Outpatient Administration of Blinatumomab Infusion and Hematopoietic Stem Cell Transplantation as Treatment for Patients with Refractory Acute Lymphoblastic Leukemia in a Pediatric Oncologic Center in Mexico
Article Information
Godoy-Fernández JF1, Cubría-Juárez MP2, Zapata-Tarrés M3, Taveras-Rodríguez O1, Vera-Hernández M4, Briseño- Rebollar EV5, Bautista-Hernández I6, Vega-Vega L7, Ellis-Irigoyen A8, Escamilla-Asiain G9, Bernabé-Gaspar LE10, Pérez-García M11*
Affiliation:
1Resident of the pediatric program for hematopoietic stem cell transplantation, Hospital Infantil Teletón de Oncología. México
2Chief of the Acute Lymphoblastic Leukemia Department, Hospital Infantil Teletón de Oncología. México
3Research Coordination, Mexican Institute of Social Services Foundation, Fundación IMSS. México
4Nurse coordinator stem cell transplantation, Hospital Infantil Teletón de Oncología. México
5Department of hematopoietic stem cell transplantation, Hospital Infantil Teletón de Oncología. México
6Chief of the Oncologic Surgery Department, Hospital Infantil Teletón de Oncología. México
7Director, Hospital Infantil Teletón de Oncología. México
8Chief of Department Oncology, Hospital Infantil Teletón de Oncología. México
9Medical Director. Hospital Infantil Teletón de Oncología. México
10Faculty of Medicine, Benemérita Universidad Autónoma de Puebla
11Chief of the hematopoietic stem cell transplantation Department, Hospital Infantil Teletón de Oncología, México
*Corresponding author: Pérez-García Martin, Chief of the hematopoietic stem cell transplantation Department, Hospital Infantil Teletón de Oncología, México.
Received: September 06, 2023; Accepted: September 13, 2023; Published: October 04, 2023
Citation: Godoy-Fernández JF, Cubría-Juárez MP, Zapata-Tarrés M, Taveras-Rodríguez O, Briseño-Rebollar EV, Bautista-Hernández I, Vega- Vega L, Ellis-Irigoyen A, Escamilla-Asiain G, Bernabé-Gaspar LE, Pérez-García M. Outpatient Administration of Blinatumomab Infusion and Hematopoietic Stem Cell Transplantation as Treatment for Patients with Refractory Acute Lymphoblastic Leukemia in a Pediatric Oncologic Center in Mexico. Journal of Biotechnology and Biomedicine. 6 (2023): 446-449.
View / Download Pdf Share at FacebookAbstract
Acute Lymphoblastic Leukemia (ALL) is the most common pediatric cancer and the most common subtype of pediatric leukemia, the high toxicity of chemotherapy, the relapse and refractory disease of many patients require the development of novel therapeutic approaches such as targeted immunotherapy in combination with Hematopoietic Stem Cell Transplantation (HSCT). These new therapeutic approaches must be taken in count to revolutionize the management of numerous ALL subtypes, improve treatment outcomes and maintain low toxicity without losing the effectiveness of the treatment. Our retrospective, longitudinal, observational, descriptive study evaluated a novel therapeutic approach in a Pediatric Oncologic Center in Mexico, the outpatient administration of blinatumomab infusion in patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). The study demonstrated that 100% of the patients went into remission after Blinatumomab infusion and there was no mortality associated.
Keywords
Acute lymphoblastic leukemia; Pediatric; Advances; Mexico; Blinatumomab; Treatment; Hematopoietic cell stem transplantation
Article Details
Introduction
Acute Lymphoblastic Leukemia (ALL) is the leading cause of cancer in the pediatric population in Mexico [1,2]. A significant percentage of these patients drop out of treatment, being this and relapses the most common cause of treatment failure. The intensification of conventional chemotherapy has improved the survival of patients with ALL; however, it involves a high rate of toxicity and death [3]. Significant advances in targeted immunotherapy and molecular therapeutics, as well as in risk stratification for therapy based on germline somatic genetic analysis and minimal residual disease (MRD) monitoring, have enabled the development of targeted and effective immunotherapies in its treatment, managing to reduce the intensity and toxicity associated with conventional chemotherapy [4-6]. Blinatumomab is a low-toxicity immunotherapy, but it does not maintain prolonged remissions; [7] while Hematopoietic Stem Cell Transplantation is a cellular immunological treatment that allows more prolonged remissions [8-11]. The administration of Blinatumomab before HSCT reduces MRD, and has favorable results in leukemia-free survival, toxicity, and overall survival. It provides a complete response rate of up to 40% in second or third relapses and contributes substantially to long-term remission of the disease, proving to be a useful treatment alternative in patients with R/R ALL [12-17].
Aim
To evaluate the use of Blinatumomab and HSCT as treatment in ambulatory patients with refractory acute lymphoblastic leukemia in terms of overall survival and disease-free survival in pediatric patients treated at Hospital Infantil Teletón de Oncología (HITO).
