Pathogenesis and the emerging Therapy of Vitiligo
Article Information
Abdur Rahim Zar1, Abdul Malik1, Asif Mahmood2, Qais Ahmad Naseer2, Li Yumei3*
1Department of Dermatology, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu 212013, China
2Department of Microbiology, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu 212013, China
3The affiliated Hospital of Jiangsu University, Zhenjiang, Jiangsu 212001, China
*Corresponding author: Li Yumei, Affiliated Hospital of Jiangsu University Zhenjiang, Jiangsu 212001, China
Received: 16 October 2019; Accepted: 22 October 2019; Published: 08 November 2019
Citation: Abdur Rahim Zar, Abdul Malik, Asif Mahmood, Qais Ahmad Naseer, Li Yumei. Pathogenesis and the emerging Therapy of Vitiligo. Archives of Clinical and Biomedical Research 3 (2019): 361-373.
View / Download Pdf Share at FacebookAbstract
Vitiligo is a common and a disfiguring autoimmune disease, which has a negative impact on patients' self-esteem and quality of life. Pathogenesis of Vitiligo involves the interaction between defects of natural and extrinsic melanocytes, immune innate to swelling and destruction of T-cell-mediated melanocyte. Vitiligo affects about 1% of the general population. The risk of the disease was 6% for siblings and 23% for identical twins treatment is not only intended to stop the disease, but also to support repigmentation through the regeneration, proliferation and migration of melanocytes. Treatment strategies for dealing with all aspects of pathogenesis and pigmentation can be very effective, and this strategy may require a combination. Current treatments generally do not imply objective autoimmunity, and emerging treatments may use more specific methods based on a deep understanding of the pathogenesis of the disease, which can provide a good safety profile with a higher effectiveness. However, as with most medical treatments, careful patient selection and monitoring should enable us to normalize the pathogenic response of vitiligo in order to achieve the internal balance of healthy individuals.
Keywords
Vitiligo; Disease; Pathogenesis; Melanocytes
Article Details
Introduction
Vitiligo is a common and disfiguring autoimmune disease, which has a negative impact on patients' self-esteem and quality of life [1, 2]. The current treatment of vitiligo is a common non-targeted immunosuppressive agent, which can only provide moderate efficacy. Developing safe and effective therapies requires a better understanding of the pathogenesis of the disease in order to identify new therapeutic targets [3]. Vitiligo is caused by a dynamic interaction between genetic and environmental risks, which can trigger an autoimmune attack on skin melanocytes.
Pathogenesis of vitiligo
Genetics
The wider observation of vitiligo in the immediate relatives of patients with vitiligo provides early evidence for its heredity. Vitiligo affects about 1% of the general population [4]. The risk of the disease was 6% for siblings and 23% for identical twins [5]. In addition, vitiligo patients and their relatives are at increased risk of other autoimmune diseases, including autoimmune thyroiditis, type 1 diabetes mellitus, pernicious anemia and Addison's disease, suggesting that vitiligo is also an autoimmune disease [6]. These early observations were later confirmed by genome-wide association (GWA) studies, which found that many common genetic variations in vitiligo patients encode congenital (NLRP1, IFIH1, casp7, c1qtnf6, trif) and adaptive immune system components (FOXP3, BACH2, CD80, CCR6, PTPN22, IL2R, alpha GZMB, HLA class I and II) [7-9].
Oxidative Stress
There is increasing evidence that melanocytes in vitiligo patients have inherent defects, which reduce their ability to cope with cellular stress [10]. Epidermal cells, including melanocytes, are often exposed to environmental pressures, such as ultraviolet radiation and various chemicals, which can increase the production of reactive oxygen species (ROS). Although healthy melanocytes can relieve these stresses, melanocytes from patients with vitiligo seem to be more vulnerable. For example, melanocytes from the skin of peripheral vitiligo exhibit dilated endoplasmic reticulum (ER) and abnormal structure of mitochondria and melanosomes, which are characteristics of increased cellular stress. High concentrations of epidermal H2O2 and decreased level of catalase (a key enzyme that protects cells from oxidative damage) were observed in the skin of vitiligo patients [11-17].
Environment
The earliest trigger for vitiligo is not entirely clear. Several studies have shown that the combination of defects in melanocytes and exposure to specific environmental factors may play a central role in the pathogenesis of diseases. This is evident in a group of factory workers who developed vitiligo in gloves after exposure to a single benzophenone, an organic chemical [18].
