Pangenome Analysis of SARS-Cov2 Strains to Identify Potential Vaccine Targets by Reverse Vaccinology
Article Information
Muhammad Haseeb*, Afreenish Amir, Aamer Ikram
National Institutes of Health Islamabad, Pakistan
*Corresponding author: Muhammad Haseeb Tariq, National Institute of Health, Islamabad, Pakistan.
Received: 5 November 2022; Accepted: 14 November 2022; Published: 09 December 2022
Citation:
Haseeb M, Amir A, Ikram A. Pangenome Analysis of SARS-Cov2 Strains to Identify Potential Vaccine Targets by Reverse Vaccinology. Journal of Bioinformatics and Systems Biology 5 (2022): 144-157.
View / Download Pdf Share at FacebookAbstract
Background: Coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) leads to respiratory failure and obstructive alveolar damage, which may be fatal in immunocompromised individuals. COVID19 pandemic has badly affected every part of world, and still situation in world is getting worse with emergence of novel variants. The aim of our study is to explore the genome of SARS-CoV2 followed by in silico reverse vaccinology analysis. This will help to identify the most putative vaccine candidate against the virus in robust manner and enables cost effective development of vaccines compared with traditional strategies.
Methods: The genomic sequencing data is retrieved from NCBI (Reference Sequence Number NC_045512.2). The sequences are explored through comparative genomics approaches by GENOMICS to find out the core genome. Comprehensive set of proteins obtained was employed in computational vaccinology approaches for prediction of best possible B and T cell epitopes through ABCpred and IEDB Analysis Resource, respectively. The multi-epitopes were further tested against human toll like receptor and cloned in E. coli plasmid vector in silico.
Findings: The designed Multiepitope Subunit Vaccine was non- allergenic, antigenic (0.6543), and non-toxic, significant connections with the Human Leukocyte Antigen (HLA) binding alleles, and collective global population coverage of 84.38%. It has 276 amino acids, consisting of an adjuvant with the aid of EAAAK linker, AAY linkers used to join the 4 CTL epitopes, GPGPG linkers used to join the 3 HTL epitopes and KK linkers used to join the 7 B-cell epitopes. MESV docking with human pathogenic toll-like receptors-3 (TLR3) exhibited a stable and high binding affinity. An in-silico codon optimization approach was used in the codon system of E. coli (
Keywords
Covid-19, SARS Cov-2, Pangenome analysis, Reverse vaccinology
Covid-19 articles; SARS Cov-2 articles; Pangenome analysis articles; Reverse vaccinology articles
Reverse vaccinology articles Reverse vaccinology Research articles Reverse vaccinology review articles Reverse vaccinology PubMed articles Reverse vaccinology PubMed Central articles Reverse vaccinology 2023 articles Reverse vaccinology 2024 articles Reverse vaccinology Scopus articles Reverse vaccinology impact factor journals Reverse vaccinology Scopus journals Reverse vaccinology PubMed journals Reverse vaccinology medical journals Reverse vaccinology free journals Reverse vaccinology best journals Reverse vaccinology top journals Reverse vaccinology free medical journals Reverse vaccinology famous journals Reverse vaccinology Google Scholar indexed journals Pangenome analysis articles Pangenome analysis Research articles Pangenome analysis review articles Pangenome analysis PubMed articles Pangenome analysis PubMed Central articles Pangenome analysis 2023 articles Pangenome analysis 2024 articles Pangenome analysis Scopus articles Pangenome analysis impact factor journals Pangenome analysis Scopus journals Pangenome analysis PubMed journals Pangenome analysis medical journals Pangenome analysis free journals Pangenome analysis best journals Pangenome analysis top journals Pangenome analysis free medical journals Pangenome analysis famous journals Pangenome analysis Google Scholar indexed journals SARS Cov-2 articles SARS Cov-2 Research articles SARS Cov-2 review articles SARS Cov-2 PubMed articles SARS Cov-2 PubMed Central articles SARS Cov-2 2023 articles SARS Cov-2 2024 articles SARS Cov-2 Scopus articles SARS Cov-2 impact factor journals SARS Cov-2 Scopus journals SARS Cov-2 PubMed journals SARS Cov-2 medical journals SARS Cov-2 free journals SARS Cov-2 best journals SARS Cov-2 top journals SARS Cov-2 free medical journals SARS Cov-2 famous journals SARS Cov-2 Google Scholar indexed journals Covid-19 articles Covid-19 Research articles Covid-19 review articles Covid-19 PubMed articles Covid-19 PubMed Central articles Covid-19 2023 articles Covid-19 2024 articles Covid-19 Scopus articles Covid-19 impact factor journals Covid-19 Scopus journals Covid-19 PubMed journals Covid-19 medical journals Covid-19 free journals Covid-19 best journals Covid-19 top journals Covid-19 free medical journals Covid-19 famous journals Covid-19 Google Scholar indexed journals MERS-CoV articles MERS-CoV Research articles MERS-CoV review articles MERS-CoV PubMed articles MERS-CoV PubMed Central articles MERS-CoV 2023 articles MERS-CoV 2024 articles MERS-CoV Scopus articles MERS-CoV impact factor journals MERS-CoV Scopus journals MERS-CoV PubMed journals MERS-CoV medical journals MERS-CoV free journals MERS-CoV best journals MERS-CoV top journals MERS-CoV free medical journals MERS-CoV famous journals MERS-CoV Google Scholar indexed journals microbiology articles microbiology Research articles microbiology review articles microbiology PubMed articles microbiology PubMed Central articles microbiology 2023 articles microbiology 2024 articles microbiology Scopus articles microbiology impact factor journals microbiology Scopus journals microbiology PubMed journals microbiology medical journals microbiology free journals microbiology best journals microbiology top journals microbiology free medical journals microbiology famous journals microbiology Google Scholar indexed journals ACE2 articles ACE2 Research articles ACE2 review articles ACE2 PubMed articles ACE2 PubMed Central articles ACE2 2023 articles ACE2 2024 articles ACE2 Scopus articles ACE2 impact factor journals ACE2 Scopus journals ACE2 PubMed journals ACE2 medical journals ACE2 free journals ACE2 best journals ACE2 top journals ACE2 free medical journals ACE2 famous journals ACE2 Google Scholar indexed journals HTL epitopes articles HTL epitopes Research articles HTL epitopes review articles HTL epitopes PubMed articles HTL epitopes PubMed Central articles HTL epitopes 2023 articles HTL epitopes 2024 articles HTL epitopes Scopus articles HTL epitopes impact factor journals HTL epitopes Scopus journals HTL epitopes PubMed journals HTL epitopes medical journals HTL epitopes free journals HTL epitopes best journals HTL epitopes top journals HTL epitopes free medical journals HTL epitopes famous journals HTL epitopes Google Scholar indexed journals MESV articles MESV Research articles MESV review articles MESV PubMed articles MESV PubMed Central articles MESV 2023 articles MESV 2024 articles MESV Scopus articles MESV impact factor journals MESV Scopus journals MESV PubMed journals MESV medical journals MESV free journals MESV best journals MESV top journals MESV free medical journals MESV famous journals MESV Google Scholar indexed journals
Article Details
1. Introduction
In China at the Wuhan city, the outbreak of an unknown cause of pneumonia in December 2019 emerged and later on by WHO in February 2020, it was designated as a pandemic Corona virus disease (COVID-19) [47]. On basis of genetic properties, the coronavirinae family contains four gene Alpha, Beta, Gamma and Delta coronavirus. In the past twenty years the two beta coronaviruses, SARS-CoV and MERS-CoV have shown epidemic, more than 10,000 combine cases has been shown by these two.[17]
The terminology “Reverse Vaccinology” suggests a whole change of act and direction in the study of vaccines. Whole genome sequencing reformed biology including microbiology.
