Oxidative Stress and Cellular Death Biomarkers Assessment in Follicular Fluid of Patients with Endometriosis Undergoing IVF/ICSI Treatment
Article Information
Albane Vandecandelaere1*, Guillaume Jedraszak2, Dorian Bosquet1, Sophian Tricotteaux1, Florence Scheffler3, Antoine Galmiche4, Arthur Foulon5, Moncef Benkhalifa1, Rosalie Cabry1
1Reproductive Medicine, Reproductive Biology & Genetics and Peritox laboratory, University Hospital & School of Medicine. Picardy University Jules Verne. Amiens. France
2Constitutional Genetics Laboratory university hospital of Amiens, France
3University Hospital of Besançon
4Biochemistry laboratory university hospital of Amiens, France
5Department of gynaecology and obstetrics, University Hospital & School of Medicine. Picardy University Jules Verne. Amiens. France
*Corresponding Author: Albane Vandecandelaere, Reproductive Medicine, Reproductive Biology 1 rond point du Pr Cabrol 80000 Amiens, France.
Received: 10 October 2023; Accepted: 19 October 2023; Published: 15 December 2023
Citation: Albane Vandecandelaere, Guillaume Jedraszak, Dorian Bosquet, Sophian Tricotteaux, Florence Scheffler, Antoine Galmiche, Arthur Foulon, Moncef Benkhalifa, Rosalie Cabry. Oxidative Stress and Cellular Death Biomarkers Assessment in Follicular Fluid of Patients with Endometriosis Undergoing IVF/ICSI Treatment. Obstetrics and Gynecology Research. 6 (2023): 299-308.
View / Download Pdf Share at FacebookAbstract
To date, the pathogenesis of endometriosis related infertility is still poorly understood. In particular, impaired oocyte quality, parameter that remains difficult to assess in current practice could be implicated in this infertility. The objective of this study was to assess oocyte quality indirectly via an oxidative stress biomarker (8-hydroxy-2-deoxyguanosine, 8OHdG) and cell death marker (cell free DNA) in mature follicular fluid of patients with endometriosis undergoing IVF/ICSI treatment. Follicular fluid from a mature follicle was analyzed for 32 patients with and 35 without endometriosis. Patients were enrolled between July 2020 and February 2023 in university hospital of Amiens. Obese patients, with poor ovarian reserve or with PCOS were excluded. 8OHdG was determined by immunohistochemistry and cell free DNA by ALU-qPCR. Cell free DNA was also performed on serum of patients before and during stimulation. These analyses were then correlated to the results of IVF outcomes. Concentration of follicular 8OHdG and cell free DNA were not different depending on the presence of endometriosis or on the presence of endometrioma. There was also no significant difference in the results of IVF treatment and embryo development depending on the presence of endometriosis. However, on the whole cohort, follicular cell free DNA was negatively correlated to oocyte maturity and follicular 8OHdG negatively correlated to fertilization rate and number of good quality embryo. We cannot claim about poor oocyte quality in endometriosis with this assay. However, it revealed follicular 8OHdG and cell free DNA as potential prognostic biomarkers for IVF/ICSI outcomes.
Keywords
Follicular fluid; Endometriosis; Oocyte quality; Oxidative stress; Cell free DNA; 8OHDG
Follicular fluid articles Follicular fluid Research articles Follicular fluid review articles Follicular fluid PubMed articles Follicular fluid PubMed Central articles Follicular fluid 2023 articles Follicular fluid 2024 articles Follicular fluid Scopus articles Follicular fluid impact factor journals Follicular fluid Scopus journals Follicular fluid PubMed journals Follicular fluid medical journals Follicular fluid free journals Follicular fluid best journals Follicular fluid top journals Follicular fluid free medical journals Follicular fluid famous journals Follicular fluid Google Scholar indexed journals Endometriosis articles Endometriosis Research articles Endometriosis review articles Endometriosis PubMed articles Endometriosis PubMed Central articles Endometriosis 2023 articles Endometriosis 2024 articles Endometriosis Scopus articles Endometriosis impact factor journals Endometriosis Scopus journals Endometriosis PubMed journals Endometriosis medical journals Endometriosis free journals Endometriosis best journals Endometriosis top journals Endometriosis free medical journals Endometriosis famous journals Endometriosis Google Scholar indexed journals Oocyte quality articles Oocyte quality Research articles Oocyte quality review articles Oocyte quality PubMed articles Oocyte quality PubMed Central articles Oocyte quality 2023 articles Oocyte quality 2024 articles Oocyte quality Scopus articles Oocyte quality impact factor journals Oocyte quality Scopus journals Oocyte quality PubMed journals Oocyte quality medical journals Oocyte quality free journals Oocyte quality best journals Oocyte quality top journals Oocyte quality free medical journals Oocyte quality famous journals Oocyte quality Google Scholar indexed journals Oxidative stress articles Oxidative stress Research articles Oxidative stress review articles Oxidative stress PubMed articles Oxidative stress PubMed Central articles Oxidative stress 2023 articles Oxidative stress 2024 articles Oxidative stress Scopus articles Oxidative stress impact factor journals Oxidative stress Scopus journals Oxidative stress PubMed journals Oxidative stress medical journals Oxidative stress free journals Oxidative stress best journals Oxidative stress top journals Oxidative stress free medical journals Oxidative stress famous journals Oxidative stress Google Scholar indexed journals Cell free DNA articles Cell free DNA Research articles Cell free DNA review articles Cell free DNA PubMed articles Cell free DNA PubMed Central articles Cell free DNA 2023 articles Cell free DNA 2024 articles Cell free DNA Scopus articles Cell free DNA impact factor journals Cell free DNA Scopus journals Cell free DNA PubMed journals Cell free DNA medical journals Cell free DNA free journals Cell free DNA best journals Cell free DNA top journals Cell free DNA free medical journals Cell free DNA famous journals Cell free DNA Google Scholar indexed journals 8OHDG articles 8OHDG Research articles 8OHDG review articles 8OHDG PubMed articles 8OHDG PubMed Central articles 8OHDG 2023 articles 8OHDG 2024 articles 8OHDG Scopus articles 8OHDG impact factor journals 8OHDG Scopus journals 8OHDG PubMed journals 