Outcome of Kidney Transplant Recipients with Graft Failure
Article Information
Rusen Cosar1*, Ulku Korkmaz2, Necdet Sut3, Alaattin Ozen4, Kamuran Ibis5, Fulya Oz-Puyan6, Eylül Senödeyici7, Sarper K?z?lkaya7, Sule Parlar1, Dilek Nurlu1, Talar Ozler1 Aydogan Yalcin8 and Gulay Durmus-Altun2
1Trakya University Faculty of Medicine, Department of Radiation Oncology, Edirne, Turkey
2Trakya University Faculty of Medicine, Department of Nuclear Medicine, Edirne, Turkey
3Trakya University Faculty of Medicine, Department of Biostatistics, Edirne, Turkey
4Eskisehir Osmangazi University Faculty of Medicine, Department of Radiation Oncology, Eskisehir, Turkey
5Istanbul University Faculty of Medicine, Department of Radiation Oncology, Istanbul, Turkey
6Trakya University Faculty of Medicine, Department of Pathology, Edirne, Turkey
7Trakya University School of Medicine, Edirne, Turkey
8Trakya University Institute of Health Sciences, Radiation Biology Department, Edirne, Turkey
*Corresponding Author: Rusen COSAR, MD. Trakya University, Faculty of Medicine, Department of Radiation Oncology, Edirne, Turkey.
Received: 29 April 2023; Accepted: 10 May 2023; Published: 25 April 2023
Citation: Ali AlShaqaq, Maher AlDemerdash, Meteb M AlBugami, Baher Elgadaa, Najib Musaied, Ibtihal Shaikh, Alwaiah AlRamdhan, Abdulnaser AlAbadi, Khaled Hamawi, Fahad E AlOtaibe, Khalid Bel’eed Akkari. Outcome of Kidney Transplant Recipients with Graft Failure. Archives of Nephrology and Urology. 6 (2023): 37-43.
View / Download Pdf Share at FacebookAbstract
Background: Kidney transplantation is the optimal choice for patients with end stage renal disease. However, most kidney grafts will fail at some point during the lifetime of the recipient. This study presents outcome data in patients with failed kidney graft.
Methods: Data from 1309 kidney transplant recipients were reviewed. Multivariable cox regression analysis was used to study the predictors of graft and patient outcomes.
Results: We identified 85 kidney transplant recipients with graft failure and matched them to 170 patients with functioning graft. Mean age of the participants was 44.9 (±15.7) years. Chronic rejection was the most common cause of graft failure (31.7%). Fifty-five patients (64.7%) return to dialysis after graft failure, 13 patients (15.3%) underwent repeat transplantation, and 17 patients (20%) died. A multivariable cox regression analysis showed that increased age was associated with worse patient survival. Graft loss was associated with the diagnosis of diabetes mellitus and hypertension.
Conclusion: Patients with kidney graft failure experience significant morbidity and mortality. Strategies to optimize outcomes of such patients are needed with a focus on maximizing opportunities for re-listing and repeat transplantation.
Keywords
Graft failure; Kidney transplant recipients; Repeat transplantation
Graft failure articles; Kidney transplant recipients articles; Repeat transplantation articles
Graft failure articles Graft failure Research articles Graft failure review articles Graft failure PubMed articles Graft failure PubMed Central articles Graft failure 2023 articles Graft failure 2024 articles Graft failure Scopus articles Graft failure impact factor journals Graft failure Scopus journals Graft failure PubMed journals Graft failure medical journals Graft failure free journals Graft failure best journals Graft failure top journals Graft failure free medical journals Graft failure famous journals Graft failure Google Scholar indexed journals Kidney transplant recipients articles Kidney transplant recipients Research articles Kidney transplant recipients review articles Kidney transplant recipients PubMed articles Kidney transplant recipients PubMed Central articles Kidney transplant recipients 2023 articles Kidney transplant recipients 2024 articles Kidney transplant recipients Scopus articles Kidney transplant recipients impact factor journals Kidney transplant recipients Scopus journals Kidney transplant recipients PubMed journals Kidney transplant recipients medical journals Kidney transplant recipients free journals Kidney transplant recipients best journals Kidney transplant recipients top journals Kidney transplant recipients free medical journals Kidney transplant recipients famous journals Kidney transplant recipients Google Scholar indexed journals Repeat transplantation articles Repeat transplantation Research articles Repeat transplantation review articles Repeat transplantation PubMed articles Repeat transplantation PubMed Central articles Repeat transplantation 2023 articles Repeat transplantation 2024 articles Repeat transplantation Scopus articles Repeat transplantation impact factor journals Repeat transplantation Scopus journals Repeat transplantation PubMed journals Repeat transplantation medical journals Repeat transplantation free journals Repeat transplantation best journals Repeat transplantation top journals Repeat transplantation free medical journals Repeat transplantation famous journals Repeat transplantation Google Scholar indexed journalsArticle Details
1. Introduction
The use of ionizing radiation for locoregional tumor control in pediatric patients demands a good balance between documented efficacy and potential long-term toxicity (1-4). Because of their immature tissues, children are highly sensitive to radiotherapy, unlike adults, besides modern photon therapy approaches, proton therapy has differences because it provides more successful survival and tumor control and reduces the rate of side effects (3). Despite successful treatment of pediatric cancers, 50% of cancer survivors have one or more delayed somatic sequelae, and one-third have serious and life-threatening complications. Therefore, there is a general consensus that childhood cancer survivors should be followed up in adolescence and adulthood (2,3). Erman et al. showed that the risk of late sequelae increased to 3.8 especially in patients who used three of the multimodal treatment approaches such as surgery, radiotherapy and chemotherapy. As a result, as the years progressed in the treatment of childhood cancer, radiotherapy has either been used in lower doses than necessary or has been abandoned in clinical practice (2).
