Outcome of COVID-19 Contracted in the First and Second Trimester of Pregnancy: A Systematic Review
Article Information
Hicham Arab1*, Rola El Rassi2
1Program Director, Maternal & Fetal Health Program, Dr Arab Medical Center, Jeddah, Saudi Arabia
2Research Associate, Maternal & Fetal Health Program, Dr Arab Medical Center, Jeddah, Saudi Arabia
*Corresponding Author: Hicham Arab, Program Director at Maternal & Fetal Health Program, Dr Arab Medical Center, Building 3848, Alhayat Plaza, Prince Saud Alfaisal Street, Jeddah 23433-8654, Saudi Arabia
Received: 23 June 2021; Accepted: 02 July 2021; Published: 07 July 2021
Citation:
Hicham Arab, Rola El Rassi. Outcome of COVID-19 Contracted in the First and Second Trimester of Pregnancy: A Systematic Review. Obstetrics and Gynecology Research 4 (2021): 140-158.
View / Download Pdf Share at FacebookAbstract
Background: Pregnant women are more susceptible to COVID-19 infection than the overall population, due to immunologic and anatomic alterations. Since January 2020, the multitude of studies published have described the outcomes of COVID-19 during the third trimester of pregnancy. The aim of this systematic review is to conduct a search of the literature on SARS-CoV-2 infection in the first and second trimester and report on its outcomes.
Methods: The review was conducted based on PRISMA guidelines. We conducted a comprehensive search in Medline, PubMed, and Embase. Articles reporting on pregnancy, perinatal and fetal outcomes of COVID-19 infected women with confirmed diagnosis were eligible. The quality of the studies was assessed using the appropriate methodology.
Results: The search resulted in 7,380 articles, of which 424 were reviewed. A total of 17 studies met our inclusion criteria. The percentage of women admitted to the hospital during the first trimester was 47%, with a reported 2% receiving respiratory support. Moreover, the percentage of first trimester women who underwent a miscarriage or abortion was 11%. The percentage of pregnant women who were hospitalized during the second trimester was 44%, while 2.5% were admitted to the ICU and 1.6% received respiratory support, whereas 1.4% were intubated or mechanically ventilated. The percentage of maternal mortality was 2% and 4% during the first trimester and second trimester, respectively.
Conclusion: Pregnant women who have been infected during the first and second trimester need to be monitored carefully. There are insufficient good-quality studies assessing those infected during early pregnancy.
Keywords
COVID-19, Pregnancy, Fetus, First Trimester, Second Trimester, Outcomes
COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Pregnancy articles Pregnancy Research articles Pregnancy review articles Pregnancy PubMed articles Pregnancy PubMed Central articles Pregnancy 2023 articles Pregnancy 2024 articles Pregnancy Scopus articles Pregnancy impact factor journals Pregnancy Scopus journals Pregnancy PubMed journals Pregnancy medical journals Pregnancy free journals Pregnancy best journals Pregnancy top journals Pregnancy free medical journals Pregnancy famous journals Pregnancy Google Scholar indexed journals Fetus articles Fetus Research articles Fetus review articles Fetus PubMed articles Fetus PubMed Central articles Fetus 2023 articles Fetus 2024 articles Fetus Scopus articles Fetus impact factor journals Fetus Scopus journals Fetus PubMed journals Fetus medical journals Fetus free journals Fetus best journals Fetus top journals Fetus free medical journals Fetus famous journals Fetus Google Scholar indexed journals First Trimester articles First Trimester Research articles First Trimester review articles First Trimester PubMed articles First Trimester PubMed Central articles First Trimester 2023 articles First Trimester 2024 articles First Trimester Scopus articles First Trimester impact factor journals First Trimester Scopus journals First Trimester PubMed journals First Trimester medical journals First Trimester free journals First Trimester best journals First Trimester top journals First Trimester free medical journals First Trimester famous journals First Trimester Google Scholar indexed journals Second Trimester articles Second Trimester Research articles Second Trimester review articles Second Trimester PubMed articles Second Trimester PubMed Central articles Second Trimester 2023 articles Second Trimester 2024 articles Second Trimester Scopus articles Second Trimester impact factor journals Second Trimester Scopus journals Second Trimester PubMed journals Second Trimester medical journals Second Trimester free journals Second Trimester best journals Second Trimester top journals Second Trimester free medical journals Second Trimester famous journals Second Trimester Google Scholar indexed journals Gestational Age articles Gestational Age Research articles Gestational Age review articles Gestational Age PubMed articles Gestational Age PubMed Central articles Gestational Age 2023 articles Gestational Age 2024 articles Gestational Age Scopus articles Gestational Age impact factor journals Gestational Age Scopus journals Gestational Age PubMed journals Gestational Age medical journals Gestational Age free journals Gestational Age best journals Gestational Age top journals Gestational Age free medical journals Gestational Age famous journals Gestational Age Google Scholar indexed journals Neonatal ICU articles Neonatal ICU Research articles Neonatal ICU review articles Neonatal ICU PubMed articles Neonatal ICU PubMed Central articles Neonatal ICU 2023 articles Neonatal ICU 2024 articles Neonatal ICU Scopus articles Neonatal ICU impact factor journals Neonatal ICU Scopus journals Neonatal ICU PubMed journals Neonatal ICU medical journals Neonatal ICU free journals Neonatal ICU best journals Neonatal ICU top journals Neonatal ICU free medical journals Neonatal ICU famous journals Neonatal ICU Google Scholar indexed journals Hypertensive Disorders of Pregnancy articles Hypertensive