Pathophysiology, Evaluation and Management of Metabolic Acidosis
Article Information
Mohammad Tinawi*
Adjunct Clinical Assistant Professor of Medicine, Nephrology Specialists, Indiana University School of Medicine, Northwest-Gary, IN, USA
*Corresponding author: Mohammad Tinawi, Nephrology Specialists, P.C., 8840 Calumet Ave, Suite 101, Munster, IN 46321, USA
Received: 02 January 2021; Accepted: 12 January 2021; Published: 22 January 2021
Citation: Mohammad Tinawi. Pathophysiology, Evaluation and Management of Metabolic Acidosis. Archives of Clinical and Biomedical Research 5 (2021): 85-109.
View / Download Pdf Share at FacebookAbstract
Metabolic acidosis is a reduction in blood pH due to a primary reduction in serum bicarbonate (HCO3−). It is associated with a secondary reduction in carbon dioxide arterial pressure (PaCO2). Metabolic acidosis can be acute or chronic. Acute metabolic acidosis results from excess organic acids as in lactic acidosis, while chronic metabolic acidosis reflects reduced renal acidification. Metabolic acidosis is further classified into anion-gap (AG-MA) and hyperchloremic (normal anion-gap [NAG-MA]) based on serum anion gap (AG). Metabolic acidosis has adverse effects on a variety of body functions. Although base administration is helpful in the management of chronic metabolic acidosis, it is controversial in acute metabolic acidosis. Treatment of the underlying cause is the cornerstone of the management of acute metabolic acidosis.
Keywords
Metabolic Acidosis; Acid-Base Disorders; Acid-Base Physiology; Hyperchloremic Metabolic Acidosis; Lactic Acidosis; Renal Tubular Acidosis
Metabolic Acidosis articles; Acid-Base Disorders articles; Acid-Base Physiology articles; Hyperchloremic Metabolic Acidosis articles; Lactic Acidosis articles; Renal Tubular Acidosis articles
Article Details
1. Introduction
Normal arterial blood pH is 7.35-7.45 while intracellular pH is 7.0-7.30 [1, 2]. A variety of intracellular and extracellular buffering systems along with renal and respiratory regulations keep arterial blood pH in this narrow range. A low blood pH defines acidemia, if serum HCO3− is low, the acidemia is due to metabolic acidosis; while if PaCO2 is high, the acidemia is due to respiratory acidosis. If serum HCO3− is low and PaCO2 are elevated, the acidemia is due to a mixed acid-base disorder, namely metabolic acidosis and respiratory acidosis as in some patients with acute asthma [3]. Therefore, a simple acid-base disorder is due to a change in either serum HCO3− or PaCO2 with subsequent compensation. A mixed acid-base disorder is the presence of 2 or 3 acid-base disorders concomitantly [4]. A diet high in animal protein leads to an acid load, while a plant-based diet will lead to an alkali load [2]. On average the body produces 15,000 mmol of carbon dioxide (CO2) daily and 1 mmol/kg/day of hydrogen (H+) [5]. The body needs to eliminate volatile acid (CO2), organic acids (ketones and lactic acid), and inorganic acids resulting from protein metabolism (phosphoric acid and sulfuric acid) [6]. Buffer systems prevents large changes in pH after an acid or alkali load. The most important buffer in the extracellular fluid (ECF) is the HCO3−/CO2 buffer. If an acid (HA) is added, HCO3− is converted to CO2 which is excreted by the lungs resulting in a small change in pH [1, 5].
HA + NaHCO3− →H2O + CO2 + NaA
The bone acts as a buffer in chronic metabolic acidosis resulting in the release of calcium (Ca2+) salts [7]. Intracellular buffers include proteins, H+/HCO3− transporters, and organic phosphate compounds. Removal of acid or alkali load from the body is dependent on the lungs (CO2 regulation) and the kidneys (HCO3− regulation). The two processes work in tandem as per the Henderson-Hasselbalch equation [1, 4, 8]. Equations 1.
|
pH = 6.1 + log [HCO3−/ (0.03 x PaCO2)] [H+] = 24 x PaCO2 / [HCO3−] Note that pH = -log [H+] = log [1/H+] |
Equations 1: Henderson Hasselbalch equation. HCO3− is in mEq/l, PaCO2 is in mm Hg, and H+ is in nEq/l. A H+ of 40 nEq/l (40 x 10-9 mol/l) corresponds to a pH of 7.40.
For example, if serum HCO3− is 10 mEq/l and PaCO2 is 23 mmHg, pH will be 7.26 (corresponding to H+ of 55 nEq/l or nmol/l) [6]. The ratio of HCO3− to PaCO2 define pH. If both change similarly, there will be no change in pH. Metabolic acidosis lowers HCO3−, the lungs increase CO2 excretion limiting the change in pH [9]. Therefore, metabolic acidosis results in hyperventilation. Serum HCO3− and PaCO2 should move in the same direction (both are down in metabolic acidosis and respiratory alkalosis, and both are up in metabolic alkalosis and respiratory acidosis). Movement of Serum HCO3− PaCO2 in the opposite direction is an indication of a mixed acid-base disorder. For every 1 mEq/l (1 mmol/l) decline in HCO3−, there is a 1.2 mm Hg decline in PaCO2. This is referred to as respiratory compensation and it usually does not result in complete normalization of pH [10]. A pH that is close to the normal range may indicate a mixed acid-base disorder, namely, metabolic acidosis and respiratory alkalosis, this scenario (anion-gap metabolic acidosis and respiratory alkalosis due to hyperventilation) is common in patients with salicylate intoxication, hepatic failure and sepsis [4].
2. Pathophysiology
2.1 Renal net acid excretion
The kidneys play a vital function in acid-base regulation. There are 3 components of renal net acid excretion (NAE), ammonium (NH4+) production, tit-ratable acid and urinary HCO3− ( HCO3−U ) [1, 2, 10].
NAE = (titratable acid + NH4+) – HCO3−U
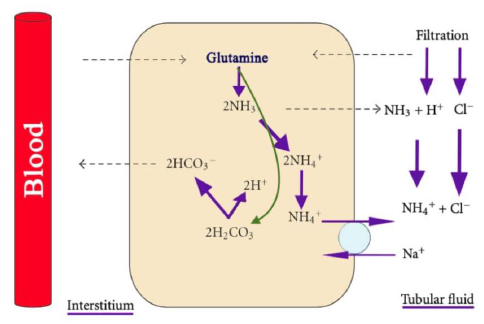
Under normal conditions all of the HCO3− filtered through the glomeruli is reabsorbed [11]. Moreover, new HCO3− is generated by the kidneys to replace the HCO3− utilized to buffer the acid load generated by the body. The proximal tubule (PT) reabsorbs 80% of filtered HCO3−, the thick ascending limb of the loop of Henle (TAL) reabsorbs 15%, the remaining 5% is reabsorbed by the cortical collecting duct (CCD) and the inner medullary collecting duct (IMCD) [1]. The most important titratable acid is phosphate which has a pKa of 6.80. Creatinine and uric acid play a lesser role as titratable acids. In chronic metabolic acidosis titratable acids do not increase significantly, while ammonium excretion in the urine does, the response takes 4-7 days [1, 5]. Therefore, the ammonia/ammonium system (NH3/NH4+) is the critical component of net acid excretion [2]. Ammonium synthesis occurs in the PT. Figure 1.
Each glutamine ion produces two NH4+ and two HCO3− ions [12]. The Na+-H+ exchanger (antiporter) NHE3 at the apical membrane of the PT transport NH4+ into the lumen [11]. The TAL reabsorbs NH4+ paracellularly (passively), via the Na+-K+-2Cl- transporter, and via the apical K+ channel. The final step of NH4+ cycle is its nonionic diffusion (secretion) into the lumen of the collecting duct [13]. Chronic acidosis and hypokalemia increase ammonium synthesis, while hyperkalemia decreases ammonium synthesis. This explains why patients with hypokalemia (especially due to hyperaldosteronism) have a concomitant metabolic alkalosis, while patients with type 4 renal tubular acidosis have hyperkalemia and metabolic acidosis [13].
Figure 1: Ammonium production in the proximal tubule from glutamine. note that the process produces HCO3−. Courtesy of Bruno and Valenti [8]. This is an open access article distributed under the Creative Commons Attribution License.