Later studies confirmed that the contact history of other phenolic and catecholic chemicals found in dyes (especially hair dyes), resins/adhesives and leather was related to vitiligo [19, 20]. Melanogenesis is a multistep process in which melanocytes produce melanin. Tyrosinase is a rate-limiting enzyme in this process, which controls melanin production by oxidizing amino acid tyrosine (a natural phenol) [21]. In vitro studies have shown that chemical phenols can act as tyrosine analogues in melanocytes, leading to high levels of cellular stress. This may include increased production of reactive oxygen species (ROS) and activation of unfolded protein response (UPR), which in turn activates congenital inflammation [22, 23].
Innate Immunity
As mentioned earlier, GWA studies in vitiligo patients showed multiple susceptibility loci associated with genes that control innate immunity [7-9]. This may lead to congenital activation disorders in response to melanocyte stress by recruiting congenital populations such as natural killer cells (NK) and by producing and releasing high levels of pro-inflammatory proteins and cytokines, including heat shock proteins (HSP), IL-1beta, IL-6 and IL-8 [22-29]. Among the larger HSP molecules, inducible HSP70 (HSP70i) is unique because it can be secreted into chaperone peptides specific to the original host cells [30]. Recently, HSP70i has been shown to play an important role in the pathogenesis of vitiligo in mice by inducing inflammatory dendritic cells (DCs). These dendritic cells themselves may be cytotoxic or may carry melanocyte-specific antigens to T cells in lymphoid tissue [24,25]. This is considered to be a key interaction between innate immunity and adaptive immunity, leading to T cell mediated autoimmune destruction of melanocytes [31].
Adaptive immunity
Ultimately, cytotoxic CD8 + T cells lead to the destruction of melanocytes [32]. Cutaneous endocrine cytokines act as an early signal to help these self-activated T cells locate stress melanocytes. This may be important because the epidermis has no blood vessels, so active mechanisms are needed to help them locate melanocytes effectively [33]. Chemokines are small, secreted proteins that act as chemokines to guide T cell migration. IFN-gamma and IFN-gamma-induced chemokines (CXCL9 and CXCL10) are highly expressed in the skin and blood of vitiligo patients and in mouse models [34-36]. In addition, IFN-gamma and CXCL10 are essential for disease progression and maintenance of mouse models [34,37]. Recently, a separate study showed that serum CXCL10 levels in vitiligo patients were not only higher than those in healthy controls, but also correlated with disease activity and significantly decreased after successful treatment, suggesting that it could be used as a biomarker for monitoring disease activity and therapeutic response [39].
Emerging treatment
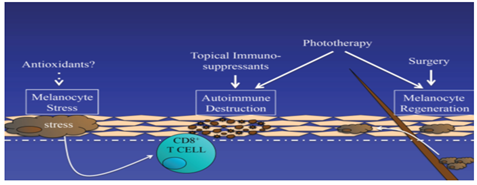
According to our current understanding of the pathogenesis of vitiligo, successful strategies for treating vitiligo should include three different approaches: reducing melanocyte stress, regulating autoimmune response and stimulating melanocyte regeneration. Existing therapies partly meet these needs, but emerging therapies may do so in a more targeted way, and combination therapies may produce better overall responses (Figure 1).
The pathogenesis of vitiligo begins with the change of melanocyte, which shows the increase of cellular stress response. This can trigger autoimmunity, destroy melanocytes, and lead to local depigmentation. Pigment re-staining requires melanocyte growth and migration, usually from hair follicles. Therefore, there are three goals to consider in the treatment of vitiligo:1) reducing melanocyte stress, 2) suppressing autoimmune targeting of melanocytes, and 3) Promote melanocyte regeneration.
Reducing melanocyte stress
The obvious decrease of catalase in epidermis of vitiligo patients and the increase of reactive oxygen species (ROS) level in diseased skin prompt people to assume that antioxidant therapy or ROS control may be an effective treatment strategy [38]. Pseudocatalase describes a treatment cream containing any amount of metal ions that converts a common reactive oxygen species, hydrogen peroxide, into water and oxygen. Early application of pseudocatalase combined with phototherapy for vitiligo seems promising [38-40]. However, they were either uncontrolled or not masked, and subsequent studies did not reproduce positive results [41-43]. It is not clear whether this strategy can be optimized or improved to develop future therapies.