Disorders of Pregnancy Research articles Hypertensive Disorders of Pregnancy review articles Hypertensive Disorders of Pregnancy PubMed articles Hypertensive Disorders of Pregnancy PubMed Central articles Hypertensive Disorders of Pregnancy 2023 articles Hypertensive Disorders of Pregnancy 2024 articles Hypertensive Disorders of Pregnancy Scopus articles Hypertensive Disorders of Pregnancy impact factor journals Hypertensive Disorders of Pregnancy Scopus journals Hypertensive Disorders of Pregnancy PubMed journals Hypertensive Disorders of Pregnancy medical journals Hypertensive Disorders of Pregnancy free journals Hypertensive Disorders of Pregnancy best journals Hypertensive Disorders of Pregnancy top journals Hypertensive Disorders of Pregnancy free medical journals Hypertensive Disorders of Pregnancy famous journals Hypertensive Disorders of Pregnancy Google Scholar indexed journals Intrauterine articles Intrauterine Research articles Intrauterine review articles Intrauterine PubMed articles Intrauterine PubMed Central articles Intrauterine 2023 articles Intrauterine 2024 articles Intrauterine Scopus articles Intrauterine impact factor journals Intrauterine Scopus journals Intrauterine PubMed journals Intrauterine medical journals Intrauterine free journals Intrauterine best journals Intrauterine top journals Intrauterine free medical journals Intrauterine famous journals Intrauterine Google Scholar indexed journals Hyperemesis Gravidarum articles Hyperemesis Gravidarum Research articles Hyperemesis Gravidarum review articles Hyperemesis Gravidarum PubMed articles Hyperemesis Gravidarum PubMed Central articles Hyperemesis Gravidarum 2023 articles Hyperemesis Gravidarum 2024 articles Hyperemesis Gravidarum Scopus articles Hyperemesis Gravidarum impact factor journals Hyperemesis Gravidarum Scopus journals Hyperemesis Gravidarum PubMed journals Hyperemesis Gravidarum medical journals Hyperemesis Gravidarum free journals Hyperemesis Gravidarum best journals Hyperemesis Gravidarum top journals Hyperemesis Gravidarum free medical journals Hyperemesis Gravidarum famous journals Hyperemesis Gravidarum Google Scholar indexed journals
Article Details
Abbreviations:
ARDS: Acute Respiratory Distress Syndrome; CDC: Centers for Disease Control and Prevention; SARS: Severe Acute Respiratory Syndrome; MERS: Middle East Respiratory Syndrome; TNF-α: Tumor Necrosis Factor- α; PROM: Premature Rupture of Membranes; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RT-PCR: Real-Time Reverse-Transcriptase Polymerase Chain Reaction; WHO: World Health Organization; ICU: Intensive Care Unit; SGA: Small-for-Gestational Age; NICU: Neonatal ICU; HDP: Hypertensive Disorders of Pregnancy; IUFD: Intrauterine Fetal Death; HG: Hyperemesis Gravidarum
1. Introduction
Human coronaviruses are among the most common pathogens that cause respiratory infection. SARS-CoV-2 has enveloped virions that measure about 50–200 nm in diameter with a single positive-sense RNA genome [1]. Coronavirus disease 2019 (COVID-19) is transmitted through respiratory droplets, physical contact, and aerosols, and there is evidence of human-to-human transmission. It seems that pregnant women are more susceptible to COVID-19 infection than the overall population, due to immunologic and anatomic alterations. Their susceptibility to respiratory infections is due to the physiologically adaptive changes of the respiratory tract, such as diaphragm elevation, increased oxygen consumption, and edema of the respiratory tract mucosa [2]; moreover, high concentrations of estrogens cause congestion and excessive secretion of airway epithelial cells [3, 4]. Additionally, COVID-19 infection is associated with maternal pyrexia, “cytokine storm” and hypercoagulability, which increases the risk of placental intervillous thrombosis and infarction, as well as maternal hypoxia secondary to acute respiratory distress syndrome (ARDS) [5]. In June 2020, the Centers for Disease Control and Prevention (CDC) reported that hospitalization with COVID-19 occurred in 31.5% of pregnant women compared with 5.8% of nonpregnant women [6]. However, there were no data on the indication for hospitalization [7].
The association between adverse obstetrical–neonatal outcomes and pathophysiological changes related to respiratory viral infection has been reported in severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) [8]. Our previous knowledge of these viral infections reminds us that the effects of such an infection during pregnancy – particularly the third trimester – has serious adverse outcomes, such as maternal deaths and increased risk of preterm birth, fetal growth restriction and perinatal mortality [9], while no evidence of vertical transmission has been found [10]. During the first trimester of pregnancy, the fetal organs start to develop, which might make maternal infections at this stage more serious compared to later stages of gestation [11, 12]. Moreover, the immune imbalance and increase in proinflammatory cells characteristic of COVID-19 infection, in addition to the relationship between tumor necrosis factor- α (TNF-α) and the reduction of nitric oxide and the increase in endothelin-1, can cause defects in placentation and other gestational processes [13]. Maternal-fetal outcomes in the second and third trimester vary between evolution to vaginal delivery, without complications and evolution to emergency cesarean delivery due to fetal distress and prematurity [14]. According to Gracia et al., pregnant patients infected with COVID-19 whether during the second or the third trimester continue to be at high obstetric risk, especially due to the premature rupture of membranes (PROM) and labor before 39 weeks. These pregnant women should be closely monitored until the moment of birth. These findings are of concern as they suggest the possibility of chronic inflammation with alteration at the placental or membrane level that triggers the culmination of pregnancy [5].