Especially, for the development of vaccine antigens through computer data base which is very sensitive while in comparison to laboratory-based assumption driven evaluation of microbes to classify elements that could display protective immunity.[26]
For identification of vaccine, computational techniques are used for reverse vaccinology. For advance experimentation, modification is crucial for their ideal use. These computational techniques help to predict antigens that are almost similar to provoke defensive responses but also describe complete detailed antigen regions and its epitopes. [40]
S protein plays an important role in facilitating entry of the virus as it is most prominent and superficial protein of the coronaviruses. Previously MERS and SARS vaccine production, the S protein, and its subunit S1 have been regularly used as vaccine antigens because of their properties to persuade neutralizing antibodies which stop infection and prevent entry into host cell. But the present coronavirus vaccines (S-protein) may have difficulties in absence of persuading full protection and likely safety issues.
Currently the vaccines of MERS/SARS were described to induce incomplete protection and neutralizing antibodies in animal models. However, it is preferred to induce sterile immunity and complete protection. Furthermore, it must be strong believed that multiple immune responses, which comprises humoral, cell-mediated, or humoral are dependable for corelates of protection than antibody titers alone. In animal models both adenovirus-based recombinant vector vaccines and whole virus vaccines expressing nucleocapsid or spike protein produced neutralizing antibody response however did not deliver fully protection. Safety is major concern for us as the effectiveness and safety of the vaccination plans have not been fully verified in clinical trials of humans , so novel approaches are required to increase the safety and efficacy of COVID-19 vaccine development. [28]
COVID-19 varies from earlier strains having numerous hazardous resides on Corona Virus receptor-binding region (especially Gln493) which deliver valuable communication with ACE2 human receptors. The change in closeness perhaps clarifies why this virus is more transmissible than other viruses. Main idea within all vaccinations is capability of vaccine to start immune response in better and exponential mode than pathogen itself. While conventional laboratory vaccines that vary on biological tests persuaded protective and neutralizing reactions in immunized animals. However, it may be hypersensitive, time consuming, costly, and foremost important involves in vitro culture of infective viruses leading us to important safety issues. Thus, a cost effective, antiallergic and highly efficient vaccine should be made for future. [1]
The aim of our study is to
- Target SARS-CoV2 by reverse vaccinology.
- The approach will identify the most putative vaccine candidates from many antigens and enables cost effective development of vaccines compared with traditional strategies.
Figure 1: Development of the vaccines by reverse vaccinology [18].
2. Materials and Methodology
The overall workflow used to develop Multiepitope vaccine against SARS-CoV2 (Table 1).
|
1 |
Viral Strain select from NCBI |
|
2 |
Retrieval of protein sequence |
|
3 |
Antigen protein screening and structure analysis |
|
4 |
Identification of B-Cell Epitopes |
|
Predicting best possible B cells epitopes using IEDB tools |
|
|
5 |
Prediction of T-Cell Epitopes |
|
Trans membrane topology prediction and antigenicity analysis |
|
|
Allergenicity assessment and population coverage analysis of proposed epitopes |
|
|
Analysis of MHC restricted alleles |
|
|
Prediction of 3d Structure of the epitopes |
|
|
Molecular docking analysis within top epitopes |
|
|
6 |
Vaccine production using proposed epitopes, adjuvants, and appropriate linkers |
|
7 |
Solubility assessment, Antigenicity and Hypersensitivity of vaccine construct. |
|
8 |
Physiochemical characterization and secondary structure analysis. |
|
9 |
Validation, prediction, and enhancement of Tertiary structure. |
|
10 |
Protein- protein docking and molecular dynamic reproduction |
|
11 |
In-silico cloning |
Table 1: Workflow used to develop Multiepitope vaccine against SARS-CoV2.
2.1 Viral strain selection and Retrieval of protein sequence:
Firstly, proteome of SARS-CoV-2 virus were extracted from GenBank of National Center for Biotechnology Information (www.ncbi.nlm.nih.gov) and for further analysis sequences of amino acids were obtained in FASTA format. The target proteins include Surface glycoprotein, Membrane Glycoprotein, Envelop Protein, Nucleocapsid and Open Read Fragments. The chemical and physical properties of selected protein were verified by Expassy Protparam tool (https://web.expasy.org/protparam/). Only stable stability profiling proteins were further analyzed and remaining were extracted out [11].
2.2 Antigen protein screening and structure analysis
The Vaxijen 2.0 (http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html) software was used for protein antigenicity. For secondary structure prediction of proteins SOPMA tool (http://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html) was used. The tertiary structures details of selected proteins were predicted by using swiss (https://swissmodel.expasy.org/interactive) and Ramachandran plot (https://swift.cmbi.umcn.nl/servers/html/ramaplot.html) [43] and errat results were checked through SAVES v6.0 (SAVESv6.0 - Structure Validation Server (ucla.edu) for protein structures verification.
2.3 Identification of B-cell epitopes
B-cell epitopes which were highly antigenic and 100% conserved in all sequences of proteins were screened out and verified by ABCpred (http://crdd.osdd.net/raghava/abcpred/) [14] then their Antigenicity was checked through Vixen 2 and allergenicity was checked through AllerTOP v. 2.0 (https://www.ddg-pharmfac.net/AllerTOP/). Default parameterswereused (maximum distance: 6 A°; minimum score: 0.5). The conformational B-cell epitopes of the proposed MESV were estimated using the Ellipro tool (http://tools.iedb.org/ellipro/) given by IEDB-AR v.2.22. To anticipate epitopes, it relies at the residual protrusion index (PI), protein structure, and neighbor residue clustering [35].
2.4 Prediction of T-Cell Epitopes
IEDB Analysis Resource (http://tools.iedb.org/mhci/) was used for the prediction of T-cell HLA class I. This server predicts peptide binders to MHC molecules from protein sequences using the MHC-I binding prediction, NetMHCpan EL 4.1. [34].Prediction of T-cell HLA class 2 was done through IEDB Analysis Resource (http://tools.iedb.org/mhcii/). A seven-allele HLA reference set was chosen: HLA-DRB1*03:01, HLA-DRB1*07:01, HLA-DRB1*15:01, HLA-DRB3*01:01, HLA-DRB3*02:02, HLA-DRB4*01:01, HLA-DRB5*01:01. HLA-DRB1*03:01, HLA-DRB1*07:01, HLA-DRB1*15:01, HLA-DR Using the IEDB Recommended, this service predicts peptide binders to MHC molecules from protein sequence [42].