Based on the antioxidant and anti-inflammatory properties of oral or local natural health products, vitamins and supplements, it has been suggested as a possible treatment [44-46]. It has been reported that the white matter polymer in plant extract improves the response of a small number of vitiligo patients to nbUVB compared with placebo [47]. A group of participants tested nbUVB with or without an "antioxidant pool" consisting of alpha-fatty acids, vitamin C, vitamin E and polyunsaturated fatty acids. They report that nbUVB combined with antioxidants is more effective for patients [48]. Greater controlled trials are needed to determine whether antioxidants are beneficial for patient management.
Regulation of autoimmunity
In the past decade, immunomodulators have made significant progress in the treatment of inflammatory skin diseases, including targeted treatment. Our latest understanding of the immune pathogenesis of vitiligo helps us identify new immune targets and develop and test new treatment methods for vitiligo.
HSP70i - group reported the role of heat shock protein HSP70i in the pathogenesis of vitiligo, suggesting that heat shock protein HSP70i is released by stress melanocytes and causes congenital inflammation in the skin [49]. Then they found that the mutant protein reduced its immunogenicity and even induced tolerance when expressed in the skin of a mouse model to prevent disease. They propose future testing, a new treatment for vitiligo [25], although the transmission of a mutant protein in the skin of patients may take some time to develop and prove safety.
IFN - gamma / CXCL10- axis is a key signaling pathway for the progression and maintenance of vitiligo. It is assumed that targeting the IFN-gamma / CXCL10 axis may be an effective therapeutic strategy [34,37]. Various antibodies and small molecule inhibitors have been developed to target components of this pathway (including IFN-gamma, CXCL10 and CXCL10 receptor CXCR3) and have been found safe in early clinical trials for the treatment of other autoimmune diseases, including psoriasis, Rheumatoid arthritis and Crohn's disease. Most trials fail to reach the end point of efficacy, possibly because interferon-gamma is not the main driving factor for these diseases. However, recent findings in patient and mouse models suggest that vitiligo is the best disease to test these research drugs [3].
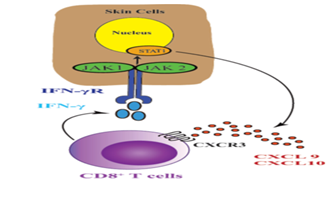
JAK-STAT signal - transduction, JAK-STAT signal is essential for the extracellular signal transduction of many cytokines including IFN-gamma to the nucleus. After ligation of cytokine receptor, Janus kinase (JAKs) phosphorylation signal transducer and transcription protein activator (STATs) are activated and target gene transcription is induced. JAK family has four members, including JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). JAK1 and JAK2 directly participate in IFN-gamma signal transduction and activate Stat1, which induces IFN-gamma-induced gene transcription including CXCL10 [50] (Figure 2).
This induces the production of CXCL9 and CXCL10, which signal through receptor CXCR3 and recruit more autologous T cells to the epidermis, leading to extensive melanocyte destruction. Targeting this cytokine pathway is a new therapeutic strategy for vitiligo.
Some small molecular JAK inhibitors with unique selectivity have been tested in patients or are under development. Interestingly, a patient with generalized vitiligo has been reported to respond to oral tofacitinib, a JAK 1/3 inhibitor approved for the treatment of moderate to severe rheumatoid arthritis [51]. Ruxolitinib, another JAK inhibitor with JAK 1/2 selectivity, has been approved by FDA for the treatment of moderate or high risk myelofibrosis and polycythemia vera [52,53]. We report a case of leukoplakia with rapid pigmentation of the face and trunk after oral administration of ruxolitinib [54]. The treatment response of these inhibitors does not seem to last long because the patient loses re-pigmentation after discontinuation of treatment [55].
Like all other immunosuppressive drugs, tofacitinib and ruxotinib may have adverse effects, including opportunistic infections and rare malignant substances. In addition, ruxotinib may income blood abnormalities including thrombocytopenia, anemia, and neutropenia [56]. Topical formulation of these drugs may provide therapeutic benefit without increasing the risk of adverse events [57]. Currently, an open label, phase 2, proof of concept clinical trial is recruiting participants to test the effectiveness of topical ruxotinib 1.5% in the treatment of vitiligo [58].