2.2 HCO3− Reabsorption in the proximal tubule
Most of HCO3− in the PT is reabsorbed via hydrogen (H+) secretion by the Na+-H+ exchanger (antiporter) NHE3 at the apical membrane [11]. NHE-3 exchanges a single Na+ ion for one H+ ion. Luminal Na+ enters the cell from the lumen while H+ exits the cell. The apical H+-ATPase plays a lesser role in H+ secretion from the PT. Two types of the enzyme carbonic anhydrase (CA) exist in the PT, lumen or membrane bound CAIV and cytosolic CAII [14]. The following reaction occurs in the lumen and is catalyzed by CAIV, Figure 2:
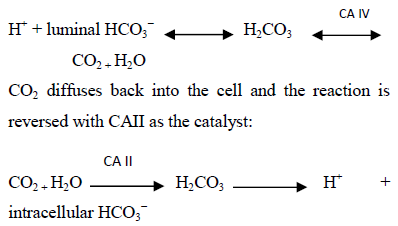
Therefore, hydrogen secretion leads to HCO3− generation in the cell. The above process replenishes H+, while HCO3− exits the cell with Na+ via the Na+- HCO3−- CO32- basolateral cotransporter (also known as Na+- HCO3− transporter, electrogenic Na+-coupled HCO3−cotransporter 1, NBCI or NBCe1) [2]. It is essential for cell voltage to be negative to drive the above processes; the basolateral Na+-K+-ATPase pump generates the required negative charge by maintaining a low intracellular Na+.
2.3 HCO3− Reabsorption in the thick ascending limb of the loop of Henle (TAL)
In the TAL apical H+ secretion is facilitated by the Na+-H+ exchanger (antiporter) NHE3 in a manner similar to the PT. HCO3− exits the cell via the Na+- HCO3−- CO32- basolateral cotransporter and the Cl--HCO3− exchanger [1, 2].
Figure 2: H+ secretion and HCO3− reabsorption in the proximal tubule. Courtesy of Bruno and Valenti [8]. This is an open access article distributed under the Creative Commons Attribution License.
2.4 HCO3− Reabsorption in the Collecting Duct (CD)
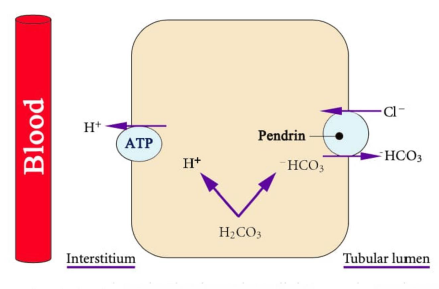
The CD consists of three segments, the cortical collecting duct (CCD), the inner medullary collecting duct (IMCD) and the outer medullary collecting duct (OMCD). The CCD has two cell types, the principal cells which reabsorb Na+ and secrete K+ under the effect of aldosterone, and the intercalated cells which regulate acid-base balance. The intercalated cells have two subtypes, a-intercalated cells which secrete H+ and b-intercalated cells which secrete HCO3−. These two subtypes are functional mirror image of each other. The medullary collecting duct only contains a-intercalated cells [1, 2]. a-intercalated cells possess two H+ transporters, a vacuolar H+-ATPase and a H+-K+-ATPase (which secrets H+ and reabsorbs K+). The generated HCO3− exits the cell via a basolateral Cl--HCO3− exchanger. b-intercalated cells generate HCO3− which exits the cell via an apical Cl--HCO3− exchanger (SLC26A4 protein [pendrin]) [15]. Figure 3. Therefore, HCO3− secretion requires luminal Cl- and is inhibited by Cl- deficiency. This is why isotonic saline (NaCl) solutions are used for correction of metabolic alkalosis. H+ exits the cell via the action of H+-ATPase located on the basolateral membrane.
Figure 3: Pendrin. b-intercalated cells generate HCO3− which exits the cell via pendrin which is an apical Cl--HCO3− exchanger. Courtesy of Bruno and Valenti [8]. This is an open access article distributed under the Creative Commons Attribution License.
3. Metabolic Acidosis
3.1 Definition
Metabolic acidosis is arterial blood pH < 7.35 due to low serum HCO3− with subsequent increased ventilation leading to a decline in PaCO2 referred to as respiratory compensation. Measurement of arterial pH is needed to make the diagnosis because low serum HCO3− can be due to metabolic acidosis or due to renal compensation for respiratory alkalosis [16]. For every 1 mEq/l (or 1 mmol/l) decline in HCO3− , the PaCO2 declines by 1.2 mmHg [17]. Table 1. Winter’s formula can also be used to calculate the respiratory compensation in metabolic acidosis [9]: Expected PaCO2 = (HCO3− x 1.5) + 8 ± 2
For example, if serum HCO3− is 14 mEq/l in a patient with metabolic acidosis, the expected PaCO2 due to respiratory compensation is: 40 – (1.2 x 10) = 28 mmHg. A normal value for HCO3− is 24 mEq/l for our purposes, therefore the decline in HCO3− is (24 - 14 = 10). Using Winter’s formula: Expected PaCO2 = (14 x 1.5) + 8 ± 2 = 29 ± 2 mmHg. A quick way to determine expected PaCO2 is to look at the last two digits of pH [17]. For example, if pH is 30, expected PaCO2 is 30 mmHg. See table 1. It is important to note that PaCO2 can rarely reach a value below 10 mmHg due to the limitations of hyperventilation. Moreover, compensation is not complete and will not result in normalization of arterial pH. Normalization of pH is usually due to concomitant respiratory alkalosis.
|
For each 1 mEq/l decline in HCO3− there is a 1.2 mm Hg decrease in PaCO2 |
|
Expected PaCO2 = (HCO3− x 1.5) + 8 ± 2 |
|
PaCO2 = the last two digits of pH on ABGs |
|
PaCO2 = (HCO3-) + 15 |
Table 1: Calculating Compensation in Metabolic Acidosis.
3.2 Diagnosis
The following steps should be followed in establishing the diagnosis of metabolic acidosis [16]:
- Obtain arterial blood gases (ABGs) and an electrolyte panel simultaneously. Metabolic acidosis is low pH due to low serum HCO3−. ABGs are needed to make accurate acid-base diagnoses. For example, the same electrolyte pattern can be seen in high anion-gap metabolic acidosis (AG-MA) and respiratory alkalosis.
- Calculate respiratory compensation and determine if it is appropriate. If PaCO2 is below the expected value, a concomitant respiratory alkalosis is suspected, while a PaCO2 above the expected value may signify respiratory acidosis.
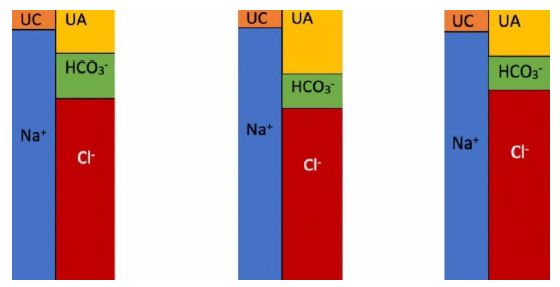
- Calculate serum anion gap (AG) using the following formula [9], Figure 4:
|
AG = (Na+) – (Cl- + HCO3−) |
Stated differently AG = unmeasured anions (UA) – unmeasured cations (UC)
UA are mostly albumin in addition to phosphate, sulfate and IgA. UC are K+, Ca+2, Mg+2 and IgG [16]. The normal value for AG is 12 ± 2 mEq/l [17]. This value may change depending on assays used to measure Na+ and Cl-. Knowledge of AG of a specific patient from a previous electrolyte panel is helpful. When an acid (HA) is added to serum, the H+ is buffered by HCO3− while the remaining anion (A) results in an anion gap. In simple AG-MA when HCO3− goes down by 1 mEq/l (due to buffering of one H+), the anion gap increases by 1 mEq/l due to the addition of (A). However, if (A) is excreted with Na+ in the urine leaving Cl- behind, NAG-MA ensues. In case of hypoalbuminemia the AG should be corrected using the formula:
|
Corrected AG = Calculated AG + 2.5 (4.5 - serum albumin in g/dl) |
Example: calculated AG is 5 mEq/l, serum albumin is 2.5 g/dl, Corrected AG = 5 + 2.5 (4.5 – 2.5) = 10 mEq/l. Therefore, anion gap decreases by 2.5 mEq/l for each 1 g/dl decline in serum albumin [16, 18]. AG differentiates high anion-gap metabolic acidosis (AG-MA) from non-anion gap (normal anion-gap or hyperchloremic) metabolic acidosis [NAG-MA]. In NAG-MA, the decline is HCO3− is accompanied by an equal rise in Cl-, therefore, the anion gap remains unchanged. In AG-MA, the decline in HCO3− is due to excess H+ which is accompanied by an unmeasured anion. (e.g., lactate).
