in patients with SCD and results in a highly thrombogenic environment. There are many mechanisms involved in hypercoagulability in SCD, including enhanced platelet function, activation of the coagulation cascade, and impaired fibrinolysis. These perturbations are due to intravascular hemolysis, externalization of highly procoagulant phosphatidylserines on the RBC membrane, NO depletion causing vasoconstriction and platelet activation and vaso-occlusion contributing to decreased blood flow and local vascular ischemic injury. The key coagulation factor, thrombin (factor IIa), is both a central protease in hemostasis and thrombosis and a key modifier of inflammation. Pharmacologic or genetic reduction of circulating prothrombin in Berkeley sickle mice significantly improves survival, ameliorates vascular inflammation, and results in markedly reduced end-organ damage [29].
Accordingly, factors both upstream and downstream of thrombin, such as the tissue factor–FX complex, fibrinogen, platelets, von Willebrand factor, FXII, high-molecular-weight kininogen, etc, also play important roles in SCD pathogenesis [29]. Asplenia – either functional or following splenectomy, is theorized to contribute to hypercoagulability and the development of PH by allowing for prolonged circulation of abnormal erythrocytes and platelet derived mediators [5]. Autopsy studies have shown microthrombotic and/or thromboembolic lesions as common findings at post-mortem in patients with SCD and PH [30]. The precise molecular mechanisms by which the coagulation system contributes to the pathogenesis of SCD remains poorly defined. Therapeutics targeting the coagulation system that spare their hemostatic function may prove beneficial.
9. Hypoxia
Hypoxia in SCD is caused by multiple factors like anemia, vaso-occlusive crisis, ventilation perfusion mismatch due to chronic thromboembolism, sleep disordered breathing and cardiac dysfunction. Upregulated hypoxic response in SCD might contribute to altered gene expression and to the development of PH [30]. Activation of HIF-1 is involved in the etiology of PH through changes in mitochondrial redox signaling, fission and numbers, and contributes to the development of a proliferative, apoptosis-resistant phenotype in pulmonary vascular cells [30]. Plasma from adults with SCD at steady-state contain elevated concentrations of pro-angiogenic factors compared to control subjects, including vascular endothelial growth factor, angiopoietin-1, basic fibroblast growth factor-D, and PlGF which will increase endothelial cell proliferation, migration and capillary-like structure formation [5]. The role of pro-angiogenic mechanisms in SCD and their consequences remain unclear, however they are likely an additional pathway to the development of PH [5]. Of interest is the reported anti-angiogenic effects of hydroxyurea that may provide additional benefits of the drug beyond impact on fetal hemoglobin [5].
Sleep-disordered breathing (SDB) with nocturnal hypoxaemia, including obstructive sleep apnoea (OSA), is common in patients with SCD. The increased prevalence of OSA and SDB in children with SCD is influenced by the high frequency of adenotonsillar hypertrophy, which is likely to be a compensatory mechanism of the immune system to functional hyposplenism [31]. Tonsillectomy and adenoidectomy (T&A) is recommended for children with documented OSA and adenotonsillar hypertrophy in the general paediatric population [31]. However, the amount of available data regarding the effect of T&A on the clinical course of SCD is still limited and further investigation is required. A study showed the association of nocturnal hypoxia(NH) with echocardiographic measurement of PH in children with SCD. Children with NH compared to non-NH had significantly worse baseline hypoxemia, higher TRJV, and higher left ventricle end-diastolic diameters. Screening for NH may help to identify children with SCD with higher morbidity risk [32].
10. Role of Genetics
The genetic basis of PH in SCD has only recently started to be studied. PH is likely to be modulated by the effects of genes that control NO and oxidant radical metabolism, cell-cell interaction, vasculogenesis, and vasoreactivity [33]. Genetic variability in adult HbF expression explains much of the disease phenotypic variability, with patients with high HbF levels exhibiting milder disease severity [4]. The role of homocysteine in SCD pathophysiology is not central, however, decreased erythrocyte life elevates the erythropoietic demand for folate and vitamin B12 thereby leading to arise in serum/plasma total homocysteine that implicate the endothelium injury. A meta-analysis conducted by B.V.K.S. Lakkakula established that the MTHFR 677C>T polymorphism is a risk factor for vascular complications in SCD [34]. Polymorphisms in genes of the transforming growth factor (TGF)-β/bone morphogenetic protein (BMP) pathway, appear to be associated with PH in SCD. Evidence of association was primarily identified for genes in the TGF superfamily, including activin A receptor type II–like 1 (ACVRL1), bone morphogenetic protein receptor 2 (BMPR2), and bone morphogenetic protein 6 (BMP6).