Since January 2020, the multitude of studies published have described the outcomes of COVID-19 during the third trimester of pregnancy. According to a recent review by Wastnedge et al., studies reporting on pregnancy outcomes in women with confirmed SARS-CoV-2 infection, whether case series or cohort studies, totaled 31, encompassing 12,260 women, most of whom were in their third trimester. Overall, the findings from these studies are reassuring and the severity of COVID-19 in pregnancy appears to be no greater than that of the general population [9]. At present, information regarding the outcomes of the infection during the first and second trimester are scarce. The aim of our systematic review is to conduct a comprehensive search of the literature regarding SARS-COV-2 infection in the first and second trimester and to report on findings relevant to maternal disease severity and clinical progression, hospital admission, placental abnor-malities, and neonatal outcome.
2. Methods
This systematic review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15].
2.1 Search strategy
We conducted a comprehensive search in Medline, PubMed, and Embase using MeSH terms, keywords, and free text words relevant to COVID-19, coronavirus, SARS-CoV-2, pregnant, pregnancy, and fetus up to February 14, 2021. We limited the search to the years 2020 and 2021. The used queries are described in Table 1. All of the retrieved articles were exported to Endnote software version X7, where duplicates were removed by using the ‘find duplicate’ function in Endnote and manually during screening. The titles and abstracts of studies retrieved via the search were screened by two reviewers. Potentially eligible selected studies were compared, and discrepancies were resolved by discussion.
|
Databases searched |
Search strategy used |
Results |
|
PubMed |
("covid 19"[All Fields] OR "covid 19"[MeSH Terms] OR "covid 19 vaccines"[All Fields] OR "covid 19 vaccines"[MeSH Terms] OR "covid 19 serotherapy"[All Fields] OR "covid 19 nucleic acid testing"[All Fields] OR "covid 19 nucleic acid testing"[MeSH Terms] OR "covid 19 serological testing"[All Fields] OR "covid 19 serological testing"[MeSH Terms] OR "covid 19 testing"[All Fields] OR "covid 19 testing"[MeSH Terms] OR "sars cov 2"[All Fields] OR "sars cov 2"[MeSH Terms] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "coronavirus"[MeSH Terms] OR "coronavirus"[All Fields] OR "coronaviruses"[All Fields] OR "sars cov 2"[MeSH Terms] OR "sars cov 2"[All Fields] OR "sars cov 2"[All Fields]) AND ("pregnancy"[MeSH Terms] OR "pregnancy"[All Fields] OR "pregnancies"[All Fields] OR "pregnancy s"[All Fields] OR "gravidity"[MeSH Terms] OR "gravidity"[All Fields] OR "pregnant"[All Fields] OR "pregnants"[All Fields] OR "fetus"[MeSH Terms] OR "fetus"[All Fields] OR "fetuses"[All Fields] OR "foetus"[All Fields]) |
3,123 |
|
Medline |
((exp Coronavirus Infections/ or Covid 19.mp.) OR (SARS-COV-2.mp.) OR (exp Coronavirus/ or coronavirus.mp.)) AND ((pregnant.mp. or exp Pregnancy/ or exp Pregnant Women/) OR (exp Fetus/ or fetus.mp.)) |
1,680 |
|
Embase |
('covid 19' OR coronavirus OR 'coronavirus disease 2019') AND ('pregnant woman' OR 'pregnancy' OR 'fetus') |
2,577 |
|
Total retrieved results |
7,380 |
|
Table 1: Search strategies applied in this review.
2.2 Inclusion and exclusion criteria
Articles reporting on pregnancy outcomes and perinatal and fetal outcomes of COVID-19 infected pregnant women with confirmed diagnosis were eligible. We excluded case reports and case series with a sample size of less than 15. Publications where infection with SARS-CoV-2 occurred in the third trimester, before or after pregnancy were also excluded. Additionally, studies that did not have clear gestational age cut-off points and those who reported findings without detailing results by trimester and where conclusions could not be made were also excluded. We also noted that some studies were conducted in the same hospital with overlapping study population and duration dates. We therefore included the umbrella study that either spanned a longer duration or had its data retrieved from a national registry or included the study with the most comprehensive information to avoid including the same data twice. Publications before January 2020 and those in languages other than English and where full text was not available were also excluded. The retrieved literature was filtered to include clinical trials, retrospective studies, prospective and observational studies, and case series of sample size above 15.
2.3 Case definition
A confirmed diagnosis for COVID-19 was based on a
positive result on real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assays of a nasopharyngeal swab sample – as per the World Health Organization (WHO) interim guidance [16] and/or diagnosed by a computed tomography chest scan were included and analyzed. A gestational age at time of diagnosis of less than 14 weeks was considered 1st trimester and between 14 and 28 weeks was considered 2nd trimester.
2.4 Outcomes assessed
Data on recent SARS-CoV-2 exposure history, clinical symptoms or signs, maternal and perinatal and fetal outcomes were collected. The primary outcome of the study was maternal mortality and morbidity, including admission to the intensive care unit (ICU), use of mechanical ventilation or death. Secondary outcomes were mode of delivery, existing maternal comorbidities, miscarriage or abortion, stillbirth, neonatal death, perinatal death, small-for-gestational age (SGA), preterm birth, admission to the neonatal ICU (NICU), vertical transmission confirmed by a positive RT-PCR assay in the neonate, congenital abnormalities, placental size, oligohydramnios, fetal investigations such as cord blood, and nasopharyngeal and amniotic fluid swabs. Miscarriage was defined as pregnancy loss before 20 weeks of gestation and stillbirth as intrauterine fetal death (IUFD) at or after 22 weeks gestation. Neonatal death was defined as death of a liveborn infant within the first 28 days postpartum, or perinatal death as in stillbirth. SGA was defined as ultrasound estimated fetal weight less than the 10th percentile. Hypertensive disorders of pregnancy (HDP), including preeclampsia, was defined as any new hypertensive disorder presenting after 20 weeks of pregnancy. For the present analysis, we interpreted missing data as absence of the specific condition or characteristics in the studied pregnant women.