AlgPred server was used for allergenicity calculation, Non-Overlapping HTL epitopes were selected manually, Toxicity checked by ToxinPred server, Antigenicity by Vaxijen server, Interferon Gamma including ability by INF epitope server. The immunogenicity of HLA Class 1 and 2 were checked through Class I Immunogenicity (http://tools.iedb.org/immunogenicity/).
2.5 Predict epitopes digesting enzymes
To predict epitopes digesting enzymes of epitopes Protein Digest server (http://db.systemsbiology.net:8080/proteomicsToolkit/proteinDigest.html) was used and to check toxicity of epitopes Toxin Pred (http://crdd.osdd.net/raghava/toxinpred/) was used. This tool was used to identify highly toxic or non-toxic peptides from peptides. It also predicts their toxicity along with all the important physio-chemical properties like hydrophobicity, charge pI etc. of peptides. And Toxic epitopes were excluded for further analysis. [14].
2.6 Population coverage analysis of proposed epitopes
For population coverage, the Population Coverage server (http://tools.iedb.org/population/) was utilized. T lymphocytes identify a complex formed by microbe epitope and a particular Major Histocompatibility Complex (MHC) molecule. Only individuals who have an MHC molecule able of binding that epitope will have a response to it. Denominated MHC limitation of T cell responses is the term given to this phenomenon. Human MHC (HLA) molecules are very polymorphic, with over a thousand known distinct alleles. Selecting several peptides with varying HLA binding specificities will allow peptide-based vaccinations or diagnostics to cover a larger patient community. The fact that various HLA types are expressed at drastically varying frequencies in various ethnicities further complicates the problem of population coverage in respect to MHC polymorphism. As a result, if not carefully considered, a vaccination or diagnostic might be developed with ethnically bias population coverage.
2.7 Evaluation of Multi-Epitope-Based Subunit Vaccine (MESV)
To develop a subunit vaccine, epitopes that are (a) highly antigenic, (b) immunogenic, (c) non-allergic, (d) non-toxin, and (e) with large population coverage are typically chosen. As a result, only those epitopes were chosen for further construction of MESV using the above criteria. To enhance the immunological response, an adjuvant added to the first Cytotoxic T Lymphocytes (CTL) epitope using the EAAAK linker. After confirming their interaction compatibility, further epitopes were connected using AAY, GPGPG, and KK linkers to retain their separate immunogenic activity. Because it is a 45 amino-acid-long peptide that works as both an immunomodulation and as well as antimicrobial agent, β-defensin was adopted as an adjuvant in this study [16].
First, using default parameters, Blastp analysis was used to check that predicted MESV sequence is not similar to the proteome of Homo sapiens [24]. Non-homologous proteins are those that have less than 37% homology. The Protparam tool was used to access the physiochemical characteristics of the designedMESV [11].
Depending on the amino acid assumptions used in the pk, Protparam estimates several physiochemical characteristics such as half-life, theoretical isoelectric point [pI], instability index, grand average hydropathy, and aliphatic index [6]. The AllerTOP v.2.0 server was used to determine the allergenicity of the MESV construct. The secondary structure of the MESV construct was examined by using the PSIPRED workbench. [24]. The alpha helices, extended chain, degree of beta folds, and random coil features of the vaccine were also analyzed in this test.
Because the proposed MESV comprised a sequence of epitopes and no relevant template was available, MESV's 3D structure was anticipated using the CABS fold server's de novo modelling approach (http://biocomp.chem.uw.edu.pl/CABSfold/) [8]. The CABS modelling technique, which merges with a multi-scale modelling pipeline with exchange replica Monte Carlo scheme, is used to build this server. A galaxy refine server was used to modify the predicted MESV 3D structure [15]. The RAMPAGE server (http://mordred.bioc.cam.ac.uk/rapper/rampage.php) was used to analyze the Ramachandran plot http://mordred.bioc.cam.ac.uk/~rapper/rampage.php [23], The PROSA web server was to check the refinement of the MESV structure, followed by analysis of structural validation [46].
The MESV structure's prediction of unbounded interactions was also evaluated using the ERRAT server (https://servicesn.mbi.ucla.edu/ERRAT/) [31].
2.8 Molecular docking of MESV with human immune receptors
The interaction between the antigenic molecule and the immune receptor molecule is critical for the proper evocation of immune response. The interaction between the MESV construct and human immune receptors was evaluated using molecular docking. TLR3 (Toll-like Receptors-3) has been studied extensively, and research have revealed that it plays a critical role in the generation of antiviral immune responses. The MESV docking with TLR3 was conducted using GRAMM-X (http://vakser.compbio.ku.edu/resources/gramm/grammx/) (PDB ID: 1ZIW) for visualization pymol was used to seethe docked complexes[36]. Furthermore, a web servercalled PDBsum (http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=index.html) was used to construct a typical sketch of interactions among docked proteins. It analyses docked molecules' amongprotein-protein interactions [21].
2.9 Immunogenicity evaluation of the vaccine construct
To validate the immunological responses of the constructed MESV, an in silico immune simulation was performed using the C-ImmSim 10.1 server (http://150.146.2.1/C-IMMSIM/index.php?page=0). The three primary components of the functional mammalian system (thymus, lymph node, and bone marrow) are all simulated in C-ImmSim [33]. The input parameters for the immune simulations are as follows: volume (10), HLA (A0101, A0101, B0702, B0702, DRB1_0101, DRB1_0101), random seed (12345), number of steps (100), number of injections set to 1. The remaining settings were presumed to be default.
2.10 In silico cloning and codon optimization
If the use of codon is different in both organisms, codon optimization is a way to enhance the translation efficacy of foreign genes in the host. After a comprehensive review of MESV features and immunological response, codon optimization and in silico cloning were carried out. The java codon adaptation tool (http://www.jcat.de/) [4] was used for MESV codon optimization to make this tool compatible with the extensively used prokaryotic expression system, E. coli K12 [39]. The other options were chosen to avoid: (i) rho-independent transcription termination, (ii) prokaryote ribosome binding sites, and (iii) restriction enzyme cleavage sites. The GC (guanine and cytosine) contents, as well as the Codon Adaptation Index (CAI) [38] were analyzed. Sticky ends of HindIII and BamHI restriction sites were attached to the start/N terminal and end/C terminal of the modified MESV sequence, respectively, to enable restriction and cloning. To assure in vitro expression, the modified nucleotide sequence of MESV was also cloned into the E. coli pET30a (+) vector using the SnapGene tool (https://www.snapgene.com/).Transcriptome data set was obtained from DDBJ SRA (DRA000400) [6]. In this entry, time course total RNA sampling during reprogramming of leaf cells of the Moss Physcomitrella patens (0, 1, 3, 6, 12 and 24 hours). Each sample has there biological replicates. We mapped transcriptome sequence data using bowtie 1.1.2 [48] since they used SOLiD sequencer. Information entropy was calculated from all count data as previously described [44].We compared culture time and information entropy of transcriptome data.