8OHDG medical journals 8OHDG free journals 8OHDG best journals 8OHDG top journals 8OHDG free medical journals 8OHDG famous journals 8OHDG Google Scholar indexed journals IVF articles IVF Research articles IVF review articles IVF PubMed articles IVF PubMed Central articles IVF 2023 articles IVF 2024 articles IVF Scopus articles IVF impact factor journals IVF Scopus journals IVF PubMed journals IVF medical journals IVF free journals IVF best journals IVF top journals IVF free medical journals IVF famous journals IVF Google Scholar indexed journals ICSI articles ICSI Research articles ICSI review articles ICSI PubMed articles ICSI PubMed Central articles ICSI 2023 articles ICSI 2024 articles ICSI Scopus articles ICSI impact factor journals ICSI Scopus journals ICSI PubMed journals ICSI medical journals ICSI free journals ICSI best journals ICSI top journals ICSI free medical journals ICSI famous journals ICSI Google Scholar indexed journals PCOS articles PCOS Research articles PCOS review articles PCOS PubMed articles PCOS PubMed Central articles PCOS 2023 articles PCOS 2024 articles PCOS Scopus articles PCOS impact factor journals PCOS Scopus journals PCOS PubMed journals PCOS medical journals PCOS free journals PCOS best journals PCOS top journals PCOS free medical journals PCOS famous journals PCOS Google Scholar indexed journals Endometriosis articles Endometriosis Research articles Endometriosis review articles Endometriosis PubMed articles Endometriosis PubMed Central articles Endometriosis 2023 articles Endometriosis 2024 articles Endometriosis Scopus articles Endometriosis impact factor journals Endometriosis Scopus journals Endometriosis PubMed journals Endometriosis medical journals Endometriosis free journals Endometriosis best journals Endometriosis top journals Endometriosis free medical journals Endometriosis famous journals Endometriosis Google Scholar indexed journals
Article Details
Abbreviations
8OHdG: 8-hydroxy-2-deoxyguanosine; DNA: Desoxyribo Nucleic Acid; IVF: In Vitro Fecondation; ICSI: Intra Cytoplasmic Spermatozoa Injection; PCOS: Polycystic Ovary Syndrom; PCR: Polymerase Chain Reaction
Introduction
Endometriosis is a benign oestrogen-dependent gynaecological pathology linked to the presence of ectopic endometrial tissue. It affects 10-18% of women of childbearing age [1] and is responsible for chronic pelvic pain and infertility [2]. It is characterized by an inflammatory and pro-oxidative peritoneal environment, which plays a role in the development and progression of the disease [3,4].
Pathogenesis of endometriosis related infertility is still poorly understood and results of clinical studies are contradictory [5,6]
Induced infertility may be linked to a poor ovarian [7] or to associated tubal pathology, but how explain infertility in endometriosis with normal reserve and tubal patency?
We ask about the impact of the inflammatory and pro-oxidant environment of the follicular microenvironment on oocyte quality [8-13].
Assessing oocyte quality is difficult, and studies often indirectly suggest a defect in oocyte quality via maturity rate, abnormality rate, mitochondrial dysfunction, or meiosis abnormalities [14-17].
However, reactive oxygen species can easily be measured in follicular fluid during IVF/ICSI, as in Terao et al.’s study which found a negative correlation between follicular oxidative stress and fertilization rate, as well as embryonic quality [18].
Thus, biomarkers of follicular oxidative stress could be developed as a new tool for indirect assessment of oocyte quality.
Moreover, in the event of oxidative stress, granulosa cells can activate various apoptosis pathways [10,19,20] and even senescence [3] necrosis [21] or ferroptosis [22].
In IVF, increased apoptosis of granulosa cells has been shown to impair oocyte maturity, fertilization rate, blastulation rate and embryo quality [23,24].
One marker for studying cell death is circulating cell-free DNA (cfDNA). CfDNA are low-molecular-weight double-stranded DNA fragments released by cell death into the various biological fluids. cfDNA has already been isolated and assayed in the follicular fluid of infertile populations.
In Scalici et al study, cfDNA was inversely correlated with follicle size and embryonic quality at J3 [25]. In infertile patients, follicular cfDNA has also been found to correlate with follicular antioxidant capacity, ovarian reserve abnormalities and chances of pregnancy [26,27].
These studies quantified cell free DNA using real-time PCR for human ALU repeats: ALU 115 and 247 primers. The interest of this technique lies in the fact that it enables us to estimate the origin of cell death between necrosis and apoptosis. Apoptosis, a programmed pathway of cell destruction, results in the release of small fragments of almost identical size (180-200 base pairs) amplified by ALU 115 primers, whereas necrosis results in the release of a variety of larger fragments amplified by ALU 115 primers and ALU 247 primers [28]. Thus, the ratio of ALU 247/ALU 115 amplification can be used to estimate the origin of cell death.
In endometriosis, the question of oocyte quality remains unsolved, and few studies have investigated the link between oxidative stress and cell death in endometriosis [29].
The aim of this study is to assess oxidative stress and cell death in follicular fluid from patients with endometriosis, and to investigate a possible correlation of these follicular biomarkers with IVF/ICSI laboratory parameters and clinical results.
Follicular oxidative stress was assessed by immunohistochemical assay of 8-hydroxy-2'-deoxyguanosine (8-OHdG) and cell death by quantification cfDNA with ALU qPCR using ALU115 and 247 primers.
cfDNA quantification was also performed on serum of patients before and during stimulation.
Materials and Methods
Ethics
This is a prospective monocentric study carried out at the Amiens University Hospital, in Reproductive medicine Department. "ENDOFIV" protocol was approved by Institutional Ethical Committee on 10/02/2020 (ID-RCB number: 2019-A02858-49). Patients gave oral consent at the time of ovarian stimulation prescription.
Study population
Patients included were women aged 18 to 40 years undergoing IVF/ICSI with or without sperm donation in the reproductive medicine department of Amiens University Hospital. 112 patients were included between 07.2020 and 03.2023.