2.5 Data extraction and assessment of quality
The full texts of all eligible articles were retrieved and screened by two reviewers, with any discrepancy resolved by discussion. The data extracted from the studies included the study design and location, the number of COVID-19 infected mothers, the number of neonates, the gestational age at the time of COVID-19 diagnosis, mode of delivery, and maternal and perinatal outcomes. The quality of the studies was also assessed using the GRADE 4 guidelines [17], which evaluates observational studies according to four distinct criteria: developing and including eligibility criteria, unflawed measurement of exposure and outcome, controlling for cofounding, and incomplete follow-up [17]. As for case series we evaluated them using the method proposed by Murad et al. [18].
3. Results
3.1 Study selection
The search resulted in 7,380 articles that were screened by title and abstract. We identified a total of 424 studies that fit our eligibility criteria and which we reviewed in full-text. The PRISMA flow diagram (Figure 1) describes the process of study review and selection. Of the 424 studies, 17 studies [7, 19-34] were selected based on our inclusion criteria. The studies included a total of 760 pregnant women, and the findings are
summarized here-in.
3.2 Characteristics of the studies
The included studies were very heterogenous, with 53% (n=9) of them being case series [20, 22-27, 30, 31], and 23% (n=4) retrospective chart reviews [20, 27, 28, 33]. The rest of the studies included were 1 observational study [7], 1 prospective cohort [31], 1 cross-sectional study [32], and 1 single-arm cohort [34]. Our search found no randomized trials in the literature (Table 2).
3.3 Population
The overall number of mothers in the abstracted studies was 2647, of which only 760, around 30% included women who were in their first or second trimester of pregnancy. A total of 205 women (27%) were in the first trimester and 555 (73%) in their second trimester of pregnancy. Pregnant women were tested with RT-PCR assay of nasopharyngeal swabs or using CT scan of lungs, mostly because of symptoms of COVID, after contact with other infected individuals, or after hospital admission for an obstetric indication.
3.4 Setting
The included studies were conducted in countries from around the world. Five of the studies took place in the United States, 3 in Italy, and the rest were conducted in Kuwait, Spain, Panama, India, Singapore, Dominican Republic, Brazil, Turkey and China (Table 2).
3.5 Quality of the studies
The results of our assessment of the methodologic quality of each eligible study are summarized in Table 3. All case series provided adequate description of selection methodology, and none of the studies conducted an adequate assessment of causality. Only 6 series [19, 21, 23, 25, 26, 30] provided an adequate ascertainment of outcome and exposure. In addition, only 3 case series [19, 23, 30] provided adequate reporting to allow other investigators to replicate the research or to allow clinical practitioners to make inferences of relevance to their practice. As for the cohorts, only 4 studies provided an adequate description of selection methodology [7, 27, 28, 32]. An appropriate assessment of measures of exposure and outcome was reported in all studies, except one [34]; while none of the studies was assessed to have adequately controlled for confounding factors. A complete follow-up was confirmed in only 3 studies [7, 20, 34]. Overall, only 1 cohort study [7] had a strong methodological design as assessed using the GRADE 4 criteria, in comparison to the other included studies, and only 3 case series [19, 23, 30] had a strong methodological design when assessed using the method proposed by Murad et al. [18].
3.6 Findings
3.6.1 During the first trimester: The overall percentage of no complications reported among pregnant women in the first trimester was 52%. The percentage of women who had been admitted to the hospital was 47%, with a reported 2% of mothers receiving respiratory support (Figure 2). Moreover, the percentage of women who underwent a miscarriage or abortion was 11%, however the majority of these pregnant women opted for an induced abortion during early pregnancy to terminate the pregnancy without any maternal indication. The reason being concerns of side-effects from drugs, radiological exam, and COVID-19. The percentage of maternal mortality during the first trimester was 2%.
3.6.2 During the second trimester
Studies reporting on outcomes in women in the second trimester of pregnancy reported complications in 48% of participants. A total of 244 pregnant women were hospitalized (44%), while a reported percentage of 2.5% were admitted to the ICU and 1.6% received respiratory support, whereas 1.4% were intubated or mechanically ventilated. Maternal mortality was reported in 22 pregnant women (4%). Of the 555 reported pregnant women in their 2nd trimester, 1.4% had HDP and 0.7% had DM. The reported fetal complications for women who had delivered during the study period ranged from miscarriage in 1.3% of the studied women, 0.7% IUFD or stillbirth, 0.4% neonatal mortality, and 1.3% NICU admissions (Figure 2). Few of the women who had contracted COVID-19 during the second trimester were reported to have delivered. Of the women who had delivered, mode of delivery was reported in 31 cases, 15 delivered via c-section and 16 via the vaginal route – 9 of whom were reported to have been induced. Preterm labor was reported in 13 cases (2.3%) of infected women.