Genetic variation in the -1 adrenergic receptor (ADRB1) was also associated with PH [33, 35]. ET-1 is one of the most potent endogenous vasoconstrictors known. The mutant T allele of a G5665T polymorphism was found to be associated with higher plasma ET-1 levels. Endothelin-1G5665T polymorphism could be considered as a molecular predictor for pulmonary dysfunction and severe vaso-occlusive crisis in SCD [36]. Polymorphism of eNOS gene is associated with unfavorable clinical outcomes, eNOS gene polymorphisms may be used as a genetic marker of prognostic value for defining patients at risk of developing severe SCD complications (acute chest syndrome,vaso occlusive crisis and pulmonary hypertension) due to impaired NO bioavailability [37].
11. Conclusion
PH is a common complication of SCD yet reversible if caught in early stages of its development. Understanding pathophysiology is one of the first steps in identifying the factors contributing to the development of PH and what all measures can be taken to prevent or treat it. As more and more studies are conducted on the pathophysiology of PH, we are giving rise to newer drugs. Also understanding the pathophysiology of this common complication of SCD will help in establishing newer biomarkers that enable early detection, predict PH severity and help monitor response to treatment.
References
- Gérald Simonneau, David Montani, David S Celermajer, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir J 53 (2019): 1801913
- Matthieu Turpin, Christelle Chantalat-Auger, Florence Parent, et al. Chronic blood exchange transfusions in the management of pre-capillary pulmonary hypertension complicating sickle cell disease. Eur. Respir. J 52 (2018): 1800272.
- Kenneth I Ataga, Charity G Moore, Cheryl A Hillery, et al. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica 93 (2008): 20-26.
- Potoka K P, Gladwin M T. Vasculopathy and pulmonary hypertension in sickle cell disease. Am. J. Physiol. Lung Cell. Mol. Physiol 308 (2015): L314-324.
- Shilo N R, Morris C R. Pathways to pulmonary hypertension in sickle cell disease: the search for prevention and early intervention. Expert Rev. Hematol 10 (2017): 875-890.
- Kalra V K, Zhang S, Malik P, et al. Placenta growth factor mediated gene regulation in sickle cell disease,” Blood Rev 32 (2018): 61-70.
- Kapetanaki M G, Gbotosho O T, Sharma D, et al. Free heme regulates placenta growth factor through NRF2-antioxidant response signaling. Free Radic. Biol. Med 143 (2019): 300-308.
- Patel N, Gonsalves C S, Malik P, et al. Placenta growth factor augments endothelin-1 and endothelin-B receptor expression via hypoxia-inducible factor-1 alpha. Blood 112 (2008): 856-865.
- Victor P Bilan, Frank Schneider, Enrico M Novelli, et al. Experimental intravascular hemolysis induces hemodynamic and pathological pulmonary hypertension: association with accelerated purine metabolism. Pulm. Circ 8 (2018): 2045894018791557.
- Bum-Yong Kang, Kathy Park, Jennifer M Kleinhenz, et al. Peroxisome Proliferator-Activated Receptor γ Regulates the V-Ets Avian Erythroblastosis Virus E26 Oncogene Homolog 1/microRNA-27a Axis to Reduce Endothelin-1 and Endothelial Dysfunction in the Sickle Cell Mouse Lung. Am. J. Respir. Cell Mol. Biol 56 (2017): 131-144.
- Schreier D A, Forouzan O, Hacker T A, et al. Increased Red Blood Cell Stiffness Increases Pulmonary Vascular Resistance and Pulmonary Arterial Pressure. J. Biomech. Eng 138 (2016): 021012.
- Irwin DC, Baek JH, Hassell K, et al. Hemoglobin-induced lung vascular oxidation, inflammation, and remodeling contribute to the progression of hypoxic pulmonary hypertension and is attenuated in rats with repeated-dose haptoglobin administration. Free Radic. Biol. Med 82 (2015): 50-62.
- Morris C R, Gladwin M T, Kato G J. Nitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disorders. Curr. Mol. Med 8 (2008): 620-632.
- Morris C R. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematol. Am. Soc. Hematol. Educ. Program (2008): 177-185.
- Jochen Steppan, Huong T Tran, Valeriani R Bead, et al. Arginase Inhibition Reverses Endothelial Dysfunction, Pulmonary Hypertension, and Vascular Stiffness in Transgenic Sickle Cell Mice. Anesth. Analg 123 (2016): 652-658.