Pelvic endometriosis was diagnosed on pelvic ultrasound or pelvic MRI. All stages of endometriosis were included; a distinction was made between superficial and deep endometriosis, and within deep endometriosis, patients with or without objectified ovarian involvement. History of endometriosis surgery was specified. At the time of puncture, we specified the presence (or not) of endometrioma on the ovary from which the follicle under analysis originated was sought.
Exclusion criteria
Patients suffering from obesity, poor ovarian reserve (defined as AMH < 1.5 ng/ml), polycystic ovary syndrome (PCOS) (defined according to Rotterdam criteria), hyperprolactinemia, viral risk management, a pelvic inflammatory disease other than endometriosis, a recent (< 6 months) upper genital infection or a COVID-19 infection at the time of stimulation were excluded. Patients whose spouse had a sperm count below 5 million/ml were also excluded.
Ovarian stimulation
All included patients received a controlled ovarian stimulation protocol for IVF with or without ICSI. Patients were stimulated with recombinant FSH alone or a combination of FSH+LH, using a long GnRH agonist treatment or short antagonist protocol. The GnRH agonist used was Decapeptyl® 0.1 mg, and the antagonists were Fyremadel® or Orgalutran®. The ovarian response war monitored by transvaginal pelvic ultrasound and serum hormonal levels. Induction of ovulation was decided by the clinical practician when at least 3 or 5 follicles (depending on the patient's age) had reached mature size. Ovulation was triggered with single injection of human chorionic gonadotrophin Ovitrelle® (1 ampoule of 250ug) or GnRH agonist Decapetyl® (3 ampoules of 0.1 mg).
Puncture was performed under general anaesthesia or spinal anaesthesia with transvaginal echo-guided puncture 36 hours after ovulation induction.
Blood and follicular fluid sampling
A first blood sample was taken on the day the prescription was given, and a second on the day of the oocyte pick up puncture. Thus, cfDNA was quantified before and during stimulation.
For each blood sample, 10 ml of venous blood was drawn into a cfDNA BCT tube STREK®. This tube contains a reagent that prevents the release of genomic DNA from blood cells. It was then transported to the laboratory at room temperature.
A clear non hematic follicular fluid was collected from a single mature follicle measuring between 18 and 20 mm on the day of oocyte pick up. The presence of an endometrioma on the puncture side was specified. The fluid was then transported to the laboratory at 37°C and recovered after usual removing cumulus oophorus complexes. The stage of maturity of the oocyte was specified.
Blood and follicular samples were centrifuged within 48 hours to limit cell contamination for serum et follicular fluid supernatant preparation.
All samples were centrifuged once at 1600g for 10 min at 4°C, avoiding exposure to light, then at 3200g for 20 min at 4°C, also away from light. After each centrifugation, only the supernatant was recovered. The final supernatant of follicular fluid and blood was then frozen at -80°C in anonymized tubes, pending analysis.
DNA extraction
DNA extraction was performed on centrifuged serum or follicular fluid manually using the QIAGEN® QIAmp Circulating Nucleic Acid Kit, following the kit instructions. 1 or 2 ml of serum or follicular fluid was used for this extraction. 200 µl of proteinase K was used protease digestion at 60°C for 30 min, followed by purification after cooling on a QIAGEN vac® mini column. DNA was then bound to a silicate-gel membrane. AVE buffer was used to recover a final solution of 25 or 50 µl of cfDNA, depending on the initial extraction volume. Finally, for each patient, 3 samples of 25 or 50 µl of cfDNA were stored at -25°C: one from blood before stimulation, one from blood during ovarian stimulation and one from follicular fluid.
Real-time PCR
A real-time quantitative PCR was performed. This is a variant of PCR allowing amplification and monitoring of this amplification in real time which can quantify very small quantities of DNA from a sample can be reliably assayed. The primers chosen were ALU 115 (5′-CCTGAGGTCAGGAGTTCGAG-3′,5′-CCCGAGTAGCTGGGATTACA-3′) and ALU 247 (5′-GTGGCTCACGCCTGTAATC-3′,5′-CAGGCTGGAGTGCAGTGG-3′), ubiquitous and highly repeated in the genome as described in Umetani et al [30].
qPCR was performed on Lightcycler 480 I (Roche®) and the thermocycler was parameterized as follows: pre-incubation at 95°C for 10 min then 35 cycles of three steps: denaturation at 95° C for 10s, hybridization at 60°C for 20s then elongation at 72° C for 30s.
A standard curve of 4 genomic DNA with different known concentration was used for quantification of cf DNA. The standard curve bounded the expected concentrations: 10 ng/ml, 1 ng/ml, 0.1 ng/ml and 0.01 ng/ml. A negative control was performed for each quantification. Each cfDNA assay of unknown concentration was performed in duplicate for each primer pair.
As cfDNA from follicular fluid was highly concentrated in primary manipulations, it was diluted 100-fold in order to be within the calibration range.
We used maximum second derivative calculation method to determine the concentration of DNA samples independent of the number of PCR cycles.
The ALU 247/ALU115 ratio was used to estimate the origin of cell death. The ALU 247 primer can only amplify large fragments derived from necrosis, whereas the ALU 115 primer amplifies all released fragments. The ALU 247/ALU 115 ratio is therefore close to "0" in the case of cell apoptosis and "1" in the case of cell necrosis.
8-hydroxy-2'-deoxyguanosine concentration
Determination of 8-hydroxy-2'-deoxyguanosine (8OHdG) was carried out on follicular fluid only, using the DNA Damage Competitive ELISA Kit (Invitrogen®).
This enzyme-linked immunosorbent assay detects DNA damage by quantifying the oxidation of the guanosine base. The assay is based on the reaction of 8-hydroxy-2'-deoxyguanosine with a specific chromogen-labeled antibody. The reaction is read with absorbance at 450 nm.
A standard curve was produced using a calibration range from 0 to 8,000 pg/ml, allowing concordance between the optical density of the reaction and the molecular concentration.