3.6.3 Placental and fetal histopathology and vertical transmission
Only one study reported on placental and histological findings. According to Mattar et al. [s29], RT-PCR was negative in a woman who had a 2nd trimester miscarriage for all tested samples: maternal serum, urine, swabs of the fetal ear, nasopharyngeal and oropharynx, fetal cord blood, and fetal and maternal placenta surfaces. In 2 women who delivered, swabs of the vagina, umbilical cord, maternal and fetal placental surfaces, amniotic fluid, and maternal blood and umbilical cord blood were negative for SARS-CoV-2, and histological examination of the placenta and umbilical cord did not reveal ischemia, necrosis or funisitis. Colostrum from one of these patients was negative for covid [29]. Of the 4 studies that tested neonates for COVID-19 upon delivery [7, 27, 31, 33], all reported negative test results, and none detected vertical transmission of SARS-CoV-2.
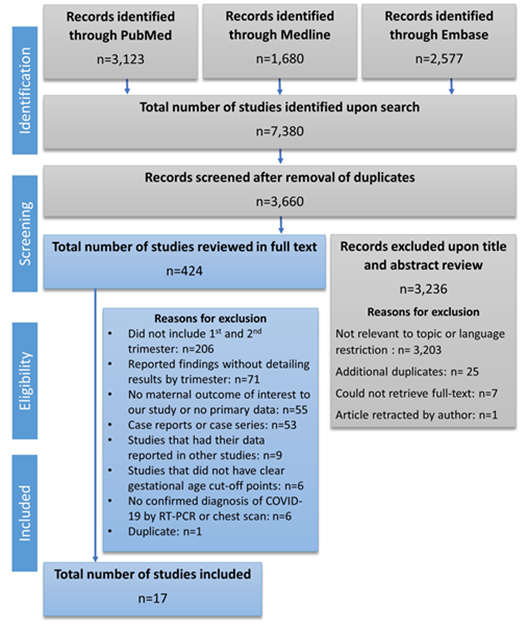
Figure 1: PRISMA flow chart for article selection process.
Table 2: Summary of the characteristics of included studies detailing maternal and fetal outcomes.
|
Cohorts/ Observational studies |
||||
|
Author, year |
Appropriate eligibility criteria |
Appropriate measure of exposure and outcome |
Adequately control confounding |
Complete follow-up |
|
Adhikari, 2020 [7] |
+ a |
+ |
- b |
+ |
|
Ayed, 2020 [20] |
- |
+ |
- |
+ |
|
Lokken, 2021 [27] |
+ |
+ |
- |
- |
|
Mahajan, 2021 [28] |
+ |
+ |
- |
? c |
|
Savasi, 2020 [30] |
- |
+ |
- |
- |
|
Takemoto, 2020 [31] |
+ |
+ |
- |
? |
|
Tug, 2020 [33] |
- |
+ |
- |
- |
|
Wang Y, 2020 [34] |
- |
? |
- |
+ |
|
Case series |
||||
|
Author, year |
Adequate selection method |
Adequate ascertainment of exposure and outcome |
Adequate assess-ment of causality |
Adequate reporting |
|
Andrikopoulou, 2020 [19] |
+ |
+ |
- |
+ |
|
Barbero, 2020 [21] |
+ |
+ |
- |
- |
|
Curi, 2020 [22] |
+ |
? |
? |
- |
|
Di Guardo, 2021 [23] |
+ |
+ |
- |
+ |
|
Giannini, 2020 [24] |
+ |
? |
- |
? |
|
Gracia, 2020 [25] |
+ |
+ |
- |
- |
|
Grechukhina, 2020 [26] |
+ |
+ |
- |
- |
|
Mattar, 2020 [29] |
+ |
- |
? |
- |
|
Mercedes, 2020 [30] |
+ |
+ |
- |
+ |
|
a + = low risk of bias; criteria met in the study design. b - = high risk of bias; criteria not met in study design. c ? = unclear risk of bias authors failed to report whether criteria was met. |
||||
Table 3: Summary of the quality assessment of the included studies.
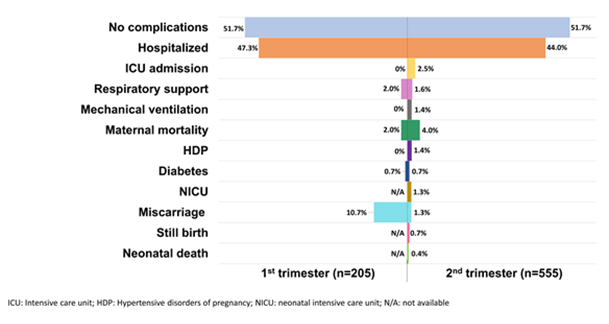
Figure 2: Outcomes of COVID-19 infected mothers in their 1st and 2nd trimester of pregnancy.
4. Discussion
In this systematic review, our main aim was to assess the data available regarding the impact of contracting COVID-19 during the first and second trimester of pregnancy. Overall, we were able to identify 17 studies that reported on women in early pregnancy who had been diagnosed with SARS-CoV-2. The evidence in general suggests that the impact of COVID-19 on both the pregnant woman and her fetus seems to be minor, although the studies were mostly case series and hence making assumptions is inappropriate. In general, pregnancy is a risk factor for hospitalization, ICU admission and death. The additional effects of contracting COVID-19 during the pregnant state, on both the pregnant woman and fetus are not well known, especially if the infection occurs during early pregnancy. Data suggests that, besides the pregnancy state, the gestational age also affects disease severity [33].