In addition to JAK inhibitors, STAT inhibitors may have similar effects. So far, seven members of the family have been identified, but only STAT1 as a homodimer participates in IFN-gamma signal transduction [50]. A previous in vitro study reported that statins inhibited STAT1 function by inhibiting HMG-CoA reductase to reduce cholesterol [59]. In addition, a vitiligo patient was reported to have improved after taking simvastatin [60]. A recent study tested systemic simvastatin in a white spot mouse model and found that it was effective in preventing and reversing disease [61]. However, we conducted a small pilot clinical trial to test that the efficacy of high dose simvastatin (80 mg per day) in patients with generalized vitiligo did not reach its primary therapeutic end point [62]. The adverse effects of limited simvastatin administration on human may be the cause of different outcomes between mice model and vitiligo patients. A study currently under way is recruiting patients to assess the benefits of Atorvastatin Combined with UVB in the treatment of active vitiligo [63]. Future studies could test local simvastatin as a method of increasing local concentration without toxicity.
Immune checkpoints - The successful application of immunotherapy in the treatment of metastatic melanoma has attracted much attention in recent years by blocking inhibitory checkpoints. Immune checkpoints are molecules that regulate T cell responses to inflammation, including cytotoxic T lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), etc. [64]. Interestingly, the response of melanoma patients to immunoassay site inhibitors is associated with the development of vitiligo [65]. It has been hypothesized that activation of these surface receptors can restore tolerance in vitiligo patients [66].
Abatacept is a fusion protein, which is composed of Fc region of immunoglobulin IgG1 and extracellular region of CTLA-4. It has been approved by FDA for the treatment of moderate to severe rheumatoid arthritis [67]. Recently, an open label, single arm, pilot study was initiated to test the efficacy of Abatacept in vitiligo patients [68]. In addition, the PD-1 ligand (PD-L1, a PD-1 agonist) is currently being developed and tested in the pre-clinical phase of inflammatory bowel disease and psoriasis [69].
Stimulation of melanocyte regeneration
α-MSH - Phototherapy is the first-line treatment for vitiligo, especially for patients with extensive diseases [1,2]. Phototherapy and combined therapy. Although the mechanism of its therapeutic effect is not fully understood, photochromism may be due to its ability to induce immunosuppression. Melanocyte stem cells can also be induced to differentiate and proliferate [70]. Alpha-melanocyte stimulating hormone (alpha-MSH) is a natural hormone that stimulates melanogenesis [71]. Alpha melanin peptide, a synthetic analogue of alpha-Melanocyte-stimulating hormone, has been approved by the European Drug Administration to alleviate photosensitivity of erythropoietic protoporphyria [72], which may also improve the efficacy of phototherapy for vitiligo [73]. Recently, we conducted a randomized, comparative multicenter trial to test the safety and efficacy of alpha-melanin tidal subcutaneous implants combined with NB-UVB in the treatment of generalized vitiligo in adults. Although side effects included nausea and excessive skin pigmentation, which led some subjects to withdraw from the trial, the combination therapy was slightly more tolerable. Compared with NB-UVB monotherapy, treatment results in faster and higher total pigmentation. This reaction is most evident in patients with dark skin [74]. It is not clear whether alpha melanopeptide has any benefit in the treatment of vitiligo.
WNT signaling - A recent study reported that the defective WNT signal of melanocytes in vitiligo patients is a way to promote the differentiation of skin melanocyte precursors. They hypothesized that this impaired signal transduction contributed to the pathogenesis of the disease, especially by inhibiting the regeneration and re-staining of melanocytes during treatment. Studies on human skin transplantation in vitro have shown that WNT activator can promote melanocyte differentiation [35]. Therapeutic WNT activation may therefore be an adjuvant therapy for vitiligo that supports melanocyte regeneration [75].