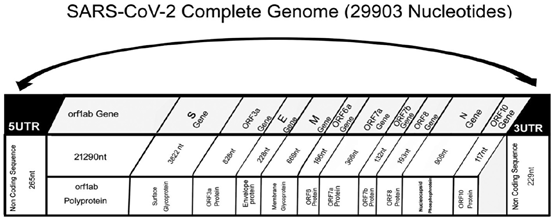
Figure 2: SARS-CoV-2 Complete Genome [19].
3. Results
Firstly, proteome of SARS-CoV-2 virus was taken from GenBank of NCBI (www.ncbi.nlm.nih.gov) and for further analysis sequences of amino acids were obtained in FASTA format. The target proteins include Surface glycoprotein, Membrane Glycoprotein, Envelop Protein, Nucleocapsid. The chemical and physical properties of selected protein was verified by Expassy Protparam tool (https://web.expasy.org/protparam/) as shown in Table 2 Only stable stability profiling proteins were further analyzed and remaining were extracted out. [12].
|
Proteins |
Molecular Weight |
Theoretical pI |
Instability index |
Stability Profiling |
Aliphatic Index |
|
Surface |
141120 |
6.32 |
32.86 |
Stable |
84.67 |
|
Membrane |
25146.6 |
9.51 |
39.14 |
Stable |
120.86 |
|
Envelop |
8365.04 |
8.57 |
36.68 |
Stable |
144 |
|
Nucleocapsid |
45696.8 |
10.09 |
55.81 |
Unstable |
52.53 |
|
ORF1A |
490043 |
6.02 |
35.02 |
Stable |
89.16 |
|
ORF1AB |
794128 |
6.31 |
33.35 |
Stable |
87.03 |
|
ORF3A |
31122.9 |
5.55 |
32.96 |
Stable |
103.42 |
|
ORF6 |
7272.54 |
4.6 |
31.16 |
Stable |
130.98 |
|
ORF7A |
13744.2 |
8.23 |
48.66 |
Unstable |
100.74 |
|
ORF7B |
5180.27 |
4.17 |
50.96 |
Unstable |
156.51 |
|
ORF8 |
13831 |
5.42 |
45.79 |
Unstable |
97.36 |
|
ORF10 |
4449.23 |
7.93 |
16.06 |
Stable |
107.63 |
Table 2: Physiochemical properties of the SARS-COV-2 proteins.
The Vaxijen 2.0 (http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html) software was used for protein antigenicity, Surface protein, ORF1A, ORF1AB, ORF3A proteins were excluded due to low antigenic values less than 0.5 as shown in below Table 3.
|
Proteins |
Antigen Prediction |
|
Surface |
0.463 |
|
Membrane |
0.51 |
|
Envelop |
0.6025 |
|
ORF1A |
0.4794 |
|
ORF1AB |
0.4625 |
|
ORF3A |
0.4945 |
|
ORF6 |
0.6131 |
|
ORF10 |
0.7185 |
Table 3: Showing that proteins were excluded due to low antigenic values less than 0.5.
For secondary structure prediction of proteins SOPMA tool (http://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html) was used as shown in Table 4. [7].
|
Proteins |
Sequence Length |
Alpha Helix |
Beta Turn |
Random Coil |
Extended Strand |
|
Membrane |
222 |
0.3486 |
0.0676 |
0.3739 |
0.2117 |
|
Envelop |
75 |
0.453 |
0.0933 |
0.2 |
0.2667 |
|
ORF6 |
61 |
0.7049 |
0.082 |
0.1148 |
0.0984 |
|
ORF10 |
38 |
0.2895 |
0.0526 |
0.2895 |
0.3684 |
Table 4: Secondary structure of COV-2 proteins.
The tertiary structures details of selected proteins were predicted by using swiss (https://swissmodel.expasy.org/interactive) and Ramcharan plot (https://swift.cmbi.umcn.nl/servers/html/ramaplot.html) as shown in in Table 5. [43] and errat results were checked through SAVES v6.0 (SAVESv6.0 - Structure Validation Server (ucla.edu)). ORF10 was excluded due to non-homologous structure and no such template was found.
|
Proteins. |
Tool used. for Modeling |
Template. |
Ramachandran. plot. |
Errat Results. |
||
|
Favored. Region |
Allowed. Region. |
Disallowed Region |
Quality. Factor |
|||
|
Envelop |
Swiss model. |
2mm4.1.A |
0.905 |
5.8%. |
0.037 |
68 |
|
Membrane |
Swiss model. |
6xdc.1.A |
0.98 |
2.0%. |
0 |
98.78. |
|
Orf6. |
Swiss model. |
5vyj.1 |
0.971 |
2.9%. |
0 |
100 |
Table 5: 3D structural details of COV-2 proteins.
B-cell epitopes which are screened out are highly antigenic and 100% conserved in all sequences of proteins were verified by ABCpred (http://crdd.osdd.net/raghava/abcpred/) then their Antigenicity was checked through Vixen 2 and allergenicity was checked through AllerTOP v. 2.0 (https://www.ddg-pharmfac.net/AllerTOP/) those epitopes were not selected further which were probably non-antigen and allergen and non-overlapping. Furthermore total 8 conformational linear epitopes of all the proteins are in shown in Table 6.
|
Proteins |
Sequence |
Position |
Epitope length |
Antigenicity |
Allergenicity |
|
Envelop Protein |
TLAILTALRLCAYCCN |
30-45 |
16 |
0.6628 |
NON-ALLERGEN |
|
Membrane Glycoprotein |
RSMWSFNPETNILLNV |
107-122 |
16 |
0.4451 |
NON-ALLERGEN |
|
RINWITGGIAIAMACL |
72-87 |
16 |
1.2392 |
NON-ALLERGEN |
|
|
PKEITVATSRTLSYYK |
165-180 |
16 |
0.5935 |
NON-ALLERGEN |
|
|
GIAIAMACLVGLMWLS |
79-94 |
16 |
0.9132 |
NON-ALLERGEN |
|
|
LVIGFLFLTWICLLQF |
22-37 |
16 |
0.9132 |
NON-ALLERGEN |
|
|
ORF6 Protein |
KVSIWNLDYIINLIIK |
23-38 |
16 |
0.5428 |
NON-ALLERGEN |
|
IKNLSKSLTENKYSQL |
37-52 |
16 |
0.4632 |
NON-ALLERGEN |
Table 6: Predicted epitopes of B-cell of Envelop, Membrane and ORF6 proteins.
3.1 T-Cell epitopes
Prediction of T-cell HLA class I was done through IEDB Analysis Resource (http://tools.iedb.org/mhci/). This server predicts peptide binders to MHC molecules from protein sequences using the MHC-I binding prediction, NetMHCpan EL 4.1. We have selected all HLA class I alleles from the selection panel of IEDB Analysis Resource for prediction of epitopes of HLA class I. The selected epitopes were Immunogenic, Antigenic and Non-toxic [34].