Materials and Methods
We conducted a retrospective, longitudinal, observational, descriptive study where we considered variables such as: age, gender, percentage of bone marrow blasts in relapse, extramedullary disease, and complete remission prior to blinatumomab administration. The number of blinatumomab cycles received and complications such as: infections, cytokine release syndrome, overall survival rate, disease-free survival rate, and cause of death. Pediatric patients between 0 and 18 years of age with a diagnosis of ALL (R/R), treated with blinatumomab and HSCT treated at HITO were included (Table 1). A multidisciplinary team composed of physicians, nurses, and pharmacists was created to address administration challenges associated with blinatumomab infusions. Although blinatumomab requires a 28-day continuous infusion, it is not necessary for patients to remain hospitalized for the entire cycle. To ensure tolerability prior to discharge, patients are monitored closely during the first 3 days of Cycle 1 for signs of cytokine release syndrome and neurological toxicities. Once discharged to the shelter, next to the hospital, the patients remain under supervision, where we check them every 72 hours for infusion pump bag change. The population was non-randomized, consecutively diagnosed with ALL (R/R) according to hospital records from January 1st, 2015, to June
1st, 2020. The statistical analysis was performed using SPSS version 25.0 software. All transplants were performed with informed consent signed by the parent or tutor responsible of the patients under 18 years of age.
Results
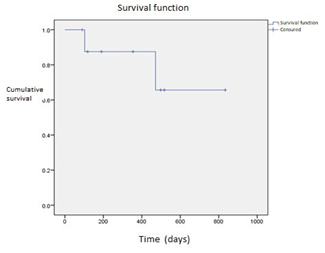
Blinatumomab was administered as treatment for R/R ALL prior to HSCT in 9 patients (Table 1). The mean age at the time of administration was 10.1 years with a SD ±4.84. The distribution of cases by gender corresponds to 66% women (n= 5) and 44% men (n=4). All patients had an oncological diagnosis of ALL and 100% of them presented refractoriness and/or relapse. In the evaluation prior to the administration of Blinatumomab, 88.8% of the patients presented positive MRD (N=8) and 11.2% of the negative cases (N=1). No patient had extramedullary disease, the number of cycles of Blinatumomab received to achieve complete remission and negative MRD were different; 4 patients received 1 cycle (44%), 3 patients received 3 cycles (33%), and 2 patients received 2 cycles (22.2%), 56% of the cases achieved deep complete remission at the end of treatment or at the last consultation, 12% achieved a first complete remission with one blinatumomab cycle and 88% required a second or third cycle to achieve it. Complications: 33% of the patients presented Cytokine Release Syndrome (n=3), with 22% being moderate grade and the most frequent (n=2) and 11% mild (n=1). 2 were admitted to the Pediatric Intensive Care Unit. Four patients died (44.4%). The most frequent cause was associated with infections: pneumonia 50% (n=2) with isolation of Pneumocystis jirovecii, septic shock with 1 case, and hemorrhagic syndrome in 1 patient. A 3-year disease- free survival and overall survival of 55% was determined
Table 1: Outpatient administration of Blinatumomab Infusion and Hematopoietic Stem Cell Transplantation as Treatment for Patients with Refractory Acute Lymphoblastic features. 2015-2020. Hospital Infantil Teletón Oncología.
|
No. |
AGE |
GENDER |
MRD |
MRD BLIN |
MRD BLIN |
MRD BLIN |
|
IR / RR |
Cycle 1 |
Cycle 2 |
Cycle 3 |
|||
|
1 |
9 |
M |
24.56% |
0.01% |
<0.01% |
- |
|
2 |
13 |
F |
6.12% |
<0.01% |
- |
- |
|
3 |
15 |
F |
18.60% |
0.21% |
0.01% |
<0.01% |
|
4 |
11 |
M |
0.21% |
<0.01% |
- |
- |
|
5 |
7 |
F |
6.80% |
<0.01% |
<0.01% |
<0.01% |
|
6 |
1 |
F |
0.01% |
0.01% |
- |
- |
|
7 |
17 |
M |
21.40% |
1.85% |
0,01% |
- |
|
8 |
10 |
F |
0.37% |
0.02% |
0.51% |
0.01% |
|
9 |
9 |
M |
4.77% |
<0.01 |
- |
- |
(Graph 1). Brown and cols conclude that the comparing high and intermediate risk first relapse of B-ALL, post reinduction treatment with blinatumomab compared with chemotherapy followed by transplant is not statistically significant with disease free survival. Our series includes only refractory ALL patients which represent a very positive result. Adverse events are like the ones described in the literature.