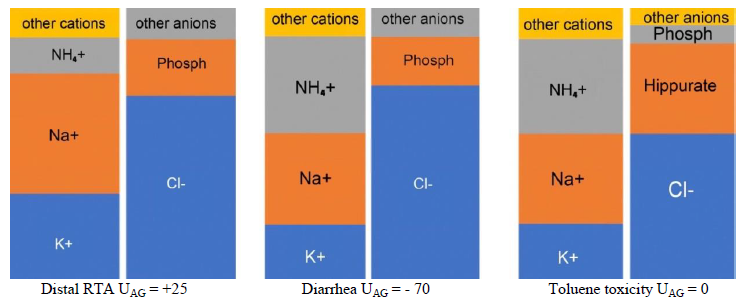
- Calculate urine anion gap (UAG) if NAG-MA is diagnosed by utilizing the following formula [16], Figure 5:
Cl-U + UA = Na+U + K+U + UC (NH4+)
UAG = UA – UC
|
UAG = (Na+U + K+U ) – Cl-U |
NH4+ concentration in the urine can be estimated using the following formula [20]:
|
Urinary NH4+ = 0.8 x (UAG) + 82 |
Urine Na+, K+, and Cl- are measured in a random (spot) urine sample. If Na+U + K+U > Cl-U in the context of NAG-MA, then urine NH4+ is low due to impaired NH4+ excretion as in renal tubular acidosis (RTAs). If Na+U + K+U < Cl-U; then urine NH4+ is sufficient and NAG-MA is due to a non-renal cause such as diarrhea [21]. Keep in mind that NH4+excretion is accompanied by chloride.
UAG can be misleading as in ketonuria (due to ketones), toluene toxicity (due to hippurate) and impaired renal acidification due to a decrease in distal delivery of Na+ [22, 23]. Ketones and hippurate in the urine will result in UAG ³ 0 despite an increase in urine NH4+. Na+U > 25 mEq/l is required for accurate testing of renal acidification. If urine acidification is impaired due to low Na+U, increasing distal delivery of Na+ by administration of normal saline or furosemide would correct the problem. Urine osmolar gap (UOG) is better than UAG in indirectly estimating urinary NH4+ [16, 24]. It can be calculated using the following formulas [25, 26], Equations 2:
|
UOG = Measured Uosm – Calculated Uosm Calculated Uosm = 2 (Na+U + K+U ) + (urine glucose/18) + (urine urea nitrogen/2.8) Urinary NH4+ = UOG/2 |
Equations 2: Urine osmolar gap. Na+ and K+ in mEq/l. Glucose and urine urea nitrogen in mg/dl. If SI units are used (mmol/l), Calculated Uosm = 2 (Na+U + K+U ) + (urine glucose) + (urine urea nitrogen).
A large Uosm indicates the presence of a missing anion such as benzoate and hippurate in toluene toxicity. Half of the urine osmolar gap is due to the missing anion. In the presence of substances such as ketones or hippurate, Uosm should not be used to estimate NH4+. Normal UOG is around 100 mOsm/kg H2O. If renal tubular function is normal, urinary NH4+ on a western diet is 30-40 mEq/d. Urinary NH4+ increases to approximately 200 mEq/d in metabolic acidosis and remains <30-40 mEq/d in patients with impaired renal acidification as in renal tubular acidoses (RTAs). Both UAG and UOG are indirect estimates of urinary NH4+. In one study UAG correlated poorly with measured NH4+ (R = 0.45), UOG performed better (R = 0.68) [27].
- Determine if the patient has a mixed acid-base disorder [16]. Table 2. If measured PaCO2 exceeds the estimated value by more than 5 mm Hg, the patient has metabolic acidosis and respiratory acidosis. This is common in patients in the critical care setting with hypercapnic respiratory failure. If blood pH is nearly normal in a patient with metabolic acidosis, a concomitant respiratory alkalosis is likely. This is common in critically ill patients with sepsis and hypoxemic respiratory failure [28]. Calculate the “delta delta [△/△]”, which is the change in AG from a normal value of 10 mEq/l compared with the change in serum HCO3− from the normal value of 24 mEq/l [16].
- Determine if the patient has a mixed acid-base disorder [16]. Table 2. If measured PaCO2 exceeds the estimated value by more than 5 mm Hg, the patient has metabolic acidosis and respiratory acidosis. This is common in patients in the critical care setting with hypercapnic respiratory failure. If blood pH is nearly normal in a patient with metabolic acidosis, a concomitant respiratory alkalosis is likely. This is common in critically ill patients with sepsis and hypoxemic respiratory failure [28]. Calculate the “delta delta [△/△]”, which is the change in AG from a normal value of 10 mEq/l compared with the change in serum HCO3− from the normal value of 24 mEq/l [16].
△ AG/△ HCO3− = (measured AG – 10) / (24 - measured serum HCO3−)
In DKA the △/△ is about 1, while in lactic acidosis it is around 1.6-1.8 [29]. If △HCO3− is large due to a large decline in HCO3−, the patient has both AG-MA and NAG-MA [17]. On the other hand, if △ HCO3− is small, the patient has both metabolic acidosis and metabolic alkalosis [28]. Note that D HCO3− can be negative in patients with combined metabolic acidosis and metabolic alkalosis.
Figure 4: Serum AG. UC is unmeasured cations; UA is unmeasured anions. Note that the number of anions and cations are equal. Drawings such as these are referred to as “Gamblegram”. The concept was first developed by an acid-base pioneer, James Lawder Gamble (1883-1959) [19]. The second diagram represents AG-MA where HCO3− is low and AG is high due to the presence of an anion such as lactate or ketone. The third diagram represents NAG-MA where HCO3− is low and Cl- is high with a normal AG.
Figure 5: Urine anion gap. The first diagram represents a patient with distal RTA. The second diagram represents a negative UAG due to diarrhea where NH4+ excretion is increased. The third diagram represents an individual with toluene toxicity. NH4+ excretion is increased but UAG is 0 due to the presence of hippurate in the urine.
|
Blood Test |
Normal |
AG-MA and respiratory acidosis |
AG-MA and NAG-MA |
AG-MA and metabolic alkalosis |
|
pH |
7.40 |
7.29 |
7.15 |
7.41 |
|
PaCO2 (mmHg) |
40 |
39 |
24 |
36 |
|
HCO3− (mEq/l) |
24 |
18 |
8 |
22 |
|
AG (mEq/l) |
10 |
16 |
18 |
27 |
|
△ AG |
0 |
6 |
8 |
17 |
|
△ HCO3− |
0 |
6 |
16 |
2 |
|
△ /△ |
N/A |
1 |
0.5 |
8.5 |
Table 2: Examples of Mixed Acid-Base Disorders.
It is noteworthy that both acute and chronic metabolic acidosis can result in hypercalcemia because H+ is buffered in the bone which leads to release of Ca+2 and calcinuria. For each 0.1 decrease in pH, ionized Ca+2 increases by 0.12 mg/dl. The reverse is true in metabolic alkalosis [30].
3.3 Case Study
A 24-year-old man brought to ER due to altered mental status. He was not taking any medications. Past medical history is unremarkable. On exam he had orthostatic drop in blood pressure and appeared weak and dehydrated. Laboratory evaluation: Na+ 132, K+ 2.0, Cl- 114, HCO3− 10 (all in mEq/l), pH 7.12, PaCO2 32 mmHg. Na+U 49, K+U 35, Cl-U 7 (all in mEq/l), urine glucose is 0, urine urea nitrogen is 252 mg/dl. Measured urine osmolality 581 mOsm/kg H2O. pH is 7.12 which indicates acidemia, HCO3− is 10, therefore the patient has metabolic acidosis.
AG = (132) – (114+10) = 8, the patient has NAG-MA.
Expected PaCO2 = (HCO3− x 1.5) + 8 ± 2 = (10 x 1.5) + 8 ± 2 = 23 ± 2
Since PaCO2 is 32 mmHg, the patient has both NAG-MA and respiratory acidosis (likely due to severe hypokalemia).
UAG = (Na+U + K+U ) – Cl-U = (49 + 35) – (7) = 77
A positive UAG is seen in RTAs, however Cl-U is very low indicating the presence of another anion in the urine. Let’s now calculate the UOG
Calculated Uosm = 2 (Na+U + K+U ) + (urine glucose/18) + (urine urea nitrogen/2.8)
Calculated Uosm = 2 (49+35 ) + (0/18) + (252/2.8) = 258 mOsm/kg H2O
UOG = 581 – 258 = 323 mOsm/kg H2O
The large urine osmolar gap is due to hippurate and benzoate. The etiology of the patient’s NAG-MA with respiratory acidosis is toluene toxicity due to glue sniffing. Toluene is metabolized to benzoic acid and hippuric acid. K+ should be replaced first. The patient needs to be hydrated with intravenous fluids (IVF) , HCO3− is added to IVF after correction of hypokalemia because HCO3− drives K+ intracellularly [31, 32].
4. Non-Anion Gap (Normal Anion Gap or Hyperchloremic) Metabolic Acidosis
4.1 Causes
The causes of NAG-MA can be broadly divided into
renal or extrarenal [4, 25] Table 3. Renal causes such as RTAs are the result of inadequate net acid excretion (inadequate NH4+ production) which results in a decline in renal HCO3− production. Chronic diarrhea is the most common extrarenal cause. Chronic diarrhea results in HCO3− loss in stool [28]. Measurement of urinary NH4+ distinguishes RTAs from extrarenal causes, however this assay is not available in most US laboratories. Urine NH4+ is low in RTAs and high in extrarenal causes. Clinicians calculate the UAG in lieu of direct measurement of urine NH4+. Direct measurement of urinary NH4+ can be done by formaldehyde titration, colorimetric or enzymatic assays. Enzymatic assay is adapted from measurement of plasma ammonia [33].