Prediction of T-cell HLA class 2 was done through IEDB Analysis Resource (http://tools.iedb.org/mhcii/). We had selected 7-allele HLA reference set: HLA-DRB1*03:01, HLA-DRB1*07:01, HLA-DRB1*15:01, HLA-DRB3*01:01, HLA-DRB3*02:02, HLA-DRB4*01:01, HLA-DRB5*01:01. The one with low adjusted rank were good binders. This server predicts peptide binders to MHC molecules from protein sequences using the IEDB Recommended. [42]. The selected epitopes were non-allergic, non-overlapping, non-toxic, Antigenic. AlgPred server was used for allergenicity calculation, Non-Overlapping HTL epitopes were selected manually, Toxicity checked by ToxinPred server, Antigenicity by Vaxijen server, Interferon Gamma including ability by INF epitope server. The immunogenicity of HLA Class 1 and 2 were checked through Class I Immunogenicity (http://tools.iedb.org/immunogenicity/).
3.2 Predict epitopes digesting enzymes
To predict epitopes digesting enzymes of epitopes Protein Digest server (http://db.systemsbiology.net:8080/proteomicsToolkit/proteinDigest.html) was used and to check toxicity of epitopes Toxin Pred (http://crdd.osdd.net/raghava/toxinpred/). This tool was used to identify highly toxic or non-toxic peptides from peptides. It also predicts their toxicity along with all the important physio-chemical properties like hydrophobicity, charge pI etc. of peptides. And Toxic epitopes were excluded for further analysis [14].
3.3 Population Coverage
Population Coverage server (http://tools.iedb.org/population/) was used for population coverage. T lymphocytes identify a complex formed by a pathogen-derived epitope and a specific Major Histocompatibility Complex (MHC) molecule. Only individuals who have an MHC molecule capable of binding that epitope will have a response to it. Denominated MHC limitation of T cell responses is the term given to this phenomenon. Human MHC (HLA) molecules are very polymorphic, with over a thousand known distinct alleles. Selecting several peptides with varying HLA binding specificities will allow peptide-based vaccinations or diagnostics to cover a larger patient community. The fact that various HLA types are expressed at drastically varying frequencies in different ethnicities further complicates the problem of population coverage in respect to MHC polymorphism. As a result, if not carefully considered, a vaccination or diagnostic might be developed with ethnically bias population coverage Table 7.
Table 7: Bias population coverage.
3.4 Selection of MESV
Only those epitopes were chosen for further construction of MESV which are a) highly antigenic, (b) immunogenic, (c) non-allergenic, (d) non-toxic, and (e) with large population coverage are typically chosen. To enhance the immunological response, an adjuvant was added to the first Cytotoxic T Lymphocytes (CTL) epitope using the EAAAK linker. After confirming their interaction compatibility, further epitopes were connected using AAY, GPGPG, and KK linkers to retain their separate immunogenic activity [16].
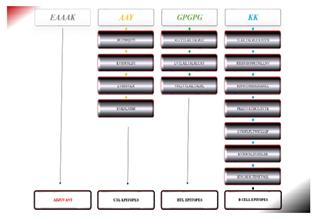
Figure 3: Graphic representation of MESV is a 276-amino-acid construct that includes an adjuvant (orange) attached to the N-terminal of MEV through the EAAAK linker (yellow). CTL epitopes were joined with AAY linkers (blue), HTL epitopes with GPGPG linkers (green), and B-cell epitopes with KK linkers (grey).
3.5 Analysis of MESV
The designed sequence was then verified for Allergenicity, Toxicity, and Antigenicity. The designed MESV was highly antigenic (0.6543), non-allergenic, and non-toxic. The molecular weight of designed vaccine was Molecular weight: 31204.78 kDa and Theoretical pI: 10.13. The estimated half-life was hours (mammalian reticulocytes, in vitro), >20 hours (yeast, in vivo) >10 hours (Escherichia coli, in vivo). The Instability Index (II) is computed to be 33.90, aliphatic index was 120.21, and Grand Average of Hydropathicity (GRAVY) was 0.329. This classifies the protein as stable. The secondary structure shows that Alpha helix is 41.79%, Beta turn is 7.50% and Extended Strand is 28.21%. ProSA-web (https://prosa.services.came.sbg.ac.at/prosa.php) was used for Z-Score which was -0.37, 3D structure of the protein and Overall quality of model was checked. [41,45] as shown in Figure 4.
3.6 Molecular docking of MESV with human immune receptors
Molecular Docking was done to evaluate the interface between human immune receptors and MESV Construct. The interaction between the antigenic molecule and immune receptor molecule is extremely important. TLR3 (Toll-like Receptors-3) has been studied extensively, and have revealed that it plays a critical role in the development of antiviral immune responses. The MESV docking with TLR3 was done by using GRAMM-X (http://vakser.compbio.ku.edu/resources/gramm/grammx/) (PDB ID: 1ZIW). Visualization of the docked complexes was done by using Pymol. Furthermore, a web server called PDBsum (http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=index.html) was used to generate the traditional schematic of interactions between docked proteins (Figure 5). It analyzes the protein-protein interactions among docked molecules.Dockthorhttps://dockthor.lncc.br/v2/index.php?tab=DOCKING&page=RESULTS&jobId=MolecularXdocking_60426ddd594 [13];[22].
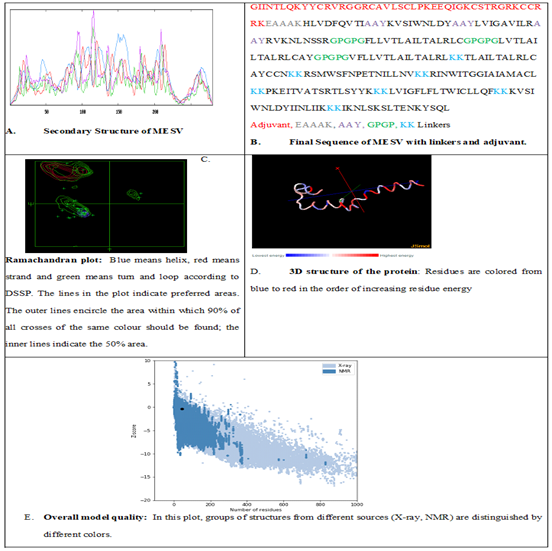
Figure 4: Structure of the protein and overall quality of model.
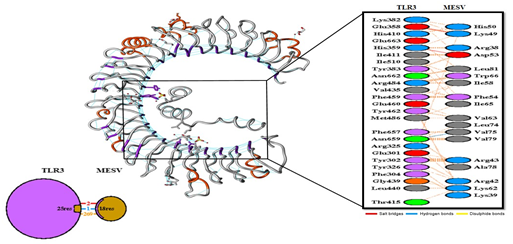
Figure 5: MESV docking with TLR3 was done by using GRAMM-X.
The number of H-bond lines between any two residues indicates the number of potential hydrogen bonds between them. For non-bonded contacts, which can be plentiful, the width of the striped line is proportional to the number of atomic contacts.