Selective sunscreen - Although it is currently the most effective treatment for vitiligo, phototherapy is still a challenge for patients. In order to receive treatment, patients usually go to a special clinic two or three times a week for a period of 1-2 years to obtain satisfactory treatment results. Although sunlight is an inexpensive alternative to phototherapy, it is difficult to monitor exposure, and non-therapeutic wavelengths of light may cause erythema and limit doses. Recently, a local formulation of 1% dimethicone has been reported to selectively block sunlight wavelengths below 300 nanometers, allowing therapeutic wavelengths to penetrate in the nbUVB range (~311-312 nanometers). A small double-blind placebo-controlled study found that the use of this cream under sunlight is safe and effective, and can induce pigmentation of skin lesions [76]. However, this needs to be confirmed in larger clinical trials, as the authors acknowledge that at certain times of the year, inadequate sunshine or physical inability to participate in public places limits their use. In addition, the cream does not block potentially harmful ultraviolet light, so this method may not be as safe as nbUVB phototherapy.
Summary
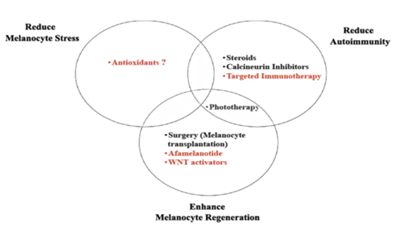
In order to better understand the pathogenesis of vitiligo, studies have shown that an optimal treatment strategy should consider three key aspects of the disease: 1) normalized melanocyte stress, 2) suppression of autoimmunity, and 3) promotion of melanocyte regeneration. Although current treatments, such as phototherapy, topical immunomodulators and surgical procedures, partially address these problems, they are carried out in a general, non-targeted manner, leading to suboptimal reactions and potential side effects.
New therapies seek to identify specific pathways through basic, transformational and clinical studies of vitiligo. To improve the efficacy and safety of patients (Figure 3). Although this is indeed a hopeful and exciting moment for vitiligo patients and their doctors, this excitement should be carefully balanced, especially as melanoma may use these same pathways to avoid immune surveillance or promote their growth. However, as with most medical treatments, careful patient selection and monitoring should enable us to normalize the pathogenic response of vitiligo in order to achieve the internal balance of healthy individuals.
Acknowledgement
We thank Abdul Malik for assistance and for comments that greatly improved the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet 386 (2015): 74-84.
- Dell'Anna MLEK, Hamzavi I, Harris J, Parsad D, Taieb A, Picardo M. Vitiligo. Nature Reviews Disease Primers 1 (2015): 1-16.
- Rashighi M, Harris JE. Interfering with the IFN-gamma/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann Transl Med 3 (2015): 343.
- Taieb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med 360 (2009): 160-169.
- Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res 16 (2003): 208-214.
- Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. J Am Acad Dermatol 74 (2016): 295-302.
- Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet 44 (2012): 676-680.
- Shen C, Gao J, Sheng Y, Dou J, Zhou F, Zheng X, et al. Genetic Susceptibility to Vitiligo: GWAS Approaches for Identifying Vitiligo Susceptibility Genes and Loci. Front Genet 7 (2016): 3.
- Spritz RA. Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J Invest Dermatol 132 (2012): 268-273.
- Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunol Rev 269 (2016): 11-25.
- Boissy RE, Liu YY, Medrano EE, Nordlund JJ. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol 97 (1991): 395-404.
- Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research 4 (1999): 91-96.
- Shalbaf M, Gibbons NC, Wood JM, Maitland DJ, Rokos H, Elwary SM, et al. Presence of epidermal allantoin further supports oxidative stress in vitiligo. Exp Dermatol 17 (2008): 761-770.
- Koca R, Armutcu F, Altinyazar HC, Gurel A. Oxidant-antioxidant enzymes and lipid peroxidation in generalized vitiligo. Clin Exp Dermatol 29 (2004): 406-409.
- Dell'Anna ML, Ottaviani M, Albanesi V, Vidolin AP, Leone G, Ferraro C, et al. Membrane lipid alterations as a possible basis for melanocyte degeneration in vitiligo. J Invest Dermatol 127 (2007): 1226-1233.
- Schallreuter KU, Wood JM, Berger J. Low catalase levels in the epidermis of patients with vitiligo. J Invest Dermatol 97 (1991): 1081-1085.
- Gibbons NC, Wood JM, Rokos H, Schallreuter KU. Computer simulation of native epidermal enzyme structures in the presence and absence of hydrogen peroxide (H2O2): potential and pitfalls. J Invest Dermatol 126 (2006): 2576-2582.
- Oliver E, Schwartz L, Warren L. Occupational leukoderma preliminary report. JAMA 113 (1939): 927-928.