Samples were diluted 5-fold for this technique. A negative control was also performed to detect any unspecific reactions.
Raw data, including those normalized by total cfDNA were then analysed.
Nuclear maturity and fertilization rates, embryo quality and clinical results
The oocyte nuclear maturity rate was defined as the number of oocytes having expelled a polar cell on the day of puncture out of the total number of cumulus-oocyte recovered. The oocyte lysis rate was defined as the number of lysed oocytes after denudation out of the number of cumulus-oocyte. The rate of abnormal oocyte morphology, in ICSI only, corresponded to oocytes with a specified morphological abnormality (granular cytoplasm, thin zona pellucida, wide perivitelline space, smooth endoplasmic reticulum or cross-linked bodies) out of the number of mature oocytes. The fertilization rate corresponded to the number of zygotes with 2 polar globules (associated with either 2 pronuclei, 0 pronuclei (syngamy) or 3 pronuclei) out of the number of oocytes with one polar globule. Blastulation rate corresponded to the number of blastocysts obtained after 5 or 6 days of culture out of the number of embryos placed in prolonged culture. The usable embryo rate corresponded to the number of embryos of sufficient quality to be transferred or frozen out of the number of zygotes. Embryo quality was defined according to the BLEFCO classification for 2- and 3-day-old embryos and the GARDNER and SHOOLCRAFT classification for blastocysts [31]. Embryos of sufficient quality for transfer or freezing are:
- 2-day embryos with 3 to 5 cells and < 10% fragmentation
- 3-day embryos with 6 to 10 cells and < 10% fragmentation
- blastocysts classified as B3 or B4, with embryonic bud and trophoblast classified as A or B.
Clinical pregnancy were confirmed by the presence of a gestational sac and fetal cardiac activity on ultrasound. Clinical pregnancies immediately following puncture (immediate transfer) or from the cohort (cryopreserved embryo) were taken into account.
Statistical analysis
A non-parametric t-test (Mann-Withney) was performed to compare means for values of interest between the endometriosis group and the control group.
Associations between parameters of interest were calculated using Pearson's correlation coefficient. The p-value was calculated for this test, taking into account the t-score calculation and the degree of freedom.
A simple linear regression model was used to assess the level of prediction of the assays in relation to the values of interest.
Orange Data Mining data visualization software was used to perform a correlation test with all possible associations in the data table.
Statistical significance is defined as p<0.05.
Results
Population characteristics
A total of 112 patients were included in this study. Analysis of all samples was carried out on 67 patients undergoing IVF-ICSI at Amiens University Hospital (figure 1). 32 patients had endometriosis, while the remaining 35 had tubal, mild male, idiopathic or societal infertility.
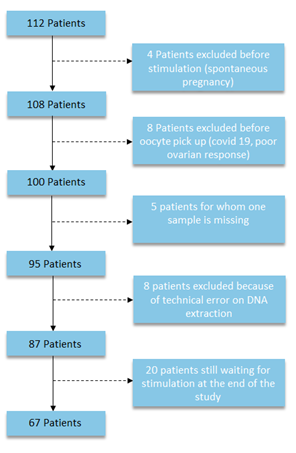
Figure 1: Flow chart des patientes analysées pour l’ADN circulant et la 8-OHdG
The mean age of the subject were 33,79 years +/- 4,46, the mean body mass index (BMI) was 23,4 +/- 4,1. Baseline characteristics of patients were compared between the 2 groups: with and without endometriosis. Groups were comparable in terms of age, BMI, AMH, FSH and oestradiol levels at the start of the cycle, and duration of infertility (Table 1). 15.6% of endometriosis patients were smokers, compared with 31% of other patients (this difference was not significant).
There was no significant difference in the sperm parameters of spouses in either group.
|
Without endometriosis |
With endometriosis |
||
|
Mean ± SD |
Mean ± SD |
p-value |
|
|
Age (years) |
33.89 ± 4.89 |
33.69 ± 4.03 |
0.841 |
|
BMI (kg/m2) |
23.42 ± 4.14 |
23.38 ± 3.99 |
0.976 |
|
FSH J2-5 (mui/L) |
7.07 ± 2.86 |
7.53 ± 2.13 |
0.834 |
|
Oestradiol J2-5 (pg/ml) |
42.28 ± 19.42 |
60.77 ± 68.38 |
0.865 |
|
AMH (ng/ml) |
3.41 ± 1.71 |
3.161 ± 1.263 |
0.911 |
|
Duration of infertility (years) |
4.42 ± 1.98 |
3.16 ± 1.78 |
0.743 |
Note: BMI: Body Mass Index, FSH: Follicle Stimulating Hormone, AMH: Anti Mullerian Hormone. Values are presented as mean+/-SD. P value= endometriosis vs without endometriosis. P<0,05 is considered statically significant.
Table 1: Baseline characteristics of the population
94% of patients with endometriosis had deep pelvic endometriosis. Among patients with deep pelvic endometriosis, 34% had undergone complete endometriosis surgery, and 31% had undergone an ovarian procedure (Figure 2).
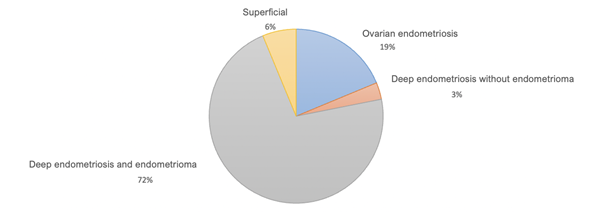
Figure 2: Population distribution by type of endometriosis
This study did not find significant difference in follicular 8OHdG assay according to the presence or absence of endometriosis. Nor was there any significant difference in the quantification of follicular and serum cfDNA according to the presence or absence of endometriosis.