Although reports of long follow-up in the surviving childhood tumor patient population are limited, it is no coincidence that the risk of late sequelae is lowest in patients with leukemia and highest in patients with kidney tumors, and this sequela is often associated with kidney damage. Because long-term renal dysfunction due to severe kidney damage is evaluated in the group of serious and fatal side effects in patients who have been treated with platinum, ifosfamide agents, and who have undergone abdominal radiotherapy or nephrectomy (5).
Irradiation to the abdomen is an integral part of treatment in most frequent extracranial pediatric tumors, such as Wilms’ tumor, neuroblastoma, non-Hodgkin’s lymphoma, and Hodgkin’s disease (6-8). In addition to radiotherapy, whole body irradiation is an alternative to cyclophosphamide in preparing leukemia patients for bone marrow transplantation. The biggest obstacle to the debate on whether kidney protection should be applied, especially so that the kidneys receive less doses, is the risk of recurrence in the kidney bed (9).
In childhood cancer survivors, the defined risk for late-onset renal failure increases 4-fold when the radiotherapy kidney dose is >15 Gy and decreases 0.8-fold when the RT cutoff dose is < 10 Gy (5). However, the use of a protective agent to be used before the application of radiotherapy, the prevention of the formation of radioprotection kidney damage in cases where it cannot be kept below 10 Gy, can reduce the rate of kidney failure, which is 8.9 times higher than its peers, and we can recommend an agent that can strengthen the hand of radiation oncologists dealing with pediatric tumors in treatment planning (6,10).
The agents used in experimental studies to prevent the emergence and development of radiation-induced nephropathy (RIN) have been agents such as geinstein, melatonin (10,11), amifostine (11-13), and angiotensin converting enzyme (ACE) inhibitors (11,15). We aimed to examine whether L-carnitine has an effect on RIN in our experiment, since the drug to be used for radioprotection will have no side effects, especially in infant rats representing the childhood tumor group.
L-carnitine is an agent with antioxidant properties that inhibits the free radical formation and helps repair membrane lipids by protecting them from damage caused by oxidation (16-19). In this context, the nephroprotective effects of L-carnitine in experimental models have been demonstrated in ischemic (20), diabetic (21) and hypertension-related models (22). In cancer treatment, it has been studied that L-carnitine has a protective effect by scavenging free radicals by increasing the activity of the superoxide dismutase enzyme, both on chemotherapy-induced organ toxicity (23-30) and the effects of ionizing radiation that causes RIN (16,31-32). However, the possible effects of L-carnitine on infancy RIN have not been thoroughly studied.
In our study, one-month-old rats after weaning from breast milk were used to examine RIN occurrence in the treatment of childhood tumors. This study aims to investigate the protective effects of L-carnitine against RIN in infant rats by using functional imaging and histopathological evaluation. Our study is the first study conducted for radioprotection on kidney tissue in infant rats.
2. Materials and Methods
2.1 Animals
Forty infant Wistar albino male rats (100±20 gr) were housed with their mothers until they reached four weeks old. Later, they were housed in rat cages with free access to a standard rodent diet and tap water under a 12-hour artificial light-dark cycle, with a mean temperature of 21 ± 2 °C and a mean humidity of 55 ± 2%. All animals were randomized to four groups. Group 1 (n = 10) was the control group and was treated with intraperitoneal injection (i.p.) of normal saline alone (10 ml/kg). Group 2 (n = 10) received L-carnitine alone. (300 mg/kg) i.p. Group 3 (n = 10) received irradiation alone (radiotherapy) and was injected with saline (10 ml/kg) i.p. 30 minutes before irradiation. Group 4 (n = 10) received L-carnitine (300 mg/kg) i.p. 30 minutes before irradiation (L-carnitine + radiotherapy).
All procedures were performed in accordance with the Declaration of Helsinki of the World Medical Association. The animal study protocol was approved by Trakya University Faculty of Medicine Institutional Animal Care and Use Committee. All experimental procedures were performed on anesthetized rats. Anesthesia was maintained with ketamine and xylazine (35 mg/kg body weight (BW) and 3 mg/kg BW intramuscular injection for infant rats, during irradiation and scintigraphic evaluation (27). Rats were followed up for three months after the experimental procedures. During the follow-up period, all rats received veterinary care.