Our study findings reveal that in the first trimester, 47% of women with a confirmed COVID-19 infection were admitted to the hospital; a very similar percentage was observed in infected women in their second trimester of pregnancy (44.0%). None of our included studies reported cases of ICU admission for women infected in their first trimester, whereas 2.5% of infected women in their second trimester were admitted to the ICU. In the largest cohort study to date, conducted in the United States with population-level data from 91,412 women, of whom 8,207 were pregnant, the pregnant state was associated with significantly increased risk of hospitalization (RR, 5.4; 95% CI, 5.1–5.6), and ICU admission (RR, 1.5; 95% CI, 1.2–1.8). A total of 31.5% of women reported hospitalization and 1.5% required ICU admission [35]. These findings however do not detail trimester of pregnancy at the time of infection. A study conducted earlier, using the same population but a smaller sample size, reported that pregnant women were less likely to require admission than nonpregnant women with COVID-19 [36]. A case series of pregnant women diagnosed with COVID-19 by RT-PCR conducted by Knight et al. [37], reported that 10% of the women studied required admission to a critical care unit [37]. Most of the women described by Knight et al. are in their third trimester and hence they might be more prone to infection or have a higher risk of displaying severe symptoms as compared to women in early pregnancy. A significant difference in number of hospital admissions was observed in pregnant women in their third trimester versus those in their first trimester (P=0.019) [38]. Our findings however suggest a higher rate of hospitalization in both the first and second trimester, with a low rate of ICU admission for the second trimester. Data however was not available for us to be able to differentiate those hospitalized for COVID-19 related illness or those admitted for pregnancy-related conditions due to universal screening policies that were implemented by some hospitals.
The risk for respiratory support appears to be higher among pregnant women than nonpregnant ones; whereby 0.5% of pregnant women required mechanical ventilation as compared to women who were not pregnant [35]. A study, conducted in Sweden, reported that infected pregnant women were four times more likely to receive mechanical ventilation than their nonpregnant counterparts [39]. In our systematic review, 2.0% of infected pregnant women in their first trimester and 1.6% of those in their second trimester, required respiratory support. However, mechanical ventilation was only required in women in their second trimester of pregnancy (1.4%). This could be attributed to the observations made by several studies that SARS-CoV-2 infection caused more severe disease and required increased monitoring with oxygen support [38, 40] in late pregnancy. In a meta-analysis conducted by Mertz et al. [41] among women of reproductive age, the pregnant state was associated with no increased risk of death from influenza [41]. The cohort by Ellington et al. [35], reports that the risk of death was the same for COVID-19 infected pregnant and nonpregnant women, at 0.2% [35]. Furthermore, a systematic review conducted by Allotey et al. [42], stated that overall, 0.02% of pregnant women from 59 studies with confirmed COVID-19 died from any cause [42]. Our review indicates maternal mortality in 2% of mothers infected in the first trimester and 4% of those infected in the second trimester. The higher reported percentage in our study can be attributed to the inclusion of the study conducted by Takemoto et al., whereby their cross-sectional study assessed clinical characteristics of pregnant and postpartum women with severe COVID-19 in Brazil and examined risk factors for mortality [32]. Their reported mortality rate was 12.7%, however it is highly probable that this number is an overestimate, as only women with “severe” symptoms were tested. Moreover, there is a possibility that much of the excess mortality, particularly in Brazil, a Low- and Middle-Income Country, could be due to inability to access critical care. In such settings, pregnant women are particularly vulnerable to the adverse impact that COVID-19 has on health services at all levels [43].
A cohort study of 1019 pregnant women by la Cour Freiesleben et al. [44] focused on possible signs of maternal SARS-CoV-2 infection in first trimester pregnancies. They reported no significant difference in nuchal translucency thickness during the first trimester scan among women infected with SARS-CoV-2 in early pregnancy as compared to women with no prior infection. Furthermore, they found no significant increased risk of pregnancy loss in women with SARS-CoV-2 infection in the first trimester [44]. To date, few studies have indicated the rate of late miscarriage. Baud et al. reported a second-trimester miscarriage in an infected pregnant woman with symptomatic COVID-19 [45]. An increased percentage of miscarriage was observed in our study in infected mothers in their first trimester of pregnancy as most mothers opted for elective termination of pregnancy due to fears of the consequences of COVID-19 and its interventions such as anti-viral medications and CT imaging. Findings of fetal outcomes included 1.3% NICU admissions, 0.7% still births and 0.4% neonatal deaths for women infected during the second trimester. In general, an influenza infection during early pregnancy has been significantly associated with preterm birth, neonatal and infant mortality [46]. Furthermore, the probability of preterm labor during hospitalization with a SARS-CoV-2 infection is significantly lower among women diagnosed in their early pregnancy in comparison to those in late pregnancy [47]. Out of the 760 pregnant women with COVID-19 in our included studies, there were no reports of neural tube defects or congenital heart disorders. Fetal organs start to develop during early pregnancy, and closely monitoring infected pregnant women for these congenital anomalies is required. However, we report additional comorbidities such as HDP in 1.4% and DM in 0.7% of infected women in the second trimester. The studies reporting on changes during pregnancy after COVID-19 infection are limited, especially those investigating impact during the early gestational period. The possible risk of complications induced by the viral infection should be considered for better obstetric care, mainly because gestational diseases are caused by interferences in the adaptive changes in the maternal organism, as well as in the formation of maternal-fetal interface–placentation.