- Fisher AA. Differential diagnosis of idiopathic vitiligo. Part III: Occupational leukoderma. Cutis 53 (1994): 278-280.
- Wu S, Li WQ, Cho E, Harris JE, Speizer F, Qureshi AA. Use of permanent hair dyes and risk of vitiligo in women. Pigment Cell Melanoma Res 28 (2015): 744-746.
- d'Ischia M, Wakamatsu K, Cicoira F, Di Mauro E, Garcia-Borron JC, Commo S, et al. Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell Melanoma Res 28 (2015): 520-544.
- Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol 132 (2012): 2601-2609.
- van den Boorn JG, Picavet DI, van Swieten PF, van Veen HA, Konijnenberg D, van Veelen PA, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest Dermatol 131 (2011): 1240-1251.
- Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol 124 (2005): 798-806.
- Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Science translational medicine 5 (2013): 174ra28.
- Levandowski CB, Mailloux CM, Ferrara TM, Gowan K, Ben S, Jin Y, et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. Proc Natl Acad Sci 110 (2013): 2952-2956.
- Yu R, Broady R, Huang Y, Wang Y, Yu J, Gao M, et al. Transcriptome analysis reveals markers of aberrantly activated innate immunity in vitiligo lesional and non-lesional skin. PLoS One 7 (2012): e51040.
- van den Boorn JG, Jakobs C, Hagen C, Renn M, Luiten RM, Melief CJ, et al. Inflammasome-Dependent Induction of Adaptive NK Cell Memory. Immunity 44 (2016): 1406-1421.
- Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Current opinion in immunology 25 (2013): 676-682.
- Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, et al. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008; 180(6):4299-4307.
- Mosenson JA, Eby JM, Hernandez C, Le Poole IC. A central role for inducible heat-shock protein 70 in autoimmune vitiligo. Exp Dermatol 22 (2013): 566-569.
- van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 129 (2009): 2220-2232.
- Rork JF, Rashighi M, Harris JE. Understanding autoimmunity of vitiligo and alopecia areata. Curr Opin Pediatr 2016
- Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Science translational medicine 6 (2014).
- Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, et al. Transcriptional Analysis of Vitiligo Skin Reveals the Alteration of WNT Pathway: A Promising Target for Repigmenting Vitiligo Patients. J Invest Dermatol 135 (2015): 3105-3114.
- Wang X, Wang Q, Wu J, Jiang M, Chen L, Zhang C, et al. Increased Expression of CXCR3 and its Ligands in Vitiligo Patients and CXCL10 as a Potential Clinical Marker for Vitiligo. Br J Dermatol 2016.
- Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol 132 (2012): 1869-1876.
- Schallreuter KU, Wood JM, Lemke KR, Levenig C. Treatment of vitiligo with a topical application of pseudocatalase and calcium in combination with short-term UVB exposure: a case study on 33 patients. Dermatology 190 (1995): 223-229.
- Schallreuter KU, Moore J, Behrens-Williams S, Panske A, Harari M. Rapid initiation of repigmentation in vitiligo with Dead Sea climatotherapy in combination with pseudocatalase (PC-KUS). Int J Dermatol 41 (2002): 482-487.
- Schallreuter KU, Kruger C, Wurfel BA, Panske A, Wood JM. From basic research to the bedside: efficacy of topical treatment with pseudocatalase PC-KUS in 71 children with vitiligo. Int J Dermatol 47 (2008): 743-753.
- Patel DC, Evans AV, Hawk JL. Topical pseudocatalase mousse and narrowband UVB phototherapy is not effective for vitiligo: an open, single-centre study. Clin Exp Dermatol 27 (2002): 641-644.
- Bakis-Petsoglou S, Le Guay JL, Wittal R. A randomized, double-blinded, placebo-controlled trial of pseudocatalase cream and narrowband ultraviolet B in the treatment of vitiligo. Br J Dermatol 161 (2009): 910-917.
- Gawkrodger DJ. Pseudocatalase and narrowband ultraviolet B for vitiligo: clearing the picture. Br J Dermatol 161 (2009): 721-722.
- Cohen BE, Elbuluk N, Mu EW, Orlow SJ. Alternative Systemic Treatments for Vitiligo: A Review. Am J Clin Dermatol 16 (2015): 463-474.