3.7 Molecular dynamics simulation
The vaccine constructs with the best molecular docking study findings gone through a molecular dynamics simulation study (Figure 6). For the molecular dynamics simulation analysis, the iMODS web-server (http://imods.Chaconlab.org/) was utilized, which is a fast, free molecular dynamics simulation server for specifying and quantifying protein flexibility.
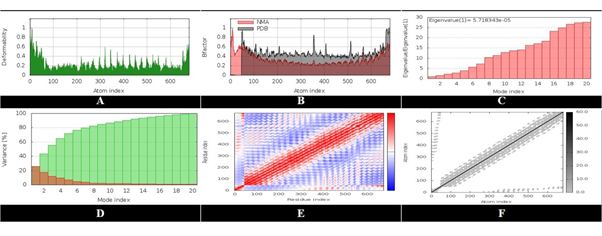
Figure 6: Analysis of molecular dynamics simulation.
3.8 Immune simulation
The vaccine formulation immune response profile was reported using the in silico technique C-ImmSim, through an online simulation server (http://150.146.2.1/C-IMMSIM/index.php). The humoral and cellular responses of a mammalian immune system to a vaccine construct are defined by C-ImmSim. Three doses of the preventive Covid vaccine's target product profile were given at various intervals of four weeks. With time periods of 1, 84, and 170, all simulation settings were set to default. The simulation volume and steps were both set to ten and one thousand, respectively (Figure 7). (random seed = 12345 with LPS-free vaccination injection). [32].
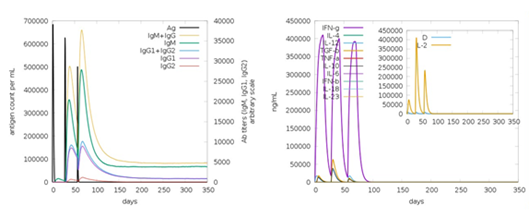
Figure 7: Immune simulation.
3.9 In-Silico cloning and codon enhancement
To enhance recombinant protein expression, a codon optimization approach was applied. Because of the genetic code's degeneracy, most amino acids may be encoded by several codons, which need codon optimization. In the codon system of E. coli (strain K12), the Java Codon Adaptation Tool (JCat) server (http://www.prodoric.de/JCat) was used to get the codon Adaptation Index (CAI) values and GC contents to evaluate the levels of protein synthesis. The GC-Content of Escherichia coli (strain K12): 50.7340272413779 and CAI-Value of the improved sequence: 0.9542834278823386. The optimum CAI value is 1.0, while a score of > 0.95 is considered good, and the GC content ranges from 30 to 70%. Beyond this range, there are negative impacts on translation and transcriptional efficiency. The optimized gene sequence for the multi-epitope vaccine was cloned in the E. coli plasmid vector pET-30a (+), with BamHI and HindIII restriction sites added to the N and C terminals, respectively. Finally, using the Snap Gene programme (https://www.snapgene.com/free-trial/), the optimized sequence of the final vaccine construct (containing restriction sites) was introduced into the plasmid vector pET-30a (+) to validate the vaccine's expression.
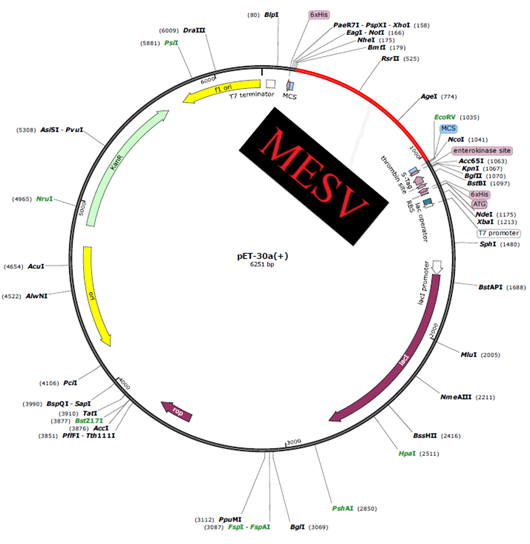
Figure 8: In-silico cloning and codon enhancement.
4. Discussion
Corona virus have long been disregarded as inconsequential microorganisms that cause human "colds." Two particularly pathogenic of corona virus, SARS-CoV and MERS-CoV, arose from cattle reservoirs in the twenty-first century and caused devastating epidemics. Recently, a new strain of CoV, formally known as SARS-COV-2, was discovered, triggering a fatal global epidemic of COVID-19Due of the constantly developing mutations, the pandemic's final dimensions and impact are presently unknown [3]. The new virus rapidly infects host cells after recombination of several virus genome particles. There is yet no effective treatment for the infection. Infection with COVID-19 is a major cause of illness and mortality around the world. Unfortunately, the lack of COVID-19 vaccines has resulted in the loss of countless valuable lives in various parts of the world. COVID-19's advent has resulted in a major global medical burden, necessitating immediate prevention actions. Researchers have been collecting data on Coronavirus in order in order to better recognize the illness's transmission, pathophysiology, and biology in order to eradicate the disease. [10].The rapid growth of databases of structural and genomic information, in combination with computational methods, contributes to the development and discovery of novel vaccination candidates. Recent advancements in bioinformatics has resulted in a variety of tools and web servers that can aid in the development of conventional vaccinations by reducing the time and cost. The creation of successful Multiepitope vaccines is difficult due to difficulties in selecting good antigen candidates and immunodominant epitopes. As a result, immunoinformatics techniques for predicting suitable antigenic epitopes of a targeted protein are critical for designing a MESV. [27].
The development of epitope-based vaccines targeting the structural proteins (M and E) of the SARS-CoV-2 was investigated in this study. These proteins are essential for the replication cycle and the structure of virus particles. [20, 37, 30].M and E proteins are required for viral entrance, replication, and particle assembly within human cells. [4 ,37].The target proteins' T- and B-cell epitopes were expected to aid the host's immunological response. The research examined at proteins at the primary, secondary, and tertiary structural levels. ABCPred predicted B-cell conserved epitopes using the IEDB analysis database. Pymol was used to visualize the position of epitopes on 3D protein structures. To determine discontinuous epitopes, the DiscoTop server was utilized. Allergenicity, toxicity, and physiochemical properties of anticipated epitopes were evaluated to increase specificity and selectivity. The peptides predicted throughout the research were stable and safe to use, according to digestion analysis.
A suitable MESV should have B-cell, HTL, and CTL epitopes and induce efficient antiviral responses against a specific virus (Zhang, 2018). Subunit vaccines of SARS-CoV-2 were created by a few groups, however they only use single protein in vaccine formulation. [1]; [2]; [6]; and the exclusive usage of CTL epitopes without regard for the significance of B cell and HTL epitopes. [25]. We have included B-cell epitopes from numerous structural proteins in addition T-cell epitopes are also important because they play a role in antibody formation and influencing its efficacy. [9]. Furthermore, antigens can quickly overcome the humoral response of memory B-cells, whereas cell-mediated immunity (T-cell immunity) often leads to long-term immunity [4]. CTL prevents pathogen spread by secreting specific antiviral cytokines and identifying and destroying infected cells [30]. As a result, the current vaccine design exceeds previously reported constructs.