The major limitation in our systematic review is the heterogeneity of the included studies, and hence no specific trend can be extrapolated from them. Thus, associations are not clear and cannot be established. However, we were able to summarize these findings with focus on maternal and fetal outcomes to provide some information on the effects of COVID-19 infection during the first or second trimesters of pregnancy. Additionally, we could not distinguish between mothers who were hospitalized for COVID-19 related illness and those admitted for pregnancy-related conditions. We also assumed that the absence of certain outcomes indicated that the outcome did not occur, and hence we might be under-estimating some outcomes that we have extracted. The findings in this systematic review suggest that pregnant women who have been infected during the first and second trimester need to be monitored carefully, as an increased number of women required hospital admission, ICU admission and receipt of respiratory support and mechanical ventilation. For example, pregnant women with hyperemesis gravidarum (HG) during their first trimester should not be left unattended as COVID-19 patients may present with gastrointestinal complaints in the absence of respiratory symptoms during the first trimester [48]. Among 1141 COVID-19 pregnant patients reported by Luo et al. [48], 16% presented with digestive symptoms which include 66% nausea and vomiting, 30% diarrhea, 25% abdominal pain, without any respiratory symptoms [48]. While Liver enzymes remain normal in pregnant women with HG, patients with COVID-19 presenting with gastrointestinal symptoms usually have abnormal liver function tests such as elevated aminotransferases and bilirubin [48]. Another highlight of this paper is the importance of conducting further research to understand the impact of COVID-19 on pregnancy in its early stages, as it will guide clinicians in patient counseling, particularly those who consider the termination of their pregnancy out of fear of COVID-19 drawbacks. Moreover, there is a need to conduct longitudinal studies to follow-up on women who contracted COVID-19 during the first or second trimester as this will allow us to draw better conclusions regarding pregnancy outcomes following the infection.
In conclusion, the acuteness of the disease phase is more pronounced depending on the pregnancy stage during which the woman contracts it. The early phases of pregnancy imply less concern for the fetus as it is still not viable, and the mother’s health remains the clinician’s utmost concern. The later stages of pregnancy bring about more obstetric complications; that coupled with a SARS-CoV-2 infection would lead to an increased risk for both the mother and the viable fetus. Despite the many studies reporting on the impact of COVID-19 infection on pregnant women and its outcomes on the fetus, there are insufficient good-quality studies assessing those infected during early pregnancy that would allow unbiased conclusions with regard to the severity of the disease or specific complications of COVID-19 in pregnant women.
Acknowledgments
The authors would like to acknowledge Ms. Joanne Khabsa for her assistance in extracting data and reviewing the quality of the included studies.
Conflict of Interest
The authors would like to report no conflict of interest.
References
- Xintian Xu, Ping Chen, Jingfang Wang, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63 (2020): 457-460.
- Hirnle L, Lysenko L, Gerber H, et al. Respiratory Function in Pregnant Women, in Neurobiology of Respiration. Springer Netherlands: Dordrecht 788 (2013): 153-160.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi 55 (2020): 166-171.
- Huijun Chen, Juanjuan Guo, Chen Wang, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet 395 (2020): 809-815.
- Anna Gracia-Perez-Bonfils, Oscar Martinez-Perez, Elisa Llurba, et al. Fetal heart rate changes on the cardiotocograph trace secondary to maternal COVID-19 infection. Eur J Obstet Gynecol Reprod Biol 252 (2020): 286-293.
- Charles Egloff, Christelle Vauloup-Fellous, Olivier Picone, et al. Evidence and possible mechanisms of rare maternal-fetal transmission of SARS-CoV-2. J Clin Virol 128 (2020): 104447.
- Emily H Adhikari, Wilmer Moreno, Amanda C Zofkie, et al. Pregnancy Outcomes Among Women With and Without Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Netw Open 3 (2020): e2029256.
- Schwartz DA. An Analysis of 38 Pregnant Women With COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. Arch Pathol Lab Med 144 (2020): 799-805.
- Elizabeth A N Wastnedge, Rebecca M Reynolds, Sara R van Boeckel, et al. Pregnancy and COVID-19. Physiol Rev 101 (2021): 303-318.
- Schwartz DA, Graham A L. Potential Maternal and Infant Outcomes from (Wuhan) Coronavirus 2019-nCoV Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 12 (2020).
- Michelle Silasi, Ingrid Cardenas, Ja-Young Kwon, et al. Viral Infections During Pregnancy. American Journal of Reproductive Immunology 73 (2015): 199-213.
- Alvarado MG, Schwartz D A. Zika Virus Infection in Pregnancy, Microcephaly, and Maternal and Fetal Health: What We Think, What We Know, and What We Think We Know. Arch Pathol Lab Med 141 (2017): 26-32.
- da Silva JVF, Silva KSC, Dos Santos Junior V E. SARS-CoV-2 in the first trimester of pregnancy: potential interference in placentation. J Matern Fetal Neonatal Med (2020): 1.
- Stefano Forestieri, Maria Antonietta Marcialis, Lucia Migliore, et al. Relationship between pregnancy and coronavirus: what we know. J Matern Fetal Neonatal Med (2020): 1-12.
- David Moher, Alessandro Liberati, Jennifer Tetzlaff, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6 (2009): e1000097.
- H.O. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) is suspected: interim guidance, 13 March 2020. World Health Organization: Geneva, Switzerland (2020).
- Gordon H Guyatt, Andrew D Oxman, Gunn Vist, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). Journal of Clinical Epidemiology 64 (2011): 407-415.
- Mohammad Hassan Murad, Shahnaz Sultan, Samir Haffar, et al. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Medicine 23 (2018): 60.
- Andrikopoulou Maria, Madden, Nigel, Wen Timothy, et al. Symptoms and Critical Illness Among Obstetric Patients With Coronavirus Disease 2019 (COVID-19) Infection. Obstet Gynecol 136 (2020): 291-299.
- Amal Ayed, Alia Embaireeg, Asmaa Benawadh, et al. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID-19 in Kuwait. BMC Pregnancy and Childbirth 20 (2020): 754.