- Szczurko O, Shear N, Taddio A, Boon H. Ginkgo biloba for the treatment of vitilgo vulgaris: an open label pilot clinical trial. BMC Complement Altern Med 11 (2011): 21.
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol 28 (2003): 285-287.
- Middelkamp-Hup MA, Bos JD, Rius-Diaz F, Gonzalez S, Westerhof W. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol 21 (2007): 942-950.
- Dell'Anna ML, Mastrofrancesco A, Sala R, Venturini M, Ottaviani M, Vidolin AP, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol 32 (2003): 631-636.
- Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res 25 (2012): 88-98.
- Villarino AV, Kanno Y, Ferdinand JR, O'Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol 194 (2015): 21-27.
- Craiglow BG, King BA. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol 151 (2015): 1110-1112.
- Mesa RA, Yasothan U, Kirkpatrick P. Ruxolitinib. Nat Rev Drug Discov 11 (2012): 103-104.
- Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 372 (2015): 426-435.
- Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol 74 (2016): 370-371.
- Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol 2015.
- Galli S, McLornan D, Harrison C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert Opin Drug Saf 13 (2014): 967-976.
- Craiglow BG, Tavares D, King BA. Topical Ruxolitinib for the Treatment of Alopecia Universalis. JAMA Dermatol 152 (2016): 490-491.
- Tufts Medical Center. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); Topical Ruxolitinib for the Treatment of Vitiligo. Available from: https://clinicaltrials.gov/ct2/show/NCT02809976 NLM Identifier: NCT02809976 [2000-
- Zhao Y, Gartner U, Smith FJ, McLean WH. Statins downregulate K6a promoter activity: a possible therapeutic avenue for pachyonychia congenita. J Invest Dermatol 131 (2011): 1045-1052.
- Noel M, Gagne C, Bergeron J, Jobin J, Poirier P. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis 3 (2004): 7.
- Agarwal P, Rashighi M, Essien KI, Richmond JM, Randall L, Pazoki-Toroudi H, et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol 135 (2015): 1080-1088.
- Vanderweil SG, Amano S, Ko W, Richmond J, Kelley M, Makredes Senna M, et al. A small double-blind, placebo-controlled, phase-II, proof-of-concept clinical trial to evaluate oral simvastatin as a treatment for vitiligo. J Am Acad Dermatol 2016.
- Centre Hospitalier Universitaire de Nice. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); Atorvastatin in Active Vitiligo. Available from: https://clinicaltrials.gov/ct2/show/NCT02432534 NLM Identifier: NCT02432534 [2000- [cited 2016 Jan 19]]
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 37 (2010): 430-439.
- Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol 72 (2015): 221-236.
- RS, N VG. Targeting CTLA-4, PD-L1 and IDO to modulate immune responses in vitiligo. Exp Dermatol 2016
- Moreland L, Bate G, Kirkpatrick P. Abatacept. Nat Rev Drug Discov 5 (2006): 185-186.
- Brigham and Women's Hospital. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); Open-label Pilot Study of Abatacept for the Treatment of Vitiligo. Available from: https://clinicaltrials.gov/ct2/show/NCT02281058 NLM Identifier: NCT02281058. [2000-
- com [Internet]. [[cited 2016 June 30]] Available from: http://www.genexine.com/m31.php.
- Bulat V, Situm M, Dediol I, Ljubicic I, Bradic L. The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Coll Antropol 35 (2011): 147-151.
- Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol 88 (2013): 76-83.
- Fabrikant J, Touloei K, Brown SM. A review and update on melanocyte stimulating hormone therapy: afamelanotide. J Drugs Dermatol 12 (2013): 775-779.
- Grimes PE, Hamzavi I, Lebwohl M, Ortonne JP, Lim HW. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol 149 (201): 68-73.
- Lim HW, Grimes PE, Agbai O, Hamzavi I, Henderson M, Haddican M, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol 151 (2015): 42-50.
- Harris JE. Melanocyte Regeneration in Vitiligo Requires WNT beneath their Wings. J Invest Dermatol 135 (2015): 2921-2923.
- Goren A, Salafia A, McCoy J, Keene S, Lotti T. Novel topical cream delivers safe and effective sunlight therapy for vitiligo by selectively filtering damaging ultraviolet radiation. Dermatol Ther 27 (2014): 195-197.