The ratios of ALU 247/115 in follicular fluid were 0.54 and 0.49 respectively in patients with and without endometriosis. These results were not significantly different according to the presence or absence of endometriosis (Table 2).
|
Without endometriosis |
With endometriosis |
||
|
Mean ± sd |
Mean ± sd |
p-value |
|
|
Follicular ALU 115 (ng/ml) |
48.64 ± 47,14 |
63.61± 65,13 |
0.78 |
|
Follicular ALU 247 (ng/Ml) |
2627 ± 26.4 |
28.2 ± 29.11 |
0.73 |
|
Serum ALU 115 before stimulation (ng/ml) |
0.45 ± 0.81 |
0.23 ± 0.19 |
0.91 |
|
Serum ALU 115 day of puncture (ng/ml) |
0.35 ± 0.27 |
0.26 ± 0.23 |
0.88 |
|
ALU247/115 ratio |
0.52 ± 0.18 |
0.51 ± 0.18 |
0.55 |
|
Follicular 8OHdG (ng/ml) |
11.61 ± 2.89 |
12.4 ± 1.81 |
0.99 |
|
Normalised follicular 8OHdG |
0.77 ± 1.1 |
0.67 ± 0.69 |
0.84 |
Note: cfDNA: cell free DeoxiriboNucleic Acid,8OHdG: 8 hydroxy-2-deoxyguanosine. Values are presented as mean+/- SD. P value= endometriosis vs without endometriosis. P<0,05 is considered statically significant.
Table 2: follicular and serum cfDNA and follicular 8-OHdG in patients with and without endometriosis
There was no correlation between follicular cfDNA and 8OHdG.
However, there was a significant positive correlation between serum cfDNA taken on the day of puncture and follicular cfDNA (coefficient= 0.37), as well as a positive correlation between follicular cfDNA and patient age (coefficient=0.29). There was also a positive correlation between follicular and serum cfDNA and BMI.
Among patients with endometriosis, the presence of an endometrioma on the side of the analysed follicle did not influence follicular cfDNA assays. 8OHdG levels in follicles punctured on the side of an endometrioma tended to be higher, but this result was not significant (p=0.087) (Table 3).
|
Without Endometrioma N=11 |
With Endometrioma |
||
|
Mean ± SD |
Moyenne ± SD |
p-value |
|
|
Follicular ALU 115 (ng/ml) |
92.37 ± 87.29 |
47.26 ± 47.51 |
0.14 |
|
Follicular ALU 247 (ng/ml) |
3877 ± 32.55 |
26.51 ± 30.22 |
0.35 |
|
Follicular 8OHdG (ng/ml) |
12.29 ± 1.79 |
12.59 ± 1.91 |
0.69 |
|
Normalised follicular 8OHdG |
0.44 ± 0.47 |
0.91 ± 0.84 |
0.087 |
Note: 8OHdG: 8 hydroxy-2-deoxyguanosine. Values are presented as mean+/- SD. P value= endometriosis vs without endometriosis. P<0,05 is considered statically significant.
Table 3: Comparison of follicular and serum cfDNA and follicular 8-OHdG in patients with endometriosis according to the presence of an endometrioma at the time of puncture
There was no difference in the results of reproductive outcome according to the presence or absence of endometriosis (table 4). The rate of abnormal oocytes in ICSI, the maturity rate, the fertilization rate, the blastulation rate and the number of usable embryos at the end of stimulation were comparable.
There were less clinical pregnancy (after immediate transfer or cumulative frozen embryo transfer) in patients with endometriosis (25%) vs other patients (37%) but this difference was not significant. It should be noted that non-pregnant patients who had not yet undergone transfer of all the embryos resulting from the attempt were not included in this analysis (18% of the cohort).
|
|
Without endometriosis |
With endometriosis |
|
|
Mean ± SD |
Mean ± SD |
p-value |
|
|
Serum E2 level at induction (pg/ml) |
2400 ± 1318 |
2312 ± 922 |
0.7 |
|
Number of mature oocytes at puncture |
7.76 ± 3.47 |
6.77 ± 3.61 |
0.33 |
|
Maturity rate (%) |
70.7 ± 21 |
70.5 ± 23 |
0.91 |
|
Abnormal oocyte rate (%) |
44.6 ± 34.6 |
49.4 ± 37 |
0.66 |
|
Fertilisation rate (%) |
75.06 ± 12 |
64± 32.07 |
0.17 |
|
Blastulation rate (%) |
62.98 ± 31.16 |
52.65 ± 41 |
0.99 |
|
No. of good quality embryo at the end of stimulation |
2.48 ± 1.85 |
1.68 ± 1.98 |
0,17 |
|
Fertilisation failure rate (%) |
2.85 |
6.25 |
NS |
|
Pregnancy resulting from the cohort (%) |
37 |
25 |
NS |
Note: E2: estradiol, Abnormal oocyte rate was assessed only for ICSI cycles (granular cytoplasm, thin zona pellucida, wide perivitelline space, smooth endoplasmic reticulum or cross-linked bodies), good quality embryo was defined according to the BLEFCO classification and GARDNER and SCHOOLCRAFT classification, Values are presented as mean+/- SD. P value= endometriosis vs without endometriosis. P<0,05 is considered statically significant.
Table 4: Reproductive outcomes
About association between follicular markers and IVF laboratory parameters: for patients with endometriosis, there was a significant negative correlation between the normalized 8OHdG assay and blastulation rate. There was no correlation between cfDNA and IVF laboratory parameters in the endometriosis group (table 5).
For the entire cohort, there was a significant negative correlation between follicular normalized 8OHdG and fertilization rate. This correlation tend to be negative in patients with endometriosis but the results did not reach significativity.
There was also a negative correlation between 8OHdG and the number of usable embryos at the end of stimulation.
It must be noted that there were either no correlation between rate of abnormal oocyte and blastulation rate and embryo result (results not shown).