2.2 Irradiation
The rats in the radiotherapy (RT) and L-carnitine + RT groups were irradiated individually with a single dose of 8 Gy (6 MV photon) at a depth of 2.25 cm through an anterior 2.5 × 2 cm single portal (with a 1.5 cm bolus) covering the left kidney in its entirety. A linear accelerator treatment unit (Varian 2100 C/D, Varian Inc., Palo Alto, CA) was used at a source skin distance of 100 cm. The dose rate was 600 MU/min. The rats were anesthetized, and then fixed on a 20×30 cm blue Styrofoam treatment couch (Med-Tec, Orange City, IA) in a prone position. The position of the kidney was determined in an X-ray scope image by measuring the distance between the tip of the nose and the middle of the kidney hilus. The distance information was used to position the center of the irradiation field to the middle of the kidney hilus in the anesthetized rat. The field’s correct positioning was confirmed for each individual rat using a therapy simulator (Mecaserto-Simics, Paris, France). Special dosimetry was performed for the irregular radiotherapy fields. The dose homogeneity across the radiotherapy field was ± 3%.
2.3 Imaging Study
At the third month after radiotherapy, all surviving animals were imaged with static DMSA cortical kidney scintigraphy to measure the damage to the cortical parenchyma in the kidney, and DTPA dynamic kidney scintigraphy to measure the deterioration in glomerular structures and possible obstructive effects.
For imaging, commercially available DMSA kit (Renocis, France) and DTPA kit (CIS, France) was prepared according to the manufacturer’s recommendations. 99mTc-DMSA was administered intravenously through the 24F cannula in the tail vein at a regular dose of 37-50 MBq (in 0.2 ml). Posteroanterior views of the abdomen of the rats were taken in the supine position (at least 300.000 counts/view) with a low-energy, high-resolution pinhole and parallel collimators in a 256 × 256 matrix format at 3 hours after the injection of Tc-99m DMSA. The relative uptake of the kidneys was quantitatively calculated by taking the geometric mean of the counts obtained by plotting the regions of interest for the kidney and background areas in the anterior and posterior images.
Dynamic scans were acquired for 20 minutes on a single-head gamma camera (Philips, Eindhoven, the Netherlands) with low-energy, high resolution, and parallel collimators in a 64×64 matrix format after the injection of 99mTc-DTPA. The time activity curve was obtained by placing regions of interest (ROIs) around the kidney and in the background, similar to the DMSA study.time to peak (Tmax) of the time activity curve and the half-time to peak activity (T1/2) were calculated automatically using the workstation DTPA evaluation software.In order to distinguish the deterioration due to the systemic effect of the treatment from the local RT effect, the right and left (RT-received kidney) counts were recorded and evaluated separately. All images were acquired using a single-head gamma camera (Philips, Eindhoven, The Netherlands).
2.4 Histopathological analysis
The left kidneys were dissected and decapsulated after imaging. They were later prefixed in formaldehyde for 24 hours for further histopathological evaluation. The kidneys were divided into halves with a central transverse section. After formalin fixation, kidneys were processed into paraffin wax tissue blocks, and thin tissue sections (4 μm) were produced using a microtome. All sections were then stained with hematoxylin and eosin (H&E) and evaluated under a light microscope. A certified pathologist who was blinded to the experimental protocol assessed the tissue sections.
To evaluate RIN, proximal tubular degeneration, proximal tubular atrophy, interstitial fibrosis, and glomerular damage were assessed microscopically. Occurrence of cytoplasmic eosinophilia, apical blebbing, loss of intercellular adhesions, cytoplasmic vacuolization, karyorrhexis, and karyolysis were defined as proximal tubular degeneration. Capillary loop collapse and dilatation of Bowman’s capsule were defined as glomerular damage. Proximal tubular degeneration, proximal tubular atrophy, interstitial fibrosis, and glomerular damage were scored as: 0 (no abnormality), 1 (weak lesions affecting <25% of the kidney samples), 2 (moderate lesions affecting 25-50% of the kidney samples), and 3 (marked lesions affecting >50% of the kidney samples).
2.5 Statistical analysis
Scintigraphy data are presented as mean (±) standard deviation (SD), and histopathological data are presented as median (min-max). Differences in the scored parameters among the four groups were analyzed with ANOVA. Intergroup comparisons were tested by post hoc Bonferroni tests. p<0.05 was considered statistically significant.
The results are expressed as median (interquartile range). The normality distribution of the variables was tested by a one-sample Kolmogorov-Smirnov test. The Kruskal-Wallis test was used to assess the statistical significance of comparisons, and the Dunn test was used for multiple comparisons when significant results were obtained. Statistics were performed with Statistica version 7.1 (Statsoft Inc., Tulsa, OK, USA). P values under 0.05 were considered statistically significant.