Patients with toluene toxicity and normal renal function will have NAG-MA due to rapid excretion of benzoate and hippurate as sodium and potassium salts. Patients with impaired renal function will develop AG-MA due to retention of benzoate and hippurate (unmeasured anions). Patients with DKA will have NAG-MA during treatment because of excretion of ketones as Na and K salts [34]. Chronic NAG-MA is associated with bone disease, muscle wasting, progression of chronic kidney disease (CKD), and impaired growth in children [35].
|
Chronic diarrhea, laxative abuse, GI losses (biliary or pancreatic secretions), high output GI fistulae |
|
Ureteral intestinal diversions (ureterosigmoidostomy, ileal conduit) |
|
Toluene toxicity due to glue or paint sniffing (excess excretion of benzoate and hippurate) |
|
Medications · Carbonic anhydrase inhibitors (topiramate, acetazolamide) · Exogenous acid: total parenteral nutrition (amino acids load), Ca chloride, arginine chloride, NH4Cl and sulfur · Excessive infusion of Cl- rich intravenous solutions such as isotonic saline |
|
Post-hypocapnic state |
|
Recovery from diabetic ketoacidosis (DKA) |
|
Advanced chronic kidney disease (CKD) |
|
RTAs · Hypokalemic: Proximal (type 2) and distal (type 1) · Hyperkalemic: voltage-dependent (hyperkalemic distal RTA) and type 4 RTA (aldosterone deficiency or resistance) |
Table 3: Causes of NAG-MA.
4.2 Extrarenal non-anion gap metabolic acidosis
The main causes are chronic diarrhea and laxative abuse. NAG-MA is the result of loss of NaHCO3 in intestinal secretions. Subsequent hypovolemia results in increased renal absorption of NaCl and increased urine NH4+ excretion [36]. Hypokalemia is common. UAG is negative, and urine pH is variable and can be over 6.0 due to the significant increase in urine NH4+ [16]. The diagnosis of chronic diarrhea is easily elicited from the history; however, patients may deny surreptitious laxative abuse. The diagnosis of laxative abuse requires a high index of suspicion, and it is more common than proximal and distal RTAs. Patients with NAG-MA due to diarrhea may require alkali and potassium replacements, especially if the underlying cause cannot be mitigated. Ureteral intestinal diversions are uncommon causes of extrarenal NAG-MA. A ureterosigmoidostomy may result in more severe NAG-MA compared to an ileal conduit due to increased contact time between the intestine and the urine. NAG-MA results from Cl- exchange for HCO3− via activation of the Cl-- HCO3− exchanger, and from absorption of urinary NH4Cl by the intestine [28]. When ammonia is metabolized in the liver, HCO3− is consumed.
4.3 Renal tubular acidoses (RTAs)
RTAs are a form of NAG-MA. All RTAs are associated with impaired NH4+ excretion. RTAs can be proximal (type 2), distal (type 1), or hyperkalemic [37]. Hyperkalemic RTA is divided into voltage-dependent RTA and type 4 RTA (hypoaldosteronism). Serum potassium measurement differentiates proximal and distal RTAs (hypokalemic RTAs) from hyperkalemic RTA. Distal RTA and proximal RTA were named type 1 and type 2 respectively based on the order of discovery of these disorders. Some authorities refer to distal RTA as classical distal RTA and to voltage-dependent RTA as generalized distal RTA with hyperkalemia or as hyperkalemic distal RTA. See table 5 for comparison of different types of RTAs [16, 38].
4.4 Proximal RTA (Type 2)
As mentioned above the PT reabsorbs 80% of filtered HCO3−. In proximal RTA the PT ability to reabsorb HCO3− is diminished. This leads to HCO3− wasting in the urine and a decrease in serum HCO3− until a new lower serum HCO3− level is established that matches the ability of the PT and subsequent nephron segments to reabsorb filtered HCO3−. Once that occurs a new steady state is established with no further HCO3− wasting in the urine [16]. Fractional excretion of HCO3− (FEHCO3-) is > 15% in this disorder:
|
FEHCO3- = (U/P) HCO3− / (U/P) Cr x 100 |
Hypokalemia is a main feature of proximal RTA due to increased distal delivery of Na+ and the activation of the renin-angiotensin-aldosterone (RAAS) system. Hyperaldosteronism is due to volume depletion resulting from HCO3− wasting [38]. Proximal RTA can be due to isolated pure bicarbonate wasting (mutations of SLC4A4 or SLC9A3/NHE3) or part of Fanconi syndrome (proximal RTA with glucosuria without hyperglycemia, phosphaturia, aminoaciduria, and uricosuria) [12]. Fanconi syndrome can be inherited as in cystinosis tyrosinemia, galactosemia, hereditary fructose intolerance, Lowe’s syndrome and Wilson’s disease or acquired as in multiple myeloma and light chain deposition disease [16]. Certain medications (tenofovir, ifosfamide, strep-tozocin, outdated tetracycline and gentamicin) and toxins (lead, cadmium, and mercury) can result in Fanconi syndrome. Recently, Fanconi syndrome due to the immune checkpoint inhibitor nivolumab has been described [39, 40].
In contrast to distal RTA, patients with proximal RTA can acidify their urine to a pH < 5.5. Once the disorder is treated with bicarbonate, urine pH increases to above 5.5 due to increased HCO3− wasting in the urine, this also worsens hypokalemia and increases fractional excretion of HCO3−(FE HCO3−) [16]. Unlike distal RTA, proximal RTA is not associated with nephrocalcinosis or nephrolithiasis. One exception is topiramate induced proximal RTA. Topiramate is a carbonic anhydrase inhibitor and it is associated with hypercalciuria, hypocitraturia and increased risk of kidney stones [41].
Proximal RTA requires treatment with a large amount of alkali, typically 10-20 mEq/kg/day, this treatment aggravates hypokalemia, therefore, potassium replacement is needed as well. Thiazide diuretics induce volume depletion and may enhance HCO3− absorption. Best treatment options include alkali-containing K+ (to replace both K+ and HCO3−) [16, 38], Table 4. For example, Polycitra-K ®, each 5 ml solution contains 398 mg of K+ in the form of potassium citrate monohydrate which provides 10 mEq of K+ and 10 mEq of HCO3−. It is also available in crystals; each 4.5 g packet provides 30 mEq of K+ and 30 mEq of HCO3−. It is critical to treat children aggressively to a normal serum HCO3− to prevent growth retardation. Adults may be treated less aggressively to a serum HCO3− around 18 mEq/l to avoid utilization of a large amount of alkali. For example, a 60 kg patient who requires 10 mEq/kg of alkali needs 600 mEq/day or 20 packets of Polycitra-K®/day.
4.5 Distal RTA (Type 1, dRTA)
Distal RTA or classical distal RTA can be genetic or acquired. Genetic dRTA is either due to mutations of the H+-ATPase subunits (autosomal recessive with deafness dRTA1-ATP6V1B1 and autosomal recessive with normal hearing dRTA2-ATP6V0A4) or due to mutations in the Cl-/ HCO3− exchanger band 3 protein (autosomal dominant due to defect in AE1 [anion exchanger 1] gene, dRTA-SLC4A1 not associated with deafness) [23, 38]. dRTA is associated with growth retardation, metabolic bone disease, muscle weakness, impaired urinary acidification, nephrocalcinosis and nephrolithiasis. Patients with carbonic anhydrase II deficiency with osteopetrosis develop both proximal and distal RTA (also called mixed proximal-distal RTA or type 3 RTA) [42]. In distal RTA there is a defect in net H+ secretion, therefore, urine pH is >5.5 even with acidosis [38].
In other words, patients with distal RTA can never acidify their urine. Urine pH is unreliable in case of a urinary tract infection, for example a urinary tract infection with urea-splitting organisms such as proteus can raise urine pH to alkaline range. Therefore, failure to acidify urine, hypercalciuria, hypocitraturia, nephrocalcinosis and nephrolithiasis (calcium phosphate stones) distinguish distal from proximal RTA [12]. In acquired distal RTA, patients can have an increase in H+ membrane permeability (back leak, gradient defect), the classic example is amphotericin B. Other patients have an acquired defect in H+-ATPase as in Sjögren’s syndrome [43]. Acquired distal RTA can be idiopathic, but it is usually secondary to another disorder, such as autoimmune disorders (Sjögren’s syndrome, systemic lupus erythematosus, and hypergammaglobulinemia), drugs (amphotericin B, lithium, foscarnet), toxins (mercury, toluene toxicity due to glue sniffing), and tubulointerstitial kidney disease (Balkan nephropathy, kidney transplantation) [16].