HLA alleles keep their responses to T-cell epitopes that are highly variable among ethnic groups. T-cell epitopes are linked with multiple alleles to increase population coverage. To anticipate the worldwide distribution of the alleles, the HTL and CTL epitopes were chosen based on their HLA alleles. The findings revealed that chosen epitopes and alleles cover a wide range of geographical regions around the world. The considered epitopes represent 84.38% of the world's population. With 93.62% of the population, Germany is the most populous country. SARS-CoV-2 epidemics occurred in large numbers in, Spain, France and Iran. Vaccine candidates are therefore critical for protecting people in these areas from SARS-CoV-2 infection. In China, where virus originally appeared and had multiple outbreaks, population coverage was 60.83 percent.
From CTL, HTL, and B cell epitopes, vaccination candidates were chosen based on their antigenicity, toxicity, immunogenicity, population coverage, and allergenicity. The MESV was created by joining the HTL, CTL, and B cell epitopes using GPGPG, AAY, and KK linkers, respectively. Linkers are introduced as a necessary component in the evolution of MESV to improve folding, stability, and expression. When used alone, multi-epitope-based vaccinations are ineffective and require adjuvant coupling. Adjuvants are components in vaccination formulations that protect against infection while also influencing immune responses, antigen development, stability, and durability. [6]. As a result, a 45-amino-acid long adjuvant-defensins was combined with a 5-amino-acid linker at the N-terminus of the EAAAK linker. The EAAAK linker is used to engage the first epitope and adjuvant to aid in efficient separation of the bi-functional fusion protein domains. The final vaccination stretch was revealed to be 276 amino acids long after adjuvant and linkers were added.
The MESV construct has been found to be stable, basic, and hydrophobic based on its physiochemical features. According to the theoretical pI value, MESV was basic, ensuring a consistent physiological pH interaction. According to the estimated aliphatic index and instability index scores, the vaccination protein appears to be stable and thermostable. Its hydrophobic nature is indicated by a positive grand average of hydropathy score. MESV has been discovered to be immunogenic, antigenic, and allergenic. These points to the epitopic vaccine's capacity to stimulate a significant immune response without causing allergic responses.
The 3D structure prediction helps give a lot of information about how important protein components are arranged in space, which is useful for studying ligand interactions, protein activities, dynamics, and other proteins.[40]. The MESV construct's desired properties significantly improved after modification. The Ramachandran plot analysis reveals that the majority of residues are found in the preferred and allowed regions, with only a few residues in the disallowed region, indicating that the model's overall quality is acceptable. The RMSD number, Poor Rotamers, Clash Score, and MolProbity all indicate that the proposed MESV construct is of high quality. To detect errors in the modelled MESV build, various structure validation methods were applied. The overall structure of the modified MESV is of good quality, as evidenced by the ERRAT quality factor (77.1822 %) and z-score (-0.37).
For an immune response to be triggered there must be a good contact between the antigen molecule and the immune receptor molecules. After then, the improved MESV construct was docked against TLR3 to see if it might trigger a rapid immunological response. In molecular docking research, stable connections between MESV and TLR3 were found, and proficient binding required less energy.
Multi-epitope vaccines including B- and T-cell epitopes should potentially activate both humoral and cellular immune responses. Our vaccination produced the most IFN- with the highest levels of IL-10 and IL-2 activity. Antibodies also defend against SARS-CoV-2 in the extracellular environment. Furthermore, because a subunit vaccination has a variety of B-cell and T-cell epitopes, the irrelevant Simpson index (D) suggests a diversified immune response.
Because of mRNA codon incompatibility, which requires codon optimization for better expression, the translation effectiveness of foreign genes within the host system differs [29].The obtained was 1.0, and the GC content (53.2%) was likewise within the optimal range, implying that increased expression in the E. coli K-12 system is achievable. MESV in silico cloning's major goal was to guide genetic engineers and molecular biologists on the predicted expression level or possible cloning sites in a specific expression system, such as the E. coli K12 system.
In this study, we used a next-generation vaccine design strategy to produce a MESV construct capable of eliciting immune responses against SARS-CoV-2. We anticipate that our vaccine will trigger both humoral and cell-mediated immune responses. The interaction and binding patterns between both the receptor and the vaccination protein remained consistent and improved. Furthermore, effective immune responses were seen in real life during immunological simulation. MESV, which was carefully built using such a technique, could thus become a valuable asset in the fight against viral diseases.
Experimental procedures are used to produce initial raw data for computational/immunoinformatics approaches. The accuracy of immunoinformatics predictions can be limited by the quality of the data and the effectiveness of the computer techniques used. To ensure the genuine potential of proposed MESV to combat COVID-19, more in vivo and in vitro research is required.
Current developments in immunological bioinformatics areas had resulted in different servers and tools that can save cost and time of traditional vaccine development. The main problem in selection of immunodominant epitopes and suitable antigen candidates remains a hurdle for researchers. Though for designing a multiple epitope vaccine the antigenic epitopes prediction of a relevant protein by immunoinformatic methods are very helpful. By using Insilco cloning we will acquire a harmless SARS-CoV2 vaccine that could trigger immune responses: Cellular, innate, and humoral. Though, the production and manufacture of vaccine is expensive and takes more time. Immunoinformatic approaches can decrease this load. Now a day’s researchers are finding different methods for development of multiepitope subunit vaccine. With the development of computational tools epitope prediction for antibodies become more meaningful.
5. Conclusion:
Current developments in immunological bioinformatics areas had resulted in different servers and tools that can save cost and time of traditional vaccine development. The main problem in selection of immunodominant epitopes and suitable antigen candidates remains a hurdle for researchers. Though for designing a multiple epitope vaccine the antigenic epitopes prediction of a relevant protein by immunoinformatic methods are very helpful. By using in silico cloning, we will acquire a harmless SARS-CoV2 vaccine that could trigger immune responses: cellular, innate, and humoral. Though, the production and manufacture of vaccine is expensive and takes more time. Immunoinformatic approaches can decrease this load. Nowadays researchers are finding different methods for development of multiepitope subunit vaccine. With the development of computational tools epitope prediction for antibodies become more meaningful.
References
- Abdelmageed MI, Abdelmoneim AH, Mustafa MI, et al. Design of a Multiepitope-Based Peptide Vaccine against the e Protein of Human COVID-19: An Immunoinformatics Approach. BioMed Research International 10 (2020): 2683286.
- Abraham Peele K, Srihansa T, Krupanidhi S, et al. Design of multi-epitope vaccine candidate against SARS-CoV-2: a in-silico study. Journal of Biomolecular Structure & Dynamics 39(10) (2021): 3793–3801.
- Abramo JM, Reynolds A, Crisp GT, et al. Individuality in music performance. In Assessment & Evaluation in Higher Education: Springer International Publishing 37 (2012): 1007/82.
- Alsaadi, EAJ, & Jones IM. Membrane binding proteins of coronaviruses. Future Virology 14(4) (2019): 275–286.