Furthermore, according to our results, follicular cfDNA (ALU115 and ALU247) had a negative significantly impact on the rate of oocyte maturity.
|
With endometriosis Correlation coefficient |
Entire cohort Correlation coefficient |
|||||
|
ALU 115 |
ALU 247 |
Normalized 8OHdG |
ALU 115 |
ALU 247 |
Normalized 8OHdG |
|
|
Number of mature oocytes |
-0.027 |
-0.062 |
-0.107 |
-0.102 |
-0.075 |
-0.006 |
|
Rate of oocyte maturity |
-0.214 |
-0.188 |
-0.028 |
-0,315* |
-0.3* |
0.267 |
|
Rate of oocytes with morphological abnormalities |
0.29 |
0.006 |
0.176 |
0.186 |
0.085 |
0.223 |
|
Fertilization rate |
0.113 |
0.065 |
-0.343 |
0.102 |
0.138 |
-0.281* |
|
Blastulation rate |
0.192 |
0.201 |
-0.440* |
-0.003 |
0.083 |
0.013 |
|
Number of good embryo quality |
0.136 |
0.063 |
-0.301 |
-0.039 |
-0.014 |
-0.287* |
Note: 8OHdG: 8 hydroxy-2-deoxyguanosine. * when results are significant, P<0,05 is considered statically significant
Table 5: correlation coefficient between 8-OHdG and follicular cfDNA on IVF results
Results of IVF/ICSI laboratory parameters were also analyzed for the whole cohort by establishing a "threshold" of high cfDNA defined by the median of cfDNA assays. Patients with cfDNA above 39 ng/ml (median quantification with ALU 115 primers) did not have significantly different IVF/ICSI laboratory outcomes from patients with follicular cfDNA below 39 ng/ml (results not shown)
Finally, on the entire cohort, 8OHdG and cfDNA were not found to be significantly different according to the presence of a pregnancy at the end of the attempt (cumulative fresh and frozen transfers).
Discussion
This monocentric cohort study analyzed a biomarkers of oxidative stress and cell death (free circulating DNA as consequences of apoptosis and or senescence) in follicular fluid from a mature follicle in a cohort of patients with normal ovarian reserve. The results were analyzed according to the presence or absence of endometriosis. We then tried to see if there is an association between these biomarkers and IVF treatment incomes.
Follicular 8OHdG assay were not different in patients with endometriosis compared with other IVF/ICSI patients. The presence of an endometrioma at the time of oocyte pick up did not affect the results either.
However, a negative correlation was found between follicular 8OHdG and the blastulation rate in patients with endometriosis, as well as the fertilization rate in the entire cohort.
Many studies reported a possible impact of follicular oxidative stress on oocyte quality [11,18]. Although oxidative stress appears to be involved in the pathophysiology of endometriosis [32] and oocyte quality, it has not yet been determined which marker(s) are the most specific and how it can predict oocyte quality and IVF/ICSI outcomes [33].
Of many follicular biomarkers of oxidative stress studied in patients with endometriosis, Da Broi et al, just found a significant increase in 8OHdG and vitamin E (antioxidant action) [34], Prieto et al found only a significantly lower level of vitamin C and Choi et al observed only a significant decrease in glutathione. However, they reported a follicular excess of pro-inflammatory cytokines (IL6 and 8) with a positive correlation between these cytokines and biomarkers of oxidative stress [35].
Seino et al. found an excess of follicular 8-OHdG in the endometriosis subgroup, but without any correlation with IVF laboratory and clinical parameters [36].
Finally, our study is consistent with Vàrnagy et al. which found no significant difference in follicular or serum 8-OHdG levels in the endometriosis group but did find a correlation between follicular 8-OHdG and embryonic quality [37].
8OHdG is a biomarker of guanosine oxidation, and therefore of oxidative stress damage to DNA. The association between oxidative stress and cell death is well described in the literature [3] [29] [38]. It is for this reason that we specifically chose this marker to be coupled to the cfDNA assay and that it was standardized by the latter so as not to over estimate the assay by the excess cf DNA itself.
However, the impact of this oxidative stress and follicular cell death on the results of ART, a fortiori in endometriosis, is not yet clearly elucidated.
Our study is the first to combine the quantification of follicular cfDNA with the assay of 8OHdG in the specific population of endometriosis.
The serum and follicular circulating DNA assays did not differ significantly according to the presence or absence of endometriosis or endometrioma.
Nor was there any correlation between cfDNA and 8OHdG levels.
On the other hand, a negative correlation was found between ALU 115, ALU 247 and the rate of oocyte maturity in the entire cohort. Furthermore, we found a positive correlation between follicular cfDNA and age of patients which argue the hypothesis of accelerate apoptosis in growing follicle of ageing women [39].
Follicular cfDNA has already been studied in infertile patients of all causes [25], in particular qPCR using the ALU 115 and 247 primers. Scalici et al. found a negative correlation between circulating DNA measured in follicular fluid and the size of follicles collected [25], Latif Khan et al. found a negative correlation between cfDNA and follicular melatonin levels (which prevent oxidative stress), oocyte maturity of the oocyte from the follicle and the chances of pregnancy [27].
Using pooled follicular fluid, Traver et al. found a significantly higher level of circulating DNA in patients with ovarian reserve disorders and evaluated it as a predictive marker of clinical pregnancy with a sensitivity of 88% and a specificity of 60% [26]. Casteilero Alves et al, found a significant elevation of ALU 247 in patients with endometriosis, PCOS and reduced ovarian reserve compared with idiopathic infertility, with a predictive effect of follicular cfDNA on embryo fragmentation rates and clinical pregnancy [40].
In contrast to these studies, our study focused specifically on the endometriosis population with normal ovarian reserve, taking care to exclude patients who may have elevated oxidative stress, such as PCOS. Almost as many endometriosis patients as "control" patients were included.
Because of well-known inflammatory and pro-oxidative profile in endometriosis, our team expected to find an increase in cfDNA and markers of oxidative stress, especially in the presence of endometriomas which produce ROS, notably via the endoplasmic reticulum [29,41].
These results also contradict the study by Toya et al, who analyzed the cell cycle using flow cytometry on granulosa cell cultures and found an increased rate of apoptosis in endometriosis. This was a technically different analysis, using cell culture but with a very small number of patients (9 endometriosis) [20].
Our study is the first to combine a follicular cfDNA quantification with simultaneous serum quantification. There was no significant difference according to the presence or absence of endometriosis and this result is not consistent with the study by Zacchariah et al. which found a significant increase in plasma cfDNA in patients with non-severe endometriosis. The DNA extraction kit used was not the same and the amplification primers for RT-PCR were not the same as in our study [42].