Clinically, patients have NAG-MA with hypokalemia, hypercalciuria and hypocitraturia. Some patients have salt wasting and nephrogenic diabetes insipidus due to severe hypokalemia. UAG is positive and urine pH is > 5.5 even in the presence of metabolic acidosis [23]. Untreated children have stunted growth. A retroperitoneal ultrasound may show nephrocalcinosis. Patients with incomplete distal RTA (those who are not acidotic and have features of distal RTA such as recurrent calcium phosphate stones and hypocitraturia) need a confirmatory test. Oral NH4Cl is given to induce metabolic acidosis.
Serial pH assays are done. Since NH4Cl is associated with gastrointestinal side effects, many clinicians give furosemide (40 mg) and fludrocortisone (1 mg orally) to stimulate distal H+ secretion. Patients with distal RTA cannot lower their pH below 5.3 [44]. Alkali treatment in distant RTA normalizes growth in children, improves hypercalciuria and hypocitraturia, and decreases kidney stone formation. Table 4. The amount required is 1-2 mEq/kg/day which is less than the amount need for proximal RTA. As in proximal RTA, giving alkali lower K+, and both alkali and K+ should be replaced [16].
|
Sodium bicarbonate (NaHCO3) tablets: 7.8 mEq in 650 mg tablet |
|
Baking soda: 60 mEq/teaspoon |
|
Potassium bicarbonate (Effer-K®): available in 10 mEq, 20 mEq, and 25 mEq effervescent tablets |
|
Potassium citrate (Urocit-K®): available in extended-release tablets: 5 mEq, 10 mEq, and 15 mEq. |
|
Polycitra-K ®: potassium citrate solution in the form of potassium citrate monohydrate, 5 ml provides 10 mEq of K+ and 10 mEq of HCO3−. It is also available in crystals; each 4.5 g packet provides 30 mEq of K+ and 30 mEq of HCO3−. |
|
Shohl’s solution (citric acid and Na+ citrate): Cytra-2®: 334 mg citric acid monohydrate/500 mg Na+ citrate dihydrate per 5 ml solution. Each mL contains 1 mEq Na+, and is equivalent to 1 mEq HCO3− |
Table 4: Alkali treatment options for RTAs.
|
Proximal RTA (type 2) |
Distal RTA (type 1) |
Hyperkalemic RTA (voltage-dependent RTA and type 4 RTA) |
|
|
Main Mechanism |
Decreased HCO3− in PT |
Decreased distal excretion of H+ |
Decreased H+ and K+ excretion in the collecting duct |
|
Serum HCO3− |
Moderately low (16-20) |
Can be significantly low (10-20) |
Moderately low (16-20) |
|
Serum K+ |
Low (<3.5 mEq/l) |
Low (<3.5 mEq/l) |
High (>5.5 mEq/l) |
|
Kidney function |
Usually normal |
Usually normal |
CKD is common |
|
Urine pH during acidosis, or post furosemide or NH4Cl |
Low (<5.5) |
High (>5.5) |
Variable, but patient can have pH <5.5 |
|
Urine citrate |
High/normal |
Low |
Low/normal |
|
Urine NH4+ |
Low/normal |
Low |
Low |
|
FEHCO3- |
10-15% with alkali tx |
2%-5% |
5%-10% |
|
Urine AG |
Negative |
Positive |
Positive |
|
Urine PCO2 (normal is >60-70 mmHg |
Normal |
Low |
Normal |
|
Urine PCO2 – blood PaCO2 (normal >20-30 mm Hg) |
Normal |
Low (except with amphotericin B) |
Low |
|
Additional features |
Fanconi syndrome |
Nephrocalcinosis, nephrolithiasis, polyuria, polydipsia |
CKD |
Table 5: Comparison of different types of RTAs.
4.6 Hyperkalemic RTA
The mechanism of hyperkalemic RTA is impaired distal Na+ reabsorption leading to hyperkalemic NAG-MA. Hyperkalemia decreases NH4+ production and transport [45]. There are two types of hyperkalemic RTA : voltage-dependent RTA (generalized distal RTA with hyperkalemia or hyperkalemic distal RTA) and type 4 RTA or hypoaldosteronism [46].
4.6.1 Voltage-dependent RTA: In this type of hyperkalemic RTA, Na+ absorption is impaired in the principal cells of the collecting tubule. This lowers the electronegativity of the lumen and subsequently both H+ and K+ secretions, resulting in hyperkalemia and NAG-MA [47]. Examples are lupus nephritis, amyloidosis, obstructive uropathy and sickle cell disease [48]. This type can result from severe decline in Na+ distal delivery as in acute kidney injury (AKI), dehydration, liver cirrhosis and heart failure (HF).
4.6.2 Type 4 RTA: This is the most prevalent type of all RTAs especially in adults. It is due to hypoaldosteronism resulting from decreased aldosterone secretion or mineralocorticoid resistance.
- Decreased aldosterone secretion: This is common in patients with diabetic kidney disease due to hyporeninemic hypoaldosteronism syndrome [49]. The typical features of hyporeninemic hypoaldosteronism syndrome include age over 65, hyperkalemia, diabetes mellitus, hypertension and NAG-MA [16]. Many commonly utilized medications inhibit aldosterone secretion such as inhibitors of the renin-angiotensin-aldosterone (RAAS inhibitors) including angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs) and direct renin inhibitors [50]. Other examples include medications that inhibit renin release from the juxtaglomerular cells such cyclosporine and tacrolimus (calcineurin inhibitors), b blockers and non-steroidal anti-inflammatory medications (NSAIDs) [51]. Heparin and ketoconazole inhibit aldosterone secretion [52]. Adrenal insufficiency (primary and secondary) is less common than the above-mentioned causes. Familial hyperkalemic hypertension (Gordon’s syndrome or pseudohypoaldosteronism type II) is a rare syndrome characterized by volume expansion, normal kidney function, hyperkalemia, hypertension and NAG-MA [53]. It is treated with thiazide diuretics. This autosomal dominant form of Gordon’s syndrome is due to mutations in the WNK4 and WNK1 kinases [12].
- Mineralocorticoid resistance (decreased aldosterone responsiveness): Mineralo-corticoid resistance can result from medications that block the collecting tubule epithelial Na+ channel (ENaC) including trimethoprim, pentamidine, amiloride and triamterene. Resistance can also be due to mineralocorticoid receptor blockers such as spironolactone, eplerenone and drospirenone (a progestin component of some birth control pills). Pseudohypoaldosteronism type I is rare [54]. It is characterized by NAG-MA, hyperkalemia and salt wasting nephropathy. Mutations in the 3 subunits of the ENaC is the cause of the autosomal recessive type of this syndrome, while mutations in the mineralocorticoid receptor gene is the cause of the autosomal dominant type [16].
4.7 Treatment of hyperkalemic RTA
The underlying causes should be treated, and offending medications should be stopped if feasible. Management of hyperkalemia is very useful because lowering K+ increases NH4+ production and allows continuation of certain medications that may have strong indications such as ACEI and ARB in renal and cardiac patients. This is achieved by implementation of low K+ diet and administration of K+ binders and alkali. Two new K+ binders (patiromer and sodium zirconium cyclosilicate) are suitable for chronic use [55]. Patients with hypertension or hypervolemia will benefit from diuretic therapy with a thiazide or a loop diuretic. Patients with aldosterone deficiency in absence of hypertension and hypervolemia may benefit from fludrocortisone (0.1 mg orally per day). Alkali therapy can be added to treat the acidosis. Adrenal insufficiency requires treatment with corticosteroids [56].
4.8 Metabolic acidosis in CKD
A study in 1038 adults with non-dialysis CKD stages 2-5 showed that the prevalence of metabolic acidosis is 7% in CKD-2, 13% in CKD-3 and 40% in CKD-4/5 [57]. Patients with CKD commonly develop NAG-MA with variable hyperkalemia once glomerular filtration rate (GFR) is < 20-25 ml/min. As renal functions worsen (GFR <15 ml/min) they may develop AG-MA (uremic acidosis) [58]. Therefore, patients with severe CKD may have NAG-MA, AG-MA or both [59]. In CKD patients, chronic metabolic acidosis is associated with hypoalbuminemia, impaired glucose tolerance, muscle wasting and metabolic bone disease [60]. Chronic metabolic acidosis increases ammoniagenesis with subsequent activation of complement, tubulointerstitial injury and worsening kidney function [61]. In participants of The Systolic Blood Pressure Intervention Trial (SPRINT), serum bicarbonate below 22 mEq/l was associated with increased cardiovascular disease risk [62]. In a study of 1240 male patients with moderate and advanced non-dialysis dependent CKD, Kovesdy et al. found that serum bicarbonate had a U-shaped association with mortality. Lowest mortality was in patients with serum bicarbonate in the range of 26-29 mEq/l and highest mortality in patients with serum bicarbonate < 22 mEq/l [63]. Some studies suggest that treatment of metabolic acidosis with oral alkali or reducing dietary acid intake may slow the decline of renal function in CKD patients [64–66]. A trial by Di Iorio et al. found that treatment of metabolic acidosis in patients with stages 3-5 CKD reduced mortality and improved renal survival [67]. It is recommended to give patients with CKD and serum HCO3− < 22 mEq/l oral bicarbonate supplementation to maintain serum HCO3− within the normal range, unless contraindicated [68]. Oral HCO3− improves muscle strength in CKD and prevent metabolic bone disease and catabolic state [69, 70]. In CKD patients, increasing the intake of fruits and vegetables and reducing dietary protein intake reduces net endogenous acid production and may prevent metabolic acidosis [71]. Patients should be monitored for hyperkalemia. Veverimer is a non-absorbed oral polymer that selectively binds hydrochloric acid. It is under study for treatment of metabolic acidosis in CKD patients [72, 73].