3. Results
3.1 Scintigraphy results
The damage caused by 8 Gy dose administration to the left kidney was significantly different for the RT group compared to the control group. We detected this difference by measuring the % relative parenchymal involvement (DMSA) for the left kidney lower in the RT group than in the control group. In addition, L-carnitine did not damage the kidney parenchyma with DMSA% similar to the control group. Although the rate of involvement was higher in the LC+RT group than in the RT group, the difference was not statistically significant (Figure 1). The DTPA Tmax value, which measures glomerular function for the left kidney, was similar to the control group in the LC and LC+RT groups. In the RT group, the lowest DTPA Tmax value was found due to loss of glomerular function. Reflecting the tubular excretion period, in DTPA T1/2, the rate of firing from the collecting system for the left kidney decreases with RT, while L-Carnitine increases this time to the level of left kidney tissue that has not received RT when given before RT (Table 1)
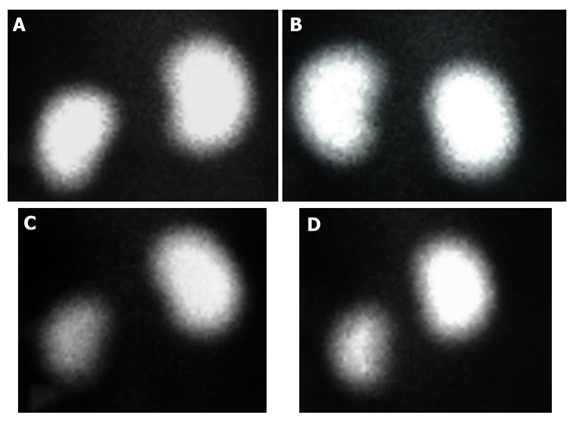
Figure 1: The posterior views of the 99mTc-DMSA scan are shown by the group. The percentages of left kidney function were similar between the RT and RT + L-Carnitine groups; Control group (Left 51%) (A), L-Carnitine group (Left 53%) (B), RT group (Left 25%) (C), RT + L-Carnitine group (Left 28%) (D)
Table I: 99mTc-DMSA and 99mTc-DTPA results of groups
In the right kidney scintigraphy findings, in which RT was not applied and the systemic effect of L-Carnitine was measured, we found that the DMSA % value in the right kidney was higher due to the decrease in the relative DMSA % in the left kidney. While DTPA Tmax and DTPA T1/2 values for the right kidney were higher in the RT group, interestingly, they were statistically significantly higher in the L-Carnitine + RT group. Interestingly, DTPA Tmax DTPA T1/2 values for the right kidney were statistically significantly higher in the L-Carnitine + RT group compared to the RT group (p=0.009, p=0.076).
3.2 Histopathological results
The histopathological findings are summarized in Table II. Histopathological evaluation of Control and L-Carnitine groups are demonstrated in Figures 2 and 3. Damage due to RT in kidney tissue has also been proven histopathologically. Tubular degeneration, interstitial fibrosis, and glomerular damage statistically significant damage were detected in the RT group. Statistically significant differences were detected for tubular degeneration, tubular atrophy, interstitial fibrosis, and glomerular damage when comparing groups using the Kruskal-Wallis test. All histopathological findings worsened with radiotherapy, and a positive effect of L-carnitine was not detected statistically. Examples of histopathological examinations of the RT and RT+ L-carnitine groups are shown in Figures 4 and 5. The degree of glomerular damage and tubular degeneration decreased when L-Carnitine was administered before RT. There was no difference in the degree of tubular atrophy and interstitial fibrosis (Table 3).
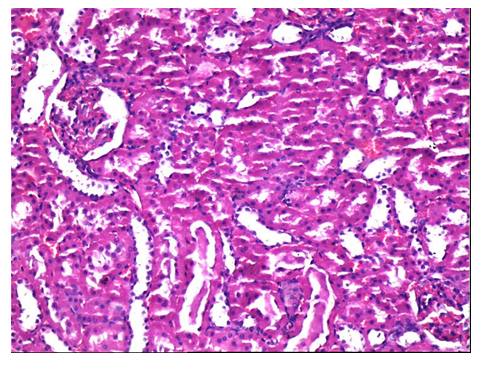
Figure 2: Histopathological evaluation of Control group. Normal appearance of the glomerulus with proximal and distal tubules (H&E x 100).
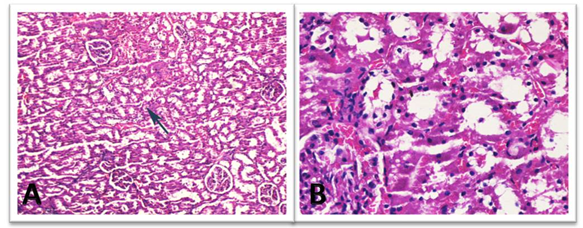
Figure 3: Histopathological evaluation of L-Carnitine group. Low (A) and High (B) power views of the healthy glomerulus. Slight vacuolar degeneration of the proximal tubules (arrow) and congestion was noted (H&E x 50, 200).
