Outcome and Impact on Adjuvant Treatment Processing Time after Mastectomy with or without Immediate Breast Reconstruction on a Large Cohort and Determination of a Postoperative Complications Predictive Score
Article Information
Léa Morante1, Sandrine Rua1, Monique Cohen1, Laura Sabiani1, Marc Martino1, Max Buttarelli1, Aurore Van Troy1, Anthony Gonçalves2, Agnès Tallet3, Aurélie Jalaguier Coudray4, Marie Bannier1, Gilles Houvenaeghel1*
1Aix-Marseille Univ, CNRS, INSERM, Institut Paoli-Calmettes, Department of Surgical Oncology, CRCM, Marseille, France
2Aix-Marseille Univ, CNRS, INSERM, Institut Paoli-Calmettes, Department of Medical Oncology, CRCM, Marseille, France
3CNRS, INSERM, Institut Paoli-Calmettes, Department of Radiotherapy, CRCM, Marseille, France
4CNRS, INSERM, Institut Paoli-Calmettes, Department of Radiology, CRCM, Marseille, France
*Corresponding Author: Gilles Houvenaeghel, Department of surgical oncology, Paoli Calmettes Institute
232 Bd de Sainte Marguerite, 13009 Marseille, France.
Received: 31 August 2023; Accepted: 13 September 2023; Published: 15 November 2023
Citation: Léa Morante, Sandrine Rua, Monique Cohen, Laura Sabiani, Marc Martino, Max Buttarelli, Aurore Van Troy, Anthony Gonçalves, Agnès Tallet, Aurélie Jalaguier Coudray, Marie Bannier, Gilles Houvenaeghel. Outcome and Impact on Adjuvant Treatment Processing Time after Mastectomy with or without Immediate Breast Reconstruction on a Large Cohort and Determination of a Postoperative Complications Predictive Score. Archives of Clinical and Medical Case Reports. 7 (2023): 390-408.
View / Download Pdf Share at FacebookAbstract
Background: Mastectomies are indicated in 12 to 40% of patients with an increase of immediate breast reconstruction (IBR) rate during the last years and complication rates between 5% to 61%. We analyzed data collected from 2016 to 2020 to assess the rate of IBR and complications, interval-time to adjuvant therapy and to establish a predictive score of postoperative complications.
Methods: We included all mastectomies performed from January 2016 to July 2020, in a retrospective analysis with prospective data collection of age, body mass index (BMI), ASA-status, diabetes, tobacco use, adjuvant treatments, year of treatment, type of mastectomy, modalities of IBR, complications and postoperative hospitalization length (POHL). We calculated a predictive score for complications.
Results: Among 2,112 mastectomies, IBR rate was 40.5%. Complication rate was 31.9% without difference between IBR and no-IBR groups. Grade 2-3 complications were significantly more frequent only for a BMI >30 (OR=1.8, p=0.002). Implant loss rate was 7.2% (44/609). A predictive score was determined with a significant increase of complications and Grade 2-3 breast complications rates (p<0.0001). The median POHL was 1 and 2 days in no-IBR and IBR groups. Intervaltime >60-days was associated only with age >75 years for adjuvant chemotherapy and age >75 years, Grade 2-3 complications and IBR for post-mastectomy radiotherapy.
Conclusions: Performing IBR was not significantly associated with complications and higher rate of interval-time >60-days for adjuvant chemotherapy. The complication predictive score can be a tool to inform patients at risks of complications and to compare results with others studies and techniques.