5. Anion-gap Metabolic Acidosis (AG-MA)
Many finds the mnemonic MUDPILES (CAT) helpful in remembering the causes of AG-MA [4]. Table 6.
|
MUDPILES (CAT) |
|
M-Methanol |
|
U-Uremia |
|
D-Diabetic ketoacidosis, alcohol ketoacidosis, starvation ketoacidosis, D-lactic acidosis |
|
P-Paraldehyde, Paracetamol (Acetaminophen), Phenformin (and metformin), Propylene glycol |
|
I-Isoniazid, Iron |
|
L-Lactic acidosis |
|
E-Ethanol and Ethylene glycol |
|
S-Salicylate |
|
C-Carbon monoxide and Cyanide |
|
A-Aminoglycosides |
|
T-Theophylline |
Table 6: Causes of Anion-Gap Metabolic Acidosis.
5.1 Lactic acidosis
Lactic acid is the final product in glucose anaerobic metabolism. Lactate accumulation results in AG-MA. Lactic acidosis is defined as lactate concentration > 4 mEq/l. There are two types of lactic acidosis, type A and type B. In some conditions both types occur simultaneously such as a patient with liver failure who develops sepsis [74]. Type A lactic acidosis is due to hypoxia or tissue ischemia as in shock (septic, cardiogenic or hypovolemic), carbon monoxide poisoning and acute hypoxia such as severe asthma [10, 72]. In type B lactic acidosis, there is no hypoxia or tissue underperfusion. Type B lactic acidosis is due to liver failure, malignancy, thiamine deficiency or toxins/medications such as cyanide, metformin, salicylate, methanol, ethylene glycol, isoniazid, propofol, propylene glycol, linezolid and isoniazid [10, 72]. Treatment of lactic acidosis is addressed at the underlying cause. Severe metabolic acidosis including lactic acidosis decreases cardiac output by decreasing contractility and blood pressure [76]. Some clinicians give HCO3− if pH is < 7.2. The goal is to raise pH to above 7.2 without normalization. No study in humans has shown improved survival with the administration of HCO3− in metabolic acidosis [77]. Therefore, administration of HCO3− to treat metabolic acidosis due to production of organic acids is controversial [78, 79]. HCO3− therapy can worsen hypervolemia, and can result in worsening of intracellular acidosis, hypernatremia, rebound metabolic alkalosis and decrease in ionized Ca2+. The decrease in ionized Ca2+ decreases cardiac contractility [80]. HCO3− is given intravenously by adding 3 ampules of sodium bicarbonate (50 mEq/ampule) to one liter of 5% dextrose in water (150 mEq NaHCO3− in 1 L of D5W). If sodium bicarbonate ampules (50 mEq in 50 ml) are given undiluted (not infused in D5W), hypernatremia may ensue due to hypertonicity.
Clinical assays for lactic acid only measure L-lactate. The diagnosis of D-lactic acidosis requires a special assay for D-lactate. D-lactic acidosis is encountered in some patients with short small bowel, as in patients post bowel resection surgery, due to large delivery of carbohydrate to the colon [81]. Bacterial overgrowth in the colon metabolizes these carbohydrates into D-lactate resulting in confusion and slurred speech after intake of a large carbohydrate meal. Antibiotics and low carbohydrate diet are helpful. In critically ill patients, elevated blood lactate in the first 24 h of intensive care unit (ICU) admission and the change in lactate over the same interval are both independently predictive of inpatient mortality [82]. Lactate assay has become routine in critically ill patients especially with sepsis, because hyperlactatemia is associated with increased mortality.
5.2 Diabetic ketoacidosis (DKA)
DKA results from insulin deficiency with subsequent metabolism of fatty acids into ketones (acetone, acetoacetic acid, and b-hydroxybutyric acid). Patients have hyperglycemia, hypovolemia due to osmotic diuresis, metabolic acidosis and multiple electrolyte abnormalities related to Na+, K+, Mg2+ and phosphate [83]. Most patients present with AG-MA, others have NAG-MA especially early in the course of DKA [49]. NAG-MA also develops during the resolution of DKA. The preferred diagnostic test is measurement of b-hydroxybutyrate. Treatment is with insulin and intravenous fluids. Electrolytes are replaced as well. HCO3− therapy is usually not needed because insulin therapy converts keto acid anions to HCO3− [78].
Some patients with DKA presents with blood glucose < 200 mg/dl. This entity is called euglycemic DKA and is important to recognize [84]. Patients who are taking sodium glucose cotransporter 2 (SGLT2) inhibitors should be monitored for the development of euglycemic DKA [85]. Starvation can lead to a mild AG-MA due to ketosis [86]. Alcoholic ketoacidosis can be severe and is usually associated with electrolyte disorders, hypovolemia and malnutrition [87]. Patients should be observed for symptoms of alcohol withdrawal. Dextrose containing intravenous fluids are helpful because they stimulate insulin release. Thiamine (preferably intravenously) should be given with such intravenous solutions.
5.3 Salicylate toxicity
Salicylic acid or Salicylate is an organic acid with a pKa of 3, this means that at physiologic pH it is almost completely dissociated. The undissociated (unionized) fraction is what determines the toxicity of salicylate. This fraction increases as pH becomes more acidemic, therefore, correction of metabolic acidosis is critical in salicylate poisoning. Salicylate toxicity is characterized by a mixed acid-base disorder (AG-MA and respiratory alkalosis) [4]. AG-MA predominates in infants and children, while respiratory alkalosis predominates in adults.
AG-MA is due to accumulation of salicylate, lactate and occasionally ketones. It is treated with alkaline intravenous fluids (sodium bicarbonate solution) to alkalinize the urine which enhances salicylic acid excretion [88]. Patients with severe toxicity or serum salicylate level > 80 mg/dl should be treated with hemodialysis.
5.4 Pyroglutamic acidosis
This is an infrequent cause of AG-MA, yet it is important to recognize. It is rarely encountered in critically ill patients treated with regular doses of acetaminophen. Patients who take acetaminophen and are at risk for this form of AG-MA include women and those with malnutrition, liver disease, alcoholism and CKD [89]. These patients have reduced levels of glutathione (due to acetaminophen and oxidative stress) which leads to accumulation of pyroglutamic acid (5-oxoproline) [90]. Patients present with altered mental status. Specialized laboratories can measure 5-oxoproline in the blood or urine.
5.5 Methanol and ethylene glycol toxicities
Both intoxications are associated with severe AG-MA and an osmolar gap. Both toxins can be ingested accidentally or as a suicide attempt. When toxins are suspected as the cause of AG-MA, osmolar gap should be calculated [74, 91, 92] Equation 3.
|
Osmolar gap = measured osmolality – calculated osmolality Calculated osmolality = 2 x [Na+] + [BUN/2.8] + [glucose/18] |
Equation 3: Osmolar gap: Na+ in mEq/l, blood urea nitrogen (BUN) and glucose in mg/dl. If SI units are used (mmol/l) the Calculated osmolality = 2 x [Na+] + [BUN] + [glucose].
The normal range for serum osmolality in adults is 280-295 mOsm/kg H2O, a value > 310 mOsm/kg H2O is a significant hyperosmolality. The lethal dose of methanol is 60-250 ml, while the lethal dose of ethylene glycol is 100 ml. Methanol is metabolized by alcohol dehydrogenase to formaldehyde which is converted to formic acid. Ethylene glycol is metabolized by the same enzyme to glycolic and oxalic acid, with subsequent formation of Ca oxalate crystals in the urine. Methanol ingestion leads to inebriation followed by abdominal pain, and occasionally seizures and coma. Blindness is due to the toxic effect of formic acid on the retina. Ethylene glycol ingestion also leads to inebriation which can progress to seizures and coma [93]. Some patients develop pulmonary edema and acute kidney injury. The drug of choice for management of methanol and ethylene glycol intoxications is intravenous fomepizole [74]. Fomepizole inhibits alcohol dehydrogenase and prevents formation of toxic metabolites. If fomepizole is unavailable intravenous ethanol (5% or 10% solution) is used. Intravenous bicarbonate is used to manage the acidosis. Hemodialysis is usually indicated because it is effective in removing both toxins and their metabolites. The usual indications for hemodialysis include osmolar gap > 10 mOsm kg H2O, serum HCO3− < 20 mEq/l, arterial pH < 7.3 and oxalate crystals in the urine (in case of ethylene glycol toxicity) [16]. Other toxic alcohols that can result in metabolic acidosis include diethylene glycol, propylene glycol, and isopropanol [93].