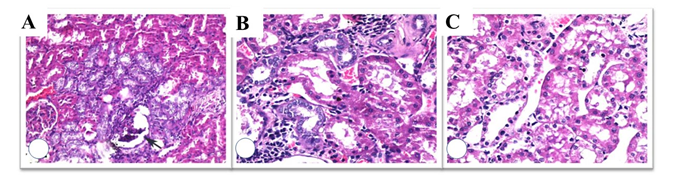
Figure 4: Histopathological evaluation of RT group; capillary loop collapse (arrow) (A), regenerative changes in the periglomerular tubules with lymphoid infiltration and fibrosis (B), vacuolar degeneration of the proximal tubules (c); additionally, nuclear enlargement and hyperchromasia of the tubular epithelial cells were observed (H&E x 100, 200, 200).
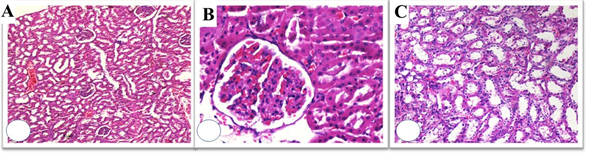
Figure 5: Histopathological evaluation of L-carnitine + RT group; Low-power view resembles Control and L-carnitine group (A), High-power view shows no glomerular damage (B), Proximal and distal tubular degeneration was reduced (C). However, nuclear enlargement and hyperchromasia were still remarkable (H&E x 50, 200, 100).
|
Control |
L-Carnitine |
Radiotherapy |
Radiotherapy + L-Carnitine |
p* |
Pairwise comparisons of histopathologic factors** |
|
|
(C) |
(LC) |
(RT) |
(RT + LC) |
|||
|
Tubular Degeneration n (%) |
1 (0 - 1) |
0.5 (0 - 1) |
2.5 (1 - 3) |
2 (1 - 3) |
<0.001 |
C vs LC, p = 1.000 |
|
C vs RT, p = 0.009 |
||||||
|
C vs RT + LC, p = 0.052 |
||||||
|
LC vs RT, p = 0.003 |
||||||
|
LC vs RT + LC, p = 0.021 |
||||||
|
RT vs RT + LC, p = 0.403 |
||||||
|
Tubular Atrophy n (%) |
0 (0 - 1) |
0 (0 - 0) |
1 (0 - 2) |
1 (0 - 2) |
0.006 |
C vs LC, p = 1.000 |
|
C vs RT, p = 0.288 |
||||||
|
C vs RT + LC, p = 0.088 |
||||||
|
LC vs RT, p = 0.103 |
||||||
|
LC vs RT + LC, p = 0.023 |
||||||
|
RT vs RT + LC, p = 1.000 |
||||||
|
Interstitial fibrosis n (%) |
0 (0 - 1) |
0 (0 - 1) |
1 (1 - 2) |
1 (0 - 2) |
0.003 |
C vs LC, p = 1.000 |
|
C vs RT, p = 0.015 |
||||||
|
C vs RT + LC, p = 0.021 |
||||||
|
LC vs RT, p = 0.115 |
||||||
|
LC vs RT + LC, p = 0.187 |
||||||
|
RT vs RT + LC, p = 1.000 |
||||||
|
Glomerular Damage n (%) |
0 (0 - 0) |
0 (0 - 0) |
1.5 (0 - 3) |
1 (0 - 2) |
0.002 |
C vs LC, p = 1.000 |
|
C vs RT, p = 0.024 |
||||||
|
C vs RT + LC, p = 0.058 |
||||||
|
LC vs RT, p = 0.024 |
||||||
|
LC vs RT + LC, p = 0.058 |
||||||
|
RT vs RT + LC, p = 1.000 |
Table II: Histopathologic results of groups
|
Results |
LC+RT |
|
|
Pathology |
Scintigraphy |
|
|
Tubular degeneration |
improved |
DTPA brought the T1/2 tubular excretion improved |
|
(less than RT group) |
||
|
Tubular atrophy |
has not changed |
|
|
Interstitial fibrosis |
has not changed |
DMSA % interstitial fibrosis improved |
|
Glomerular damage |
improved |
DTPATmax glomerular function improved |
LC+RT: L-Carnitine+Radiotherapy
Table 3: Interpretation of scintigraphy and pathology results in left kidney with RT
3.3 Survival
The numbers of rats in all groups were the same at the beginning of the study. However, death occurred in 50% (n=5) of the rats in the RT group, and 10% (n=1) of the rats died in the RT + L-Carnitine group at the end of the study period (Figure 6).