Keywords
Breast Cancer; Reconstruction; Complications; Chemotherapy; Radiotherapy
Breast Cancer Articles; Reconstruction Articles; Complications Articles; Chemotherapy Articles; Radiotherapy Articles
COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Breast Cancer articles Breast Cancer Research articles Breast Cancer review articles Breast Cancer PubMed articles Breast Cancer PubMed Central articles Breast Cancer 2023 articles Breast Cancer 2024 articles Breast Cancer Scopus articles Breast Cancer impact factor journals Breast Cancer Scopus journals Breast Cancer PubMed journals Breast Cancer medical journals Breast Cancer free journals Breast Cancer best journals Breast Cancer top journals Breast Cancer free medical journals Breast Cancer famous journals Breast Cancer Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals Reconstruction articles Reconstruction Research articles Reconstruction review articles Reconstruction PubMed articles Reconstruction PubMed Central articles Reconstruction 2023 articles Reconstruction 2024 articles Reconstruction Scopus articles Reconstruction impact factor journals Reconstruction Scopus journals Reconstruction PubMed journals Reconstruction medical journals Reconstruction free journals Reconstruction best journals Reconstruction top journals Reconstruction free medical journals Reconstruction famous journals Reconstruction Google Scholar indexed journals Ultrasound articles Ultrasound Research articles Ultrasound review articles Ultrasound PubMed articles Ultrasound PubMed Central articles Ultrasound 2023 articles Ultrasound 2024 articles Ultrasound Scopus articles Ultrasound impact factor journals Ultrasound Scopus journals Ultrasound PubMed journals Ultrasound medical journals Ultrasound free journals Ultrasound best journals Ultrasound top journals Ultrasound free medical journals Ultrasound famous journals Ultrasound Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Complications articles Complications Research articles Complications review articles Complications PubMed articles Complications PubMed Central articles Complications 2023 articles Complications 2024 articles Complications Scopus articles Complications impact factor journals Complications Scopus journals Complications PubMed journals Complications medical journals Complications free journals Complications best journals Complications top journals Complications free medical journals Complications famous journals Complications Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals Radiotherapy articles Radiotherapy Research articles Radiotherapy review articles Radiotherapy PubMed articles Radiotherapy PubMed Central articles Radiotherapy 2023 articles Radiotherapy 2024 articles Radiotherapy Scopus articles Radiotherapy impact factor journals Radiotherapy Scopus journals Radiotherapy PubMed journals Radiotherapy medical journals Radiotherapy free journals Radiotherapy best journals Radiotherapy top journals Radiotherapy free medical journals Radiotherapy famous journals Radiotherapy Google Scholar indexed journals
Article Details
1. Introduction
Since the 1990s, there has been a steady increase in breast-conserving surgeries associated with the development of oncoplasty. Nevertheless, total mastectomies for breast cancer (BC) are still indicated for 12 to 30% of patients and up to 40% [1-4]. It was 12.2% in a large French cohort of invasive BC [5]. The complication rates varies between 5% and 61% in the literature [6]. However, it is difficult to compare results between the different studies because of the large disparities in immediate breast reconstruction (IBR) rates and techniques, the complications reported, the indications for mastectomies and the monitoring time. However, increased body mass index (BMI) and smoking were reported factors to increase the risk of complications as well as previous radiotherapy and operative time [7]. IBR rate increase during the last years [8] in order to improve quality of life [9] and implant-based reconstruction was the most commonly performed procedure [10-12]. Several new procedures are been developed, as robotic procedures [13-16], pre-pectoral implant-IBR with or without mesh [7, 17-20]. Moreover, in recent year’s nipple sparing mastectomy (NSM) increase for prophylactic mastectomies [21], for local recurrence [22] and for primary BC [23, 24]. Generally, the NSM studies reported better aesthetic results than skin sparing mastectomy (SSM) and better quality of life [25-27]. NSM with IBR is consider today as a valid procedure for prophylactic mastectomy [21, 28-31] and an acceptable option for breast cancer (BC) therapeutic mastectomy [32-34]. The time to delivery adjuvant therapy after mastectomy is a key point to optimized oncologic treatments and has been few analyzed specifically for mastectomies with or without IBR [35]. In this study, we report our experience at the Paoli Calmettes Institute, by analyzing the data collected over 55 months from 2016 to 2020 to assess IBR rate, complication rate