6. Conclusions
- The kidneys guard against the development of metabolic acidosis by reclaiming filtered HCO3− mainly in the PT and by acid excretion (NH4+ production).
- Oral alkali treatment of metabolic acidosis in CKD patients has the potential of slowing the rate of decline of renal function.
- The four major causes of AG-MA are lactic acidosis, ketoacidosis, renal failure and toxins. The two major causes of NAG-MA are proximal and distal RTAs, and GI loss of HCO3−.
- Treatment of lactic acidosis is directed at the underlying cause. The use of intravenous bicarbonate is controversial.
- The kidneys and the lungs maintain normal acid-base status in the body.
- Renal tubular acidosis is due to tubular defects resulting in failure to excrete enough H+ or reabsorb filtered HCO3−.
Conflicts of Interest
The authors declares no conflict of interest.
References
- Lee Hamm L, Nakhoul N, Hering-Smith
- Acid-base homeostasis. Clin J Am Soc Nephrol 10 (2015): 2232-2242.
- Palmer BF. Normal Acid-Base Balance. In: Feehally J, Floege J, Tonelli M, et al. (eds) Comprehensive Clinical Nephrology. Elsevier Inc (2018): 142-148.
- Mountain RD, Heffner JE, Brackett NC, Sahn S A. Acid-base disturbances in acute asthma. Chest 98 (1990): 651-655.
- Narins RG, Emmett M. Simple and mixed acid-base disorders: A practical approach. Med (United States) 59 (1980): 161-187.
- Raphael KL. Metabolic Acidosis in CKD: Core Curriculum 2019. Am J Kidney Dis 74 (2019): 263-275.
- Morris CG, Low J. Metabolic acidosis in the critically ill: Part 1. Classification and pathophysiology. Anaesthesia 63 (2008): 294-301.
- Lemann J, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol - Ren Physiol 285 (2003): F811-F832.
- Bruno CM, Valenti M. Acid-base disorders in patients with chronic obstructive pulmonary disease: A pathophysiological review. J Biomed Biotechnol 2012 (2012).
- Seifter JL. Integration of acid-base and electrolyte disorders. N Engl J Med 371 (2014): 1821-1831.
- Palmer BF. Metabolic Acidosis. In: Feehally J, Floege J, Tonelli M, et al. (eds) Comprehensive Clinical Nephrology. Elsevier Inc (2018): 149-159.
- Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9 (2014): 1627-1638.
- Quigley R, Wolf M. Renal Tubular Acidosis. In: Avner E, Harmon W, Niaudet P, et al. (eds) Pediatric Nephrology. Springer (2016): 1273-1306.
- Weiner ID, Verlander JW. Renal ammonia metabolism and transport. Compr Physiol 3 (2013): 201-220.
- Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int 71 (2007): 103-115.
- Wagner CA. Pendrin-A New Target for Diuretic Therapy?. J Am Soc Nephrol 27 (2016): 3499-3501.
- Hamm LL, DuBose TD. Disorders of Acid-Base Balance. In: Yu ASL, Chertow GM, Luyckx, VA, et al. (eds) Brenner & Rector’s The Kidney. Philadelphia: Elsevier Inc (2020): 496-536.
- Palmer BF. Approach to Fluid and Electrolyte Disorders and Acid-Base Problems. Prim Care Clin Off Pract 35 (2008): 195-213.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med 26 (1998): 1807-1810.
- Harvey AMG. Classics in clinical science: James L. Gamble and ‘gamblegrams’. Am J Med 66 (1979): 904-906.
- Goldstein MB, Bear R, Richardson RMA, Halperin M L. The urine anion gap: A clinically useful index of ammonium excretion. Am J Med Sci 292 (1986): 198-202.
- Batlle DC, Hizon M, Cohen E, Gutterman C, Gupta R. The Use of the Urinary Anion Gap in the Diagnosis of Hyperchloremic Metabolic Acidosis. N Engl J Med 318 (1988): 594-599.
- Battle D, von Riotte A, Schlueter W. Urinary Sodium in the Evaluation of Hyperchloremic Metabolic Acidosis. N Engl J Med 316 (1987): 140-144.
- Gil-Peña H, Mejía N, Santos F. Renal tubular acidosis. J Pediatr 164 (2014): 691-698.
- Dyck R F, Asthana S, Kalra J, West M L, Massey K L. A modification of the urine osmolal gap: An improved method for estimating urine ammonium. Am J Nephrol 10 (1990): 359-362.
- Berend K, De Vries APJ, Gans ROB. Physiological approach to assessment of acid-base disturbances. N Engl J Med 371 (2014): 1434-1445.
- Halperin ML, Kamel KS, Goldstein MB. Fluid, Electrolyte, and Acid-Base Physiology A Problem-Based Approach. Fourth. Philadelphia: Elsevier Inc (2010).
- Ha LY, Chiu WW, Davidson JS. Direct urine ammonium measurement: Time to discard urine anion and osmolar gaps. Ann Clin Biochem 49 (2012): 606-608.
- Emmett M. Diagnosis of Simple and Mixed Disorders. In: DuBose TD, Lee Hamm L (eds) Acid-Base and Electrolyte Disorders A Companion to Brenner & Rector’s The Kidney. Philadelphia: Elsevier Inc (2002): 41-53.
- Rudkin SE, Grogan TR, Treger RM. The Δ Anion Gap/Δ Bicarbonate Ratio in Early Lactic Acidosis: Time for Another Delta? Kidney360. Epub ahead of print (2020).
- Tinawi M. Disorders of Calcium Metabolism: Hypocalcemia and Hypercalcemia. Cureus 13 (2021): e12420.
- Tinawi M. Diagnosis and Management of Hyperkalemia. Arch Clin Biomed Res 4 (2020): 153-168.
- Fraley DSD, Adler S. Correction of hyperkalemia by bicarbonate despite constant blood pH. Kidney Int 12 (1977): 354-360.
- Katagawa K, Nagashima T, Inase N, Kanayama M, Chida M, Sasaki S, et al. Urinary ammonium measurement by the auto-analyzer method. Kidney Int 36 (1989): 291-294.
- Adrogué H J, Wilson H, Boyd 3rd A E, Suki W N, Eknoyan G. Plasma Acid-Base Patterns in Diabetic Ketoacidosis. N Engl J Med 307 (1982): 1603-1610.
- Mirela Dobre, Wei Yang, Jing Chen, Paul Drawz, Lee Hamm L, Edward Horwitz, et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 62 (2013): 670-678.
- Giacomo Garibotto, Antonella Sofia, Cristina Robaudo, Stefano Saffioti, Maria Rita Sala, Daniela Verzola, et al. Kidney protein dynamics and ammoniagenesis in humans with chronic metabolic acidosis. J Am Soc Nephrol 15 (2004): 1605-1615.
- Palmer BF, Kelepouris E, Clegg DJ. Renal Tubular Acidosis and Management Strategies: A Narrative Review. Adv Ther. Epub ahead of print (2020).
- DuBose TD, McDonald GA. Renal Tubular Acidosis. In: DuBose TD, Lee Hamm L (eds) Acid-Base and Electrolyte Disorders A Companion to Brenner & Rector’s The Kidney. Philadelphia: Elsevier Inc (2002): 189-206.
- Tinawi M, Bastani B. A case of Fanconi syndrome as a complication of treatment with a checkpoint inhibitor in a patient with hepatocellular carcinoma. J Nephropathol 9 (2020): e19.
- Tinawi M, Bastani B. Nephrotoxicity of Immune Checkpoint Inhibitors: Acute Kidney Injury and Beyond. Cureus 12 (2020): e12204.
- Ankur Sinha, Phone Oo, Muhammad U Asghar, Hira A Cheema, Sanwal S Mehta, Joshua C Leinwand, et al. Type II Renal Tubular Acidosis Secondary to Topiramate: A Review. Cureus 10 (2018): e3635.
- Rastegar A. Attending rounds: Patient with hypokalemia and metabolic acidosis. Clin J Am Soc Nephrol 6 (2011): 2516-2521.
- DeFranco P E, Haragsim L, Schmitz P G, Bastani B, Li J P. Absence of vacuolar H+-ATPase pump in the collecting duct of a patient with hypokalemic distal renal tubular acidosis and Sjogren’s syndrome. J Am Soc Nephrol 6 (1995): 295-301.
- Nasser A Dhayat, Michael W Gradwell, Ganesh Pathare, Manuel Anderegg, Lisa Schneider, David Luethi, et al. Furosemide/Fludrocortisone Test and Clinical Parameters to Diagnose Incomplete Distal Renal Tubular Acidosis in Kidney Stone Formers. Clin J Am Soc Nephrol 12 (2017): 1507-1517.