- Bacchetta R, Gregori S, & Roncarolo MG .CD4+ regulatory T cells: mechanisms of induction and effector function. Autoimmunity Reviews 4(8) (2005): 491–496.
- Bhattacharya M, Sharma AR, Patra P, et al. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. Journal of Medical Virology 92(6) (2020): 618–631.
- Bjellqvist B, Hughes GJ, Pasquali C, et al. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14(10) (1993): 1023–1031.
- Blanchet C, Combet C, Geourjon C, et al. MPSA: Integrated system for multiple protein sequence analysis with client/server capabilities. Bioinformatics 16(3) (2000): 286–287.
- Blaszczyk M, Jamroz M, Kmiecik S, et al. CABS-fold: Server for the de novo and consensus-based prediction of protein structure. Nucleic Acids Research 41(2013): 406-11.
- Cooper NR, & Nemerow GR. The role of antibody and complement in the control of viral infections. The Journal of Investigative Dermatology 83(1984): 121-127.
- Douglas MG, Kocher JF, ScobeyT, et al. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information 501(2020): 186-190.
- Gasteiger E, Hoogland C, Gattiker A, et al. Protein Identification and Analysis Tools on the ExPASy Server. In JM Walker (Ed.) The Proteomics Protocols Handbook 52(2005): 571–607.
- Gasteiger E, Hoogland C, Gattiker A, et al. The Proteomics Protocols Handbook. The Proteomics Protocols Handbook10 (2005):571–608.
- Guedes IA, Barreto AMS, Marinho D, et al. New machine learning and physics-based scoring functions for drug discovery. Scientific Reports 11(1)(2021):1–19.
- Gupta S, Kapoor P, Chaudhary K, et al. In Silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 8(9) (2013):0073957.
- Heo L, Park H, & Seok C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Research 41(2013):384-8.
- Hoover DM, Wu Z, Tucker K, et al. Antimicrobial characterization of human β-defensin 3 derivatives. Antimicrobial Agents and Chemotherapy 47(9) (2003): 2804–2809.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395(10223)(2020): 497–506.
- Kanampalliwar AM, Soni R, Girdhar A, et al. Reverse vaccinology: Basics and applications. Journal of Vaccines and Vaccination 4(6) (2013): 2–7.
- Khailany RA, Safdar M, & Ozaslan M. Genomic characterization of a novel SARS-CoV-2. In Gene Reports 19(2020): 100682.
- Kirchdoerfer RN, Cottrell CA, Wang N, et al. Pre-fusion structure of a human coronavirus spike protein. Nature 531(7592)(2016): 118–121.
- Laskowski RA, Jablonska J, Pravda L, et al. PDBsum: Structural summaries of PDB entries. Protein Science 27(1)(2018): 129–134.
- Lohning AE, Levonis SM, Williams-Noonan B, et al. A Practical Guide to Molecular Docking and Homology Modelling for Medicinal Chemists. In Current Topics in Medicinal Chemistry 17(2017): 30110827.
- Lovell SC, Davis IW, Arendall III WB, et al. Structure validation by Cα geometry: Φ,ψ and Cβ deviation. Proteins: Structure, Function, and Bioinformatics 50(3) (2003): 437–450.
- Mahram A, & Herbordt MC. Fast and accurate NCBI BLASTP: Acceleration with multiphase FPGA-based prefiltering. Proceedings of the International Conference on Supercomputing 10(2010): 73–82.
- Mishra D.T Cell Epitope-Based Vaccine Design for Pandemic Novel Coronavirus 2019-nCoV 10(2020): 12029523.
- Mora M, Veggi D, Santini L, et al. Reverse vaccinology. In Drug Discovery Today 8(2003): 2689-8
- Nain Z, Karim MM, Sen MK, et al. Structural basis and designing of peptide vaccine using PE-PGRS family protein of Mycobacterium ulcerans-An integrated vaccinomics approach. Molecular Immunology 120(2020): 146–163.
- Ong E, Wong MU, Huffman A, et al. COVID-19 Coronavirus Vaccine Design Using Reverse Vaccinology and Machine Learning. Frontiers in Immunology 11(2020): 1581.
- Pandey RK, Bhatt TK, & Prajapati VK. Novel Immunoinformatics Approaches to Design Multi-epitope Subunit Vaccine for Malaria by Investigating Anopheles Salivary Protein. Scientific Reports 8(1) (2018): 1–11.
- Pohl R, Gilman R, Miller GA, et al. Muonic hydrogen and the proton radius puzzle. Annual Review of Nuclear and Particle Science 63(2013): 175–204.
- Qamar MTU, Shokat Z, Muneer I, et al. Multiepitope-based subunit vaccine design and evaluation against respiratory syncytial virus using reverse vaccinology approach. Vaccines 8(2) (2020): 1–27.
- Rapin N, Lund O, Bernaschi M, et al. Computational immunology meets bioinformatics: The use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE 5(4) (2010): 0009862.
- Rapin N, Lund O, Bernaschi M, et al. Computational Immunology Meets Bioinformatics: The Use of Prediction Tools for Molecular Binding in the Simulation of the Immune System. PLOS ONE 5(4) (2010) : 1–14.
- Reynisson B, Alvarez B, Paul S, et al. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Research 48(2020): 449–454.
- Sars-cov- A. Computational Determination of Potential Multiprotein.10 (2021): 1–17.
- Schoeman D, Fielding BC, Arias-Reyes C, et al. Journal Pre-proof Does the pathogenesis of SAR-CoV-2 virus decrease at high-altitude? Does the pathogenesis of SAR-CoV-2 virus decrease at high-altitude? Corresponding authors. Cell Research 9(1) (2020): 278–280.
- Siu YL, Teoh KT, Lo J, et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. Journal of Virology 82(22) (2008): 11318–11330.
- Song HC, Seo MY, Stadler K, et al. Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. Journal of Virology 78(19) (2004): 10328–10335.
- Tahir Ul Qamar M, Maryam A, Muneer I, et al. Computational screening of medicinal plant phytochemicals to discover potent pan-serotype inhibitors against dengue virus. Scientific Reports 9(1) (2019):
- Tahir ul Qamar M, Rehman A, Ashfaq UA, et al. Designing of a next generation multiepitope based vaccine (MEV) against SARS-COV-2: Immunoinformatics and in silico approaches 10(2020): 1–36.
- Virology S, Fever D, Fever Y, et al. Recognition of Errors in Three-Dimensional Structures of Rep Proteins of Geminivirus Strains by Using ProSA-Web. SciFed Virology Research Journal 1(1) (2017): 1–3.
- Wang P, Sidney J, Dow C, et al. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Computational Biology 4(4)(2008): 1000048.
- Waterhouse A, Bertoni M, Bienert S, et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Research 46(2018): 296–303.
- Wei Ji, Wei Wang, Xiaofang Zhao, et al. Cross-species transmission of the newly identified.pdf 92(2020): 433-440.
- Wiederstein M, & Sippl M J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Research 35(2007): 407–410.
- Wiederstein M, & Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Research 35(2007): 407-410.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 395(10229)(2020): 1054–1062.