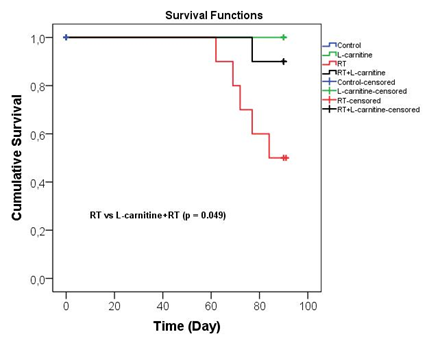
Figure 6: Comparison of the survival rates of the groups
4. Discussion
In our study, 4-week-old rats were studied, as it was expected that the rats would suck breast milk for 3 weeks after birth and start normal feeding in the most effective way, in order to represent childhood tumors. Our study is the first study of the infant period for radioprotection against RIN. Therefore, we tried to interpret the results we obtained with great care and care. It was very important to determine the functional kidney values of the 3rd month after RT. However, as seen in the survival results, the number of baby rats in the RT group decreased by 50%. Survival was found to be statistically significantly different between L-Carnitine+RT and RT groups (p=0.049). Unfortunately, this situation prevented us from showing the protective effect that L-Carnitine deserved in scintigraphic measurements between the two groups at the level of statistical significance (Figure 6). Therefore, we tried to interpret the scintigraphy results of our surviving baby rats very carefully. According to the scintigraphic findings, L-Carnitine preserved the parenchymal damage (DMSA %) and glomerular function (DTPA T max) caused by RT in baby rats whose left kidney was treated with RT (Table 1). In the DTPA ½ period, which is an indicator of the excretion rate in the kidney and gives information about tubular function, L-Carnitine moved the left kidney to the level of kidney tissue that had never received RT. It may be supportive to confirm the findings in the next study with the measurement of tubular function, a tubular agent, MAG-3 (mercaptoacetyltriglycine).
Histopathologically, L-Carnitine was shown to protect the kidney receiving radiotherapy from glomerular damage and interstitial fibrosis. The absence of histopathological difference in terms of tubular damage (atrophy and degeneration) may be the reason why the rats were sacrificed in the 3rd month. If our experiment had had a longer follow-up, it could have been demonstrated in tubular damage.
RT was applied to the left kidney, but scintigraphic differences were found between the groups in the values of the right kidney. L-carnitine administered before radiotherapy was also able to protect the contralateral kidney. DTPA max (p = 0.009) and DTPA ½ (p = 0.076) values in the right kidney were found to be significant and close to significance (Table 1). We can explain this result as increasing the workload of the right kidney against RIN that develops due to RT in the left kidney. The right kidney did not receive RT, but by compensating the deficiency of the left kidney and taking L-Carnitine support, it may have contributed to the longer life of the infant rats.
L-Carnitine is an agent that prevents the formation of free radicals and acts by increasing the activity of the superoxide dismutase enzyme from damage caused by oxidation (33). The protective effect of L-Carnitine on organ toxicity and RIN due to chemotherapy used in ischemic, diabetic and hypertension models as well as cancer treatment has been demonstrated by experimental studies (18-27).
The kidney is a highly metabolic organ and is particularly vulnerable to damage caused by oxidative stress. DNA damage caused by oxidative stress in the acute phase of radiation-induced tissue damage is an important pathomechanism in the progression of chronic kidney disease. Oxidative stress occurs when reactive oxygen species (ROS) outweighs antioxidants (34). When superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) enzymes fail as antioxidants in the cellular repair mechanism, apoptosis induced by reactive oxygen radicals comes into play as a last resort. Upregulation of Bax and Bcl-2 in the inner layer of mitochondria, activation of caspase proteases, activation of tumor necrosis factor (TNF-α) and transforming growth factor (TGF-β) results in apoptotic death. (34,35). RIN is characterized by a chronic progressive decrease in renal function and involves complex and dynamic interactions between glomerular, tubular and interstitial cells. It has been shown that glomerular damage precedes tubular damage in RIN. Therefore, prevention of progressive glomerular damage using protective agents is vital for the management of RIN (16-18).
In pediatric tumors, RT is either abandoned or given in limited doses due to its long-term side effects. Therefore, radioprotection in pediatric tumors becomes much more important in other age tumor groups (36-38). For example; in the treatment of childhood leukemia, the degree of kidney damage due to RT may increase with whole body irradiation and chemotherapy treatment (39,40). In children under 5 years of age, glomerular filtration rate begins to decrease with doses of 12-14 Gy in 1.25-1.5 Gy/fraction in the radiation treatment of the whole kidney. has been reported to cause 50% loss of kidney function. In another study on Wilm's tumor, no damage was found when the RT dose was between 11-14 Gy, while the death rate due to kidney failure increased as the RT dose increased (>12 Gy) (41,42).
L-carnitine also plays an important role in fatty acid oxidation by introducing active long-chain fatty acids into the mitochondrial matrix. In our study, we hypothesized that L-Carnitine might have protected the contralateral kidney systemically by activating this mechanism, and this will be the subject of our future studies (33). They showed that the effects of L-Cartine on the PI3K/AKT/PTEN apoptosis signaling pathway are responsible for its renoprotective effects in chronic tacrolimus nephropathy in vivo (24).