- Karin P Potoka, Katherine C Wood, Jeffrey J Baust, et al. Nitric Oxide-Independent Soluble Guanylate Cyclase Activation Improves Vascular Function and Cardiac Remodeling in Sickle Cell Disease. Am. J. Respir. Cell Mol. Biol 58 (2018): 636-647.
- Katherine C Wood, Brittany G Durgin, Heidi M Schmidt, et al. Smooth muscle cytochrome b5 reductase 3 deficiency accelerates pulmonary hypertension development in sickle cell mice. Blood Adv 3 (2019): 4104-4116.
- Kato G J, Wang Z, Machado R F, et al. Endogenous nitric oxide synthase inhibitors in sickle cell disease: abnormal levels and correlations with pulmonary hypertension, desaturation, haemolysis, organ dysfunction and death. Br. J. Haematol 145 (2009): 506-513.
- Luis E F Almeida, Sayuri Kamimura, Celia M de Souza Batista, et al. Sickle cell disease subjects and mouse models have elevated nitrite and cGMP levels in blood compartments, Nitric Oxide Biol. Chem 94 (2020): 79-91.
- Ofori-Acquah S F. Sickle cell disease as a vascular disorder. Expert Rev. Hematol 13 (2020): 645-653.
- Gregory J Kato, Sabrina Martyr, William C Blackwelder, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br. J. Haematol 130 (2005): 943-953.
- Iakovos Armenis, Vassiliki Kalotychou, Revekka Tzanetea, et al. Reduced peripheral blood superoxide dismutase 2 expression in sickle cell disease. Ann. Hematol 98 (2019): 1561-1572.
- Claudia R Morris, Jung H Suh, Ward Hagar, et al. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood 111 (2008): 402-410.
- Enrico M Novelli, Lynda Little-Ihrig, Heather E Knupp, et al. Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am. J. Physiol. Lung Cell. Mol. Physiol 316 (2019): L1150-L1164.
- Villagra J, Shiva S, Hunter L A, et al. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood110 (2007): 2166-2172.
- Katherine Redinus, Jin Hyen Baek, Ayla Yalamanoglu, et al. An Hb-mediated circulating macrophage contributing to pulmonary vascular remodeling in sickle cell disease. JCI Insight 4 (2019).
- Fatima Anjum, Jason Lazar, James Soh, et al. Dysregulation of ubiquitin-proteasome pathway and apolipoprotein A metabolism in sickle cell disease-related pulmonary arterial hypertension. Pulm. Circ 3 (2013): 851-855.
- Adly A A, Ismail E A, Andrawes N G, et al. Soluble Fas/FasL ratio as a marker of vasculopathy in children and adolescents with sickle cell disease. Cytokine 79 (2016): 52-58.
- Nasimuzzaman M, Malik P. Role of the coagulation system in the pathogenesis of sickle cell disease. Blood Adv 3 (2019): 3170-3180.
- Gordeuk V R, Castro O L, Machado R F. Pathophysiology and treatment of pulmonary hypertension in sickle cell disease. Blood 127 (2016): 820-828.
- Ilaria Liguoro, Michele Arigliani, Bethany Singh, et al. Beneficial effects of adenotonsillectomy in children with sickle cell disease. ERJ Open Res 6 (2020).
- Mondal P, Stefek B, Sinharoy A, et al. The association of nocturnal hypoxia and an echocardiographic measure of pulmonary hypertension in children with sickle cell disease. Pediatr. Res 85 (2019): 506-510.
- Steinberg M H. Genetic etiologies for phenotypic diversity in sickle cell anemia. ScientificWorldJournal 9 (2009): 46-67.
- Lakkakula B V K S. Association between MTHFR 677C>T polymorphism and vascular complications in sickle cell disease: A meta-analysis. Transfus. Clin. Biol. J. Soc. Francaise Transfus. Sang 26 (2019): 284-288.
- Allison E Ashley-Koch, Laine Elliott, Melanie E Kail, et al. Identification of genetic polymorphisms associated with risk for pulmonary hypertension in sickle cell disease. Blood 111 (2008): 5721-5726.
- Khorshied M M, Mohamed N S, Hamza R S, et al. Protein Z and Endothelin-1 genetic polymorphisms in pediatric Egyptian sickle cell disease patients. J. Clin. Lab. Anal 32 (2018).
- Yousry S M, Ellithy H N, Shahin G H. Endothelial nitric oxide synthase gene polymorphisms and the risk of vasculopathy in sickle cell disease. Hematol. Amst. Neth 21 (2016): 359-367.