Discussion
Acute lymphoblastic leukemia is the most common type of cancer in children and unfortunately, about 15% of children with high-risk B-ALL relapse after chemotherapy treatment, so there is a need for new treatment options for these patients [18]. Blinatumomab is an adequate bridge therapy to consolidate transplantation in these patients, since it has been shown to be more effective and is associated with a lower number of toxicities and less severe complications, compared to intensive chemotherapy, allowing to obtain favorable hematological conditions for the performance of a transplant in terms of complete remission and residual disease, with satisfactory clinical tolerance. The international literature describes an overall survival of 2 years with blinatumomab use of 71. 3% versus 58.4% without the use of it [19] however, it is a treatment of high cost and difficult access for developing countries, such as Mexico. This series of patient cases represents a clear example of this situation. HITO is the unique specialized pediatric cancer hospital in Mexico. It was inaugurated in 2013 and began offering the HSCT service in 2014. At the beginning the number of HSCT procedures were approximately 4 to 5 per year. Through the years, this has been changing for the better, growing and improving the quality of care, allowing us to offer HSCT services to more patients. In 2019, the opportunity arises to
introduce blinatumomab as a treatment in our institution. Nine patients were included in the present study. Comparing our results with other series is difficult; mainly because of the number of patients and because we do not randomize them. However, comparing the overall survival of these patients with those who do not have access to blinatumomab, as is the case with most of the patients in developing countries, the use of blinatumomab gives us a better prognosis and clearly a statistically better incentive for our country and institution. Probably all patients with positive MRD would have died. In Mexico this experience is an important success and challenge for other institutions to replicate.
Conclusion
Despite important advances in the treatment of ALL, HSCT remains the therapy of choice for patients with failed induction in first remission, relapsed, and refractory ALL. Administering Blinatumomab prior to HSCT with the goal of achieving a negative MRD, deep molecular remission provides a favourable prognosis with better leukemia- free survival rates, overall survival, and less toxicity. The highlights are that 100% of the patients went into remission after Blinatumomab infusion and there was no mortality associated. Our results represent an innovation in Mexico for pediatric patients with refractory ALL and a background to generate experience in a medical group.
Conflicts of interest
None of the authors have a conflict of interest to disclose.
Acknowledgments
None.
References
- Rivera-Luna R, Shalkow-Klincovstein J, Velasco- Hidalgo L, et al. Descriptive Epidemiology in Mexican children with cancer under an open national public health insurance program. BMC Cancer 14 (2014): 790.
- Reyes-León A, Juárez-Velázquez R, Medrano-Hernández A, et al. Expression of Ik6 and Ik8 Isoforms and Their Association with Relapse and Death in Mexican Children with Acute Lymphoblastic PLoS One 10 (2015): e0130756.
- Malard F, Mohty Acute lymphoblastic leukaemia. Lancet 395 (2020): 1146-1162.
- Mullighan CG, Phillips LA, Su X, et Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 322 (2008): 1377-1380.
- Bailey LC, Lange BJ, Rheingold SR, et al. Bone-marrow relapse in paediatric acute lymphoblastic Lancet Oncol 9 (2008): 873-883.
- Inaba H, Pui Immunotherapy in pediatric acute lymphoblastic leukemia. Cancer Metastasis Rev 38 (2019): 595-610.
- Yu J, Wang W, Huang Efficacy and safety of bispecific T-cell engager (BiTE) antibody blinatumomab for the treatment of relapsed/refractory acute lymphoblastic leukemia and non-Hodgkin's lymphoma: a systemic review and meta-analysis. Hematology 24 (2019): 199- 207.
- Maloney KW, Gore Agents in Development for Childhood Acute Lymphoblastic Leukemia. Pediatric Drugs 20 (2017): 111-120.
- Bailey LC, Lange BJ, Rheingold SR, et al. Bone-marrow relapse in paediatric acute lymphoblastic Lancet Oncol 9 (2008): 873-883.
- Sureda A, Bader P, Cesaro S, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant 50 (2015): 1037-1056.
- Locatelli F, Schrappe M, Bernardo ME, et al. How I treat relapsed childhood acute lymphoblastic leukemia. Blood 120 (2012): 2807-2816.
- Rafei H, Kantarjian HM, Jabbour Recent advances in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma 60 (2019): 2606-2621.
- August KJ, Guest EM, Lewing K, et al. Treatment of children with relapsed and refractory acute lymphoblastic leukemia with mitoxantrone, vincristine, pegaspargase, dexamethasone, and bortezomib. Pediatr Blood Cancer 67 (2020): e28062.
- Mustafa O, Abdalla K, AlAzmi AA, et al. FLAG/FLAG- IDA regimen for children with relapsed/refractory acute leukemia in the era of targeted novel therapies. J Oncol Pharm Pract 25 (2019): 1831-1838.
- Keating AK, Gossai N, Phillips CL, et Reducing minimal residual disease with blinatumomab prior to HCT for pediatric patients with acute lymphoblastic leukemia. Blood Adv 3 (2019): 1926-1929.
- Majhail NS, Farnia SH, Carpenter PA, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Biol Blood Marrow Transplant 21 (2015): 1863-1869.
- Brown PA, Ji L, Xu X, et al. Effect of Postreinduction Therapy Consolidation With Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults With First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical JAMA 325 (2021): 833-842.
- Bhojwani D, Pui Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol 14 (2013): e205-e217.
- Freyer DR, Devidas M, La M, et al. Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children's Oncology Blood 117 (2011): 3010-3015.