Carnitine is an agent that can be administered orally and intravenously and has minimal side effects. Carnitine therapy has been used as a replacement treatment for both hereditary and acquired disorders and for preventing oil oxidation and ketogenesis in preterm newborn infants (26,43-45).
Our study is the first model in which radioprotection on kidney tissue is investigated in infant rats. However, our study had some limitations. The first and perhaps the most important limitation is the attempt to generate the RIN model with single-dose irradiation rather than through conventional methods. The second limitation of the study is that L-carnitine was given as a single dose, and its antioxidant effect on the tissue was not evaluated using molecular methods. The third limitation of the study is the apoptotic processes were not investigated. Functional studies could have been performed, while further elucidation of the mechanism is an aim of further studies. Since infant rats were included in this study, performing serological tests by taking blood samples from rats in addition to an invasive procedure such as scintigraphy was not performed because it would disturb the continuity of the experiment. However, serological tests such as (creatine, BUN) and genetic markers may be used in future studies, in which different L-carnitine doses and follow-up times may be tested in infant rats.
5. Conclusion and Recommendations
As an antioxidant and free radical scavenger, L-carnitine has been shown to have protective effects against oxidative damage in several organs and tissues, including the kidney, as well as protective effects against damage to the cell membran. L-Carnitine was able to protect against glomerular damage and interstitial fibrosis due to RT in baby rats, and it provided prolongation of survival by supporting the compensation mechanism of the contralateral kidney. In our future study investigating the protective mechanism of L-carnitine on RIN in infant rats, Bax, BCL2, Caspase 3, TNF-κ and TGF-β levels will be examined both in tissue and serum.
Authors’ contributions
The authors confirm their contributions to the paper as follows: study conception and design: RC, AO, GDA, SP, and FOP; the acquisition, analysis and interpretation of results: NS, RC, AO, KI, DN, UK, and TO; manuscript draft preparation: RC, AO, KI, DN, UK, and TO. All authors reviewed the results and approved the final version of the manuscript.
Funding
There is no funding for this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The study was performed after obtaining ethical clearance from Trakya University and all animal experiments were conducted according to the guidelines of the Institutional Animal Ethics Committee.
Consent for publication
This manuscript does not contain any person’s data.
Competing interests
The authors declare that they have no conflicts of interest.
Acknowledgment
Not applicable
References
- Barr RD and Sala A. Quality-adjusted survival: A rigorous assessment of cure after cancer during childhood and adolescence. Pediatr Blood Cancer 44(2005): 201-204.
- Erman N, Todorovski L, & Jereb B. Late somatic sequelae after treatment of childhood cancer in Slovenia. BMC Res Notes 254 (2012).
- Yeh JM, Nekhlyudov L, Goldie SJ, et al. A model- based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med 152 (2010): 409-417.
- Steinmeier T, Schulze Schleithoff S, Timmermann B. Evolving Radiotherapy Techniques in Paediatric Oncology. Clin Oncol (R Coll Radiol) 31(2019):142-150.
- Dieffenbach BV, Liu Q, Murphy AJ, et al., Late-onset kidney failure in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Eur J Cancer 155(2021): 216-226.
- Oeffinger KC, Mertens AC, Sklar CA, et al. Childhood Cancer Survivor Study. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355(2006): 1572-82.
- Kooijmans EC, Bökenkamp A, Tjahjadi NS, et al. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev 3(2019):CD008944.
- Pater L, Melchior P, Rübe C, et al. Wilms tumor. Pediatr Blood Cancer 68 (2021): e28257.
- Lawton CA, Cohen EP, Murray KJ, et al. Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant 20(1997): 1069-74.
- Singh VK, Seed TM. Pharmacological management of ionizing radiation injuries: current and prospective agents and targeted organ systems. Expert Opin Pharmacother 21(2020): 317-337.
- Obrador E, Salvador R, Villaescusa JI, et al. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines 8(2020): 461.
- Canyilmaz E, Uslu GH, Bahat Z, et al. Comparison of the effects of melatonin and genistein on radiation-induced nephrotoxicity: Results of an experimental study. Biomed Rep 4(2016): 45-50.
- Kaldir M, Cosar-Alas R, Cermik TF, et al. Amifostine use in radiation-induced kidney damage. Preclinical evaluation with scintigraphic and histopathologic parameters. Strahlenther Onkol 184(2008): 370-5.
- Cosar R, Yurut-Caloglu V, Eskiocak S, et al., Radiation-induced chronic oxidative renal damage can be reduced by amifostine. Med Oncol 29(2012): 768-75.
- Kalman NS, Zhao SS, Anscher MS, et al. Current Status of Targeted Radioprotection and Radiation Injury Mitigation and Treatment Agents: A Critical Review of the Literature. Int. J. Radiat. Oncol.Biol. Phys 98(2017): 662-682.
- Mansour HH. Protective role of carnitine ester against radiation-induced oxidative stress in rats. Pharmacol Res 54(2006): 165-171.