We felt that it would be methodologically interesting to evaluate the serum and follicular quantification simultaneously, as they were potentially related. And although there was a positive correlation between follicular and serum cfDNA in this study, the assay ratio is around 100 times higher in follicular fluid, which meant that this bias could be ruled out [43].
One limitation of our study can be the initial volume of follicular fluid used for DNA extraction, which may have been insufficient and therefore gave rise to greater variability, the freezing time (48 hours) and thawing, which may have altered the integrity of the circulating DNA. The extraction with purification technique also had the potential to modify the results of the crude DNA concentrations, but it was carried out in a strictly identical manner throughout the cohort [44].
Finally, as in the case of autoimmune diseases, it is possible that patients with endometriosis, in response to activation of the immune system, have increased production of DNase, which may counteract the circulating DNA assay and bias this technique for assessing cell death [45,46].
About the origin of cell death, ALU 247/ALU115 ratio was on average 0.51 in cases of endometriosis and 0.52 in the absence of endometriosis, indicating a non-exclusively apoptotic origin.
Latif Khan et al. described a ratio ranging from 0 to 0.59 with an average of 0.129 indicating more apoptotic event [27].
Thus, there appear to be other cell death pathways associated with apoptosis, such as senescence, necrosis or ferroptosis, which would require other methods of evaluation [3,47].
Ferroptosis is a cell death pathway mediated by the oxidative role of iron. It appears to be of particular interest in the pathology of endometriosis, given the impact of the presence of excess iron in the oxidative stress of endometriosis. It would therefore be interesting to assess the activity of ferroptosis in follicular fluid by targeting the regulators of lipid peroxidation [48].
Finally, 8OHdG and follicular circulating DNA have nevertheless proved to be interesting predictive markers for IVF management results.
ALU 115 and 247 were negatively correlated with oocyte maturity, reinforcing the role of granulosa cells in oogenesis and folliculogenesis [9].
8OHdG was negatively correlated with the fertilization rate and the number of good embryo quality in the entire cohort, whereas the rate of abnormal oocytes, determined morphologically, was not correlated with embryo quality.
This supports the idea that analysis of oocyte quality (maturation and competency) using morphological criteria is subjective and should be supplemented by biological markers [49].
Although we excluded any other factor in favor of an inflammatory process or involving pelvic oxidative stress in the "control" population and the groups were comparable, patients who served as "controls" remained infertile patients with a potential excess of peritoneal oxidative stress.
It could also be critical to compare the data coming from a mature follicle with the results of an entire oocyte cohort, but we felt that this was more interesting than analyzing a pool of follicular fluid coming from follicles of different grade of maturity.
Another limitation of this study is that the mature follicle was defined with ultrasonography and that oocyte maturity, linked to local constraints, could not be authenticated in 30% of cases.
In conclusion, follicular 8OhdG, serum and follicular cell free DNA were not significantly different in deep pelvic endometriosis patients with normal ovarian reserve compared with other infertile patients. In endometriosis, excess cell death within the follicular fluid has not yet been clearly demonstrated in the literature, and the question of oocyte quality remains open.
However, follicular 8OHdG and follicular cfDNA have been shown to be potential predictive markers of IVF outcomes in this cohort of patients. These biomarkers could potentially help to assess oocyte quality. More studies are needed to evaluate their predictive value for pregnancy outcome by selecting the more competent embryo for implantation and clinical outcomes improvement.
Acknowledment
The authors express sincere appreciation to the participants who took part in this study mainly our ART and Genetics teams members
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- Moradi Y, Shams-Beyranvand M, Khateri S, et al. A systematic review on the prevalence of endometriosis in women. Indian J Med Res. mars 154 (2021): 446-454.
- D’Hooghe TM, Debrock S, Hill JA, et al. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. mai 21 (2003): 243-254.
- Lin X, Dai Y, Tong X, et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. févr 30 (2020): 101431.
- Samimi M, Pourhanifeh MH, Mehdizadehkashi A, et al. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J Cell Physiol 234 (2019): 19384-19392.
- Murta M, Machado RC, Zegers-Hochschild F, et al. Endometriosis does not affect live birth rates of patients submitted to assisted reproduction techniques: analysis of the Latin American Network Registry database from 1995 to 2011. J Assist Reprod Genet. août 35 (2018): 1395-1399.
- Harb HM, Gallos ID, Chu J, et al. The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta-analysis. BJOG Int J Obstet Gynaecol 120 (2013): 1308-1320.
- Kitajima M, Defrère S, Dolmans MM, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril 96 (2011): 685-691.
- Scutiero G, Iannone P, Bernardi G, et al. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid Med Cell Longev [Internet] [cité 15 juin 2020]: 2017.
- Du YB, Gao MZ, Shi Y, et al. Endocrine and inflammatory factors and endometriosis-associated infertility in assisted reproduction techniques. Arch Gynecol Obstet. janv 287 (2013): 123-130.
- Goud PT, Goud AP, Joshi N, et al. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil Steril. juill 102 (2014): 151-159.
- Karuputhula NB, Chattopadhyay R, Chakravarty B, Chaudhury K. Oxidative status in granulosa cells of infertile women undergoing IVF. Syst Biol Reprod Med. avr 59 (2013): 91-98.
- Singh AK, Chattopadhyay R, Chakravarty B, et al. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod Toxicol Elmsford N. déc 42 (2013): 116-124.
- Sanchez AM, Somigliana E, Vercellini P, et al. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact. J Steroid Biochem Mol Biol. janv 155 (2016): 35-46.
- Lu X, Wu Y, Gao XH, et al. Effect of letrozole on estradiol production and P450 aromatase messenger RNA expression of cultured luteinized granulosa cells from women with and without endometriosis. Fertil Steril. juill 98 (2012): 131-135.
- Giorgi VSI, Da Broi MG, Paz CCP, et al. N-Acetyl-Cysteine and l-Carnitine Prevent Meiotic Oocyte Damage Induced by Follicular Fluid From Infertile Women With Mild Endometriosis. Reprod Sci Thousand Oaks Calif. mars 23 (2016): 342-351.