- Autumn N Harris, Richard Grimm P, Hyun-Wook Lee, Eric Delpire, Lijuan Fang, Jill W Verlander, et al. Mechanism of hyperkalemia-induced metabolic acidosis. J Am Soc Nephrol 29 (2018): 1411-1425.
- Karet F. Mechanisms in Hyperkalemic Renal Tubular Acidosis. J Am Soc Nephrol 20 (2009): 251-254.
- Juliana Menegussi, Luiza Sarmento Tatagiba, Júlia Guasti P Vianna, Antonio Carlos Seguro, Weverton Machado Luchi. A physiology-based approach to a patient with hyperkalemic renal tubular acidosis. J Bras Nefrol 40 (2018): 410-417.
- Maud Cazenave, Vincent Audard, Jean-Philippe Bertocchio, Anoosha Habibi, Stéphanie Baron, Caroline Prot-Bertoye, et al. Tubular acidification defect in adults with sickle cell disease. Clin J Am Soc Nephrol 15 (2020): 16-24.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 373 (2015): 548-559.
- Raebel MA. Hyperkalemia Associated with Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers. Cardiovasc Ther 30 (2012): e156-e166.
- Ben Salem C, Badreddine A, Fathallah N. Drug-induced hyperkalemia. Drug Saf 37 (2014): 677-692.
- Oster J, Singer I, Fishman L. Heparin-induced aldosterone suppression and hyperkalemia. Am J Med 98 (1995): 575-586.
- McCormick J, Ellison DH. Nephron Remodeling Underlies Hyperkalemia in Familial Hyperkalemic Hypertension. J Am Soc Nephrol 28 (2017): 2555-2557.
- Edwige-Ludiwyne Hubert, Raphaël Teissier, Fábio L Fernandes-Rosa, Michel Fay, Marie-Edith Rafestin-Oblin, Xavier Jeunemaitre, et al. Mineralocorticoid Receptor Mutations and a Severe Recessive Pseudohypoaldosteronism Type 1. J Am Soc Nephrol 22 (2011): 1997-2003.
- Tinawi M. Potassium Binders. Arch Intern Med Res 3 (2020): 141-145.
- Stefan R Bornstein, Bruno Allolio, Wiebke Arlt, Andreas Barthel, Andrew Don-Wauchope, Gary D Hammer, et al. Diagnosis and treatment of primary adrenal insufficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 101 (2016): 364-389.
- Olivier Moranne, Marc Froissart, Jerome Rossert, Cedric Gauci, Jean-Jacques Boffa, Jean Philippe Haymann, et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 20 (2009): 164-171.
- Widmer B, Gerhardt RE, Harrington JT, Cohen JJ. Serum Electrolyte and Acid Base Composition: The Influence of Graded Degrees of Chronic Renal Failure. Arch Intern Med 139 (1979): 1099-1102.
- Wallia R, Greenberg A, Piraino B, Mitro R, Puschett J B. Serum Electrolyte Patterns in End-Stage Renal Disease. Am J Kidney Dis 8 (1986): 98-104.
- Kraut JA. Effect of metabolic acidosis on progression of chronic kidney disease. Am J Physiol Ren Physiol 300 (2011): F828-F829.
- Wesson DE, Buysse JM, Bushinsky DA. Mechanisms of metabolic acidosis–induced kidney injury in chronic kidney disease. J Am Soc Nephrol 31 (2020): 469-482.
- Mirela Dobre, Nicholas M Pajewski, Srinivasan Beddhu, Michel Chonchol, Thomas H Hostetter, Ping Li, et al. Serum bicarbonate and cardiovascular events in hypertensive adults: results from the Systolic Blood Pressure Intervention Trial. Nephrol Dial Transpl 35 (2019): 1377-1384.
- Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24 (2009): 1232-1237.
- Goraya N, Wesson DE. Does correction of metabolic acidosis slow chronic kidney disease progression? Curr Opin Nephrol Hypertens 22 (2013): 193-197.
- Sankar D Navaneethan, Jun Shao, Jerry Buysse, David A Bushinsky. Effects of treatment of metabolic acidosis in CKD: A systematic review and meta-analysis. Clin J Am Soc Nephrol 14 (2019): 1011-1020.
- Ione de Brito-Ashurst, Mira Varagunam, Martin J Raftery, Muhammad M Yaqoob. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20 (2009): 2075-2084.
- Biagio R Di Iorio, Antonio Bellasi, Kalani L Raphael, Domenico Santoro, Filippo Aucella, Luciano Garofano, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol 32 (2019): 989-1001.
- Andrassy KM. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int 3 (2013): 1-150.
- Krieger NS, Frick KK, Bushinsky DA. Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens 13 (2004): 423-436.
- Matthew K Abramowitz, Michal L Melamed, Carolyn Bauer, Amanda C Raff, Thomas H Hostetter. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol 8 (2013): 714-720.
- Julia J Scialla, Lawrence J Appel, Brad C Astor, Edgar R Miller 3rd, Srinivasan Beddhu, Mark Woodward, et al. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol 6 (2011): 1526-1532.
- Donald E Wesson, Vandana Mathur, Navdeep Tangri, Yuri Stasiv, Dawn Parsell, Elizabeth Li, et al. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension. Lancet 394 (2019): 396-406.
- Brady C, Chemaly ER, Lohr JW, Parker MD. Veverimer: an advance in base therapy for metabolic acidosis. Ann Transl Med (2020).
- Morris CG, Low J. Metabolic acidosis in the critically ill: Part 2. Causes and treatment. Anaesthesia 63 (2008): 396-411.
- Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 371 (2014): 2309-2319.
- Hanna Schotola, Karl Toischer, Aron F Popov, André Renner, Jan D Schmitto, Jan Gummert, et al. Mild metabolic acidosis impairs the β-adrenergic response in isolated human failing myocardium. Crit Care 16 (2012): R153.
- Boris Jung, Thomas Rimmele, Charlotte Le Goff, Gérald Chanques, Philippe Corne, Olivier Jonquet, et al. Severe metabolic or mixed acidemia on intensive care unit admission: incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Crit Care 15 (2011): R238.
- Ingelfinger JR, Kamel KS, Halperin ML. Acid-base problems in diabetic ketoacidosis. N Engl J Med 372 (2015): 546-554.
- Sabatini S, Kurtzman NA. Bicarbonate therapy in severe metabolic acidosis. J Am Soc Nephrol 4 (2009): 692-695.
- Boyd JH, Walley KR. Is there a role for sodium bicarbonate in treating lactic acidosis from shock?. Curr Opin Crit Care 14 (2008): 379-383.
- Davide G A M Bianchetti, Giacomo S Amelio, Sebastiano A G Lava, Mario G Bianchetti, Giacomo D Simonetti, Carlo Agostoni, et al. D-lactic acidosis in humans: systematic literature review. Pediatr Nephrol 33 (2018): 673-681.
- Alistair Nichol, Michael Bailey, Moritoki Egi, Ville Pettila, Craig French, Edward Stachowski, et al. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care 15 (2011): R242.
- Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: An update of its etiology, pathogenesis and management. Metabolism 65 (2016): 507-521.
- Modi A, Agrawal A, Morgan F. Euglycemic Diabetic Ketoacidosis: A Review. Curr Diabetes Rev 13 (2016): 315-321.
- Anne L Peters, Elizabeth O Buschur, John B Buse, Pejman Cohan, Jamie C Diner, Irl B Hirsch. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 38 (2015): 1687-1693.
- Kamel KS, Lin SH, Cheema-Dhadli S, Marliss E B, Halperin M L. Prolonged total fasting: A feast for the integrative physiologist. Kidney Int 53 (1998): 531-539.
- Höjer J. Severe metabolic acidosis in the alcoholic: Differential diagnosis and management. Hum Exp Toxicol 15 (1996): 482-488.
- Palmer BF, Clegg DJ. Salicylate Toxicity. N Engl J Med 382 (2020): 2544-2555.
- Emmett M. Acetaminophen toxicity and 5-oxoproline (pyroglutamic acid): A tale of two cycles, one an ATP-depleting futile cycle and the other a useful cycle. Clin J Am Soc Nephrol 9 (2014): 191-200.
- Andrew Z Fenves, Haskell M Kirkpatrick 3rd, Viralkumar V Patel, Lawrence Sweetman, Michael Emmett. Increased anion gap metabolic acidosis as a result of 5-oxoproline (pyroglutamic acid): a role for acetaminophen. Clin J Am Soc Nephrol 1 (2006): 441-447.
- Faria DK, Mendes ME, Sumita NM. The measurement of serum osmolality and its application to clinical practice and laboratory: Literature review. Jornal Brasileiro de Patologia e Medicina Laboratorial (2017): 38-45.
- Vujovic P, Chirillo M, Silverthorn DU. Learning (by) osmosis: An approach to teaching osmolarity and tonicity. Adv Physiol Educ 42 (2018): 626-635.
- Kraut JA, Kurtz I. Toxic alcohol ingestions: Clinical features, Diagnosis, and management. Clin J Am Soc Nephrol 3 (2008): 208-225.