- Brizel DM. Pharmacologic approaches to radiation protection. J Clin Oncol 25(2007): 4084-4089.
- Khana HA and Alhomidaa AS. A review of the logistic role of L-carnitine in the management of radiation toxicity and radiotherapy side effects. J. Appl. Toxicol 31(2011): 707-713.
- Liu Y, Yan S, Ji C, et al. Metabolomic changes and protective effect of (L)-carnitine in rat kidney ischemia/reperfusion injury. Kidney Blood Press Res 35(2012): 373-381.
- Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. ClinPharmacokinet 51(2021): 553-72.
- Fan JP, Kim D, Kawachi H, et al. Ameliorating effects of L-carnitine on diabetic podocyte injury. J Med Food 13(2010):1324-30.
- Zambrano S, Blanca AJ, Ruiz-Armenta MV, et al. L-carnitine attenuates the development of kidney fibrosis in hypertensive rats by upregulating PPAR-γ. Am J Hypertens 27(2014): 460-70.
- Boonsanit D, Kanchanapangka S and Buranakarl C. L-carnitine ameliorates doxorubicin-induced nephrotic syndrome in rats. Nephrology 11(2006): 313-320.
- Zheng HL, Zhang HY, Zhu CL, et al. L-Carnitine protects against tacrolimus-induced renal injury by attenuating programmed cell death via PI3K/AKT/PTEN signaling. Acta Pharmacol Sin 42(2021): 77-87.
- Kopple JD, Ding H, Letoha A, et al. L-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol Dial Transplant 17(2002): 2122-31.
- Boonsanit D, Kanchanapangka S and Buranakarl C. L-carnitine ameliorates doxorubicin-induced nephrotic syndrome in rats. Nephrology (Carlton) 11(2006): 313-320.
- Chang B, Nishikawa M, Sato E, et al. L-Carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem Biophys 405(2002): 55-64.
- Ebrahim OFA, Nafea OE, Samy W, et al. L-carnitine suppresses cisplatin-induced renal injury in rats: impact on cytoskeleton proteins expression. Toxicol Res (Camb) 10(2021): 51-59.
- Origlia N, Migliori M, Panichi V, et al. Protective effect of L-propionylcarnitine in chronic cyclosporine-a induced nephrotoxicity. Biomed Pharmacother 60(2006): 77-81.
- Jafari A, Dashti-Khavidaki S, Khalili H, et al. Potential nephroprotective effects of l-carnitine against drug-induced nephropathy: a review of literature. Expert Opin Drug Saf 12(2013):523-43.
- Caloglu M, Yurut-Caloglu V, Durmus-Altun G, et al. Histopathological and scintigraphic comparisons of the protective effects of L-carnitine and amifostine against radiation-induced late renal toxicity in rats. Clin Exp Pharmacol Physiol 36(2009): 523-30.
- Laiakis EC, Mak TD, Anizan S, et al. Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat Res 181(2014): 350-61.
- Hoppel C. The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis 41(2003): 4-12.
- Klaus R, Niyazi M and Lange-Sperandio B. Radiation-induced kidney toxicity: molecular and cellular pathogenesis. Radiat Oncol 16(2021): 43.
- Daenen K, Andries A, Mekahli D, et al. Oxidative stress in chronic kidney disease. Pediatr Nephrol 34(2019): 975-91.
- Seth R, Singh A, Seth S, et al. Late effects of treatment in survivors of childhood cancers: A single-centre experience. Indian J Med Res 146 (2017): 216-23.
- Suh E, Stratton KL, Leisenring WM, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol 21(2020): 421-435.
- Lawton CA, Cohen EP, Barber-Derus SW, et al. Late renal dysfunction in adult survivors of bone marrow transplantation. Cancer 67(1991): 2795-800.
- Frisk P, Bratteby L, Carlson K, et al.Renal function after autologous bone marrow transplantation in children: a long-term prospective study.Bone Marrow Transplant 29(2002): 129-136.
- Esiashvili N, Chiang KY, Hasselle MD, et al. Renal toxicity in children undergoing total body irradiation for bone marrow transplant. Radiother Oncol 90(2009): 242-246.
- Mitus A, Tefft M, Fellers FX. Long-term follow-up of renal functions of 108 children who underwent nephrectomy for malignant disease. Pediatrics 44(1969): 912-921.
- Peschel RE, Chen M, Seashore J. The treatment of massive hepatomegaly in stage IV-S neuroblastoma. Int J Radiat Oncol Biol Phys 7(1981): 549-553.
- Tein I. Carnitine transport: pathophysiology and metabolism of known molecular defects. J Inherit Metab Dis 26(2003): 147-169.
- Kalaiselvi T and Panneerselvam C. Effect of L-carnitine on the status of lipid peroxidation and antioxidants in aging rats. The Journal of Nutritional Biochemistry 9(1998): 575-581.
- Gramignano G, Lusso MR, Madeddu C, et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 22(2006): 136-45.