- Patricia Barbero, Laura Mugüerza, Ignacio Herraiz, et al. SARS-CoV-2 in pregnancy: characteristics and outcomes of hospitalized and non-hospitalized women due to COVID-19. The Journal of Maternal-Fetal & Neonatal Medicine (2020): 1-7.
- Berenice Curi, Alexander Sabre, Israel Benjamin, et al. Coronavirus infection in a high-risk obstetrical population of the South Bronx, New York. Am J Obstet Gynecol MFM 2 (2020): 100203.
- Federica Di Guardo, Flavia Maria Di Grazia, Luisa Maria Di Gregorio, et al. Poor maternal-neonatal outcomes in pregnant patients with confirmed SARS-Cov-2 infection: analysis of 145 cases. Arch Gynecol Obstet 303 (2021): 1483-1488.
- Alberto Giannini, Alessandro Mantovani, Cesare Vezzoli, et al. Lung ultrasound for pregnant women admitted to ICU for COVID-19 pneumonia. Minerva Anestesiol 86 (2020): 1248-1249.
- Vigil-De Gracia P, Caballero L C, Sánchez J, et al. Pregnancies recovered from SARS-CoV-2 infection in second or third trimester: obstetric evolution. Ultrasound Obstet Gynecol 56 (2020): 777-778.
- Olga Grechukhina, Victoria Greenberg, Lisbet S Lundsberg, et al. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. American Journal of Obstetrics & Gynecology MFM 2 (2020): 100246.
- Erica M Lokken, Emily M Huebner, Gray Taylor G, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol 225 (2021): 77.e1-77.e14.
- Niraj N Mahajan, Munira Ansari, Chaitanya Gaikwad, et al. Impact of SARS-CoV-2 on multiple gestation pregnancy. Int J Gynaecol Obstet 152 (2021): 220-225.
- Citra Nz Mattar, Shirin Kalimuddin, Sapna P Sadarangani, et al. Pregnancy Outcomes in COVID-19: A Prospective Cohort Study in Singapore. Ann Acad Med Singap 49 (2020): 857-869.
- Brisandy Ruiz Mercedes, Ayna Serwat, Lena Naffaa, et al. New-onset myocardial injury in pregnant patients with coronavirus disease 2019: a case series of 15 patients. Am J Obstet Gynecol 224 (2021): 387.e1-387.e9.
- Valeria M Savasi, Francesca Parisi, Luisa Patanè, et al. Clinical Findings and Disease Severity in Hospitalized Pregnant Women With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol 136 (2020): 252-258.
- Takemoto M, Menezes M O. Clinical characteristics and risk factors for mortality in obstetric patients with severe COVID-19 in Brazil: a surveillance database analysis 127 (2020): 1618-1626.
- Tug N, Yassa M. Pregnancy worsens the morbidity of COVID-19 and this effect becomes more prominent as pregnancy advances 17 (2020): 149-154.
- Yuanyuan Wang, Lian Chen, Tianchen Wu, et al. Impact of Covid-19 in pregnancy on mother's psychological status and infant's neurobehavioral development: a longitudinal cohort study in China. BMC Med 18 (2020): 347.
- Sascha Ellington, Penelope Strid, Van T Tong, et al. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-June 7, 2020. MMWR. Morbidity and mortality weekly report 69 (2020): 769-775.
- Asma Tekbali, Amos Grünebaum, Abraham Saraya, et al. Pregnant vs nonpregnant severe acute respiratory syndrome coronavirus 2 and coronavirus disease 2019 hospital admissions: the first 4 weeks in New York. American journal of obstetrics and gynecology 223 (2020): 126-127.
- Marian Knight, Kathryn Bunch, Nicola Vousden, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. Bmj 369 (2020): m2107.
- Francesca Crovetto, Fàtima Crispi, Elisa Llurba, et al. Seroprevalence and presentation of SARS-CoV-2 in pregnancy. The Lancet 396 (2020): 530-531.
- Collin J, Byström E. Public Health Agency of Sweden's Brief Report: Pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden 99 (2020): 819-822.
- Marissa Berry, Amanda Wang, Shannon M Clark, et al. Clinical Stratification of Pregnant COVID-19 Patients based on Severity: A Single Academic Center Experience 38 (2021): 515-522.
- Dominik Mertz, Calvin Ka-Fung Lo, Lyubov Lytvyn, et al. Pregnancy as a risk factor for severe influenza infection: an individual participant data meta-analysis. BMC infectious diseases 19 (2019): 683-683.
- John Allotey, Elena Stallings, Mercedes Bonet, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ (Clinical research ed.) 370 (2020): m3320-m3320.
- Pierre Buekens, Jackeline Alger, Gérard Bréart, et al. A call for action for COVID-19 surveillance and research during pregnancy. The Lancet Global Health 8 (2020): e877-e878.
- La Cour Freiesleben N, Egerup P, Hviid K V R, et al. SARS-CoV-2 in first trimester pregnancy: a cohort study. Hum Reprod 36 (2021): 40-47.
- David Baud, Gilbert Greub, Guillaume Favre, et al. Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. Jama 323 (2020): 2198-2200.
- Dorélien A. The Effects of In Utero Exposure to Influenza on Birth and Infant Outcomes in the US. Popul Dev Rev 45 (2019): 489-523.
- Gulersen M, Blitz M J. Clinical Implications of SARS-CoV-2 Infection in the Viable Preterm Period 37 (2020): 1077-1083.
- Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19). Clin Gastroenterol Hepatol 18 (2020): 1636-1637.