- Xu B, Guo N, Zhang X min, et al. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci Rep. 29 mai 5 (2015): 10779.
- Orazov MR, Radzinsky VY, Ivanov II, et al. Oocyte quality in women with infertility associated endometriosis. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol 35 (2019): 24-26.
- Terao H, Wada-Hiraike O, Nagumo A, et al. Role of oxidative stress in follicular fluid on embryos of patients undergoing assisted reproductive technology treatment. J Obstet Gynaecol Res. sept 45 (2019): 1884-1891.
- Roberts RA, Laskin DL, Smith CV, et al. Nitrative and oxidative stress in toxicology and disease. Toxicol Sci Off J Soc Toxicol. nov 112 (2009): 4-16.
- Toya M, Saito H, Ohta N, et al. Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil Steril. févr 73 (2000): 344-350.
- Bonfoco E, Krainc D, Ankarcrona M, et al. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1 août 92 (1995): 7162-7166.
- Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 25 mai 149 (2012): 1060-1072.
- Corn CM, Hauser-Kronberger C, Moser M, et al. Predictive value of cumulus cell apoptosis with regard to blastocyst development of corresponding gametes. Fertil Steril. sept 84 (2005): 627-633.
- Høst E, Gabrielsen A, Lindenberg S, et al. Apoptosis in human cumulus cells in relation to zona pellucida thickness variation, maturation stage, and cleavage of the corresponding oocyte after intracytoplasmic sperm injection. Fertil Steril. mars 77 (2002): 511-515.
- Scalici E, Traver S, Molinari N, et al. Cell-free DNA in human follicular fluid as a biomarker of embryo quality. Hum Reprod Oxf Engl. déc 29 (2014): 2661-2669.
- Traver S, Scalici E, Mullet T, Molinari N, Vincens C, Anahory T, et al. Cell-free DNA in Human Follicular Microenvironment: New Prognostic Biomarker to Predict in vitro Fertilization Outcomes 10 (2015): e0136172.
- Latif Khan H, Bhatti S, Latif Khan Y, et al. Cell-free nucleic acids and melatonin levels in human follicular fluid predict embryo quality in patients undergoing in-vitro fertilization treatment. J Gynecol Obstet Hum Reprod. 28 août (2019): 101624.
- Marzese DM, Hirose H, Hoon DSB. Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients. Expert Rev Mol Diagn. nov 13 (2013): 827-844.
- Kunitomi C, Harada M, Takahashi N, et al. Activation of endoplasmic reticulum stress mediates oxidative stress-induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol Hum Reprod. déc (2019): 40-52.
- Umetani N, Kim J, Hiramatsu S, et al. Increased integrity of free circulating DNA in sera of patients with colorectal or periampullary cancer: direct quantitative PCR for ALU repeats. Clin Chem. juin 52 (2006): 1062-1069.
- Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. juin 11 (1999): 307-311.
- Carvalho LFP, Abrão MS, Biscotti C, et al. Oxidative cell injury as a predictor of endometriosis progression. Reprod Sci Thousand Oaks Calif. juin 2013; 20 (2013): 688-698.
- Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. avr 44 (2008): 280-287.
- Da Broi MG, de Albuquerque FO, de Andrade AZ, et al. Increased concentration of 8-hydroxy-2’-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res 366 (2016): 231-242.
- Choi YS, Cho S, Seo SK, et al. Alteration in the intrafollicular thiol-redox system in infertile women with endometriosis. Reprod Camb Engl. févr 149 (2015): 155-162.
- Seino T, Saito H, Kaneko T, et al. Eight-hydroxy-2’-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril. juin 77 (2002): 1184-1190.
- Várnagy Á, Koszegi T, Györgyi E, et al. Levels of total antioxidant capacity and 8-hydroxy-2’-deoxyguanosine of serum and follicular fluid in women undergoing in vitro fertilization: focusing on endometriosis. Hum Fertil Camb Engl. sept 23 (2020): 200-208.
- Tsai-Turton M, Luong BT, Tan Y, et al. Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol Sci Off J Soc Toxicol. juill 98 (2007): 216-230.
- Bozkurt B, Erdem M, Mutlu MF, et al. Comparison of age-related changes in anti-Müllerian hormone levels and other ovarian reserve tests between healthy fertile and infertile population. Hum Fertil Camb Engl. sept 19 (2016): 192-198.
- Casteleiro Alves MM, Oliani L, Almeida M, et al. Cell-Free DNA as a New Biomarker of IVF Success, Independent of Any Infertility Factor, Including Endometriosis. Diagn Basel Switz. janv 13 (2023): 208.
- Sanchez AM, Viganò P, Somigliana E, et al. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. avr 20 (2014): 217-230.
- Zachariah R, Schmid S, Radpour R, et al. Circulating cell-free DNA as a potential biomarker for minimal and mild endometriosis. Reprod Biomed Online. mars 18 (2009): 407-411.
- Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. juin 82 (2010): 1021-1029.
- Huebner H, Lubrich H, Blum S, et al. Comparison of methods for isolation and quantification of circulating cell-free DNA from patients with endometriosis. Reprod Biomed Online. nov 43 (2021): 788-798.
- Lauková L, Konecná B, Janovicová L, et al. Deoxyribonucleases and Their Applications in Biomedicine. Biomolecules. 11 juill 10 (2020): 1036.
- Janovicová L, Conka J, Lauková L, et al. Variability of endogenous deoxyribonuclease activity and its pathophysiological consequences. Mol Cell Probes. oct 65 (2022): 101844.
- Jancar N, Kopitar AN, Ihan A, et al. Effect of apoptosis and reactive oxygen species production in human granulosa cells on oocyte fertilization and blastocyst development. J Assist Reprod Genet. mars 24 (2007): 91-97.
- Sun Y, Chen P, Zhai B, et al. The emerging role of ferroptosis in inflammation. Biomed Pharmacother Biomedecine Pharmacother. juill 127 (2020): 110108.
- Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. févr 17 (2011): 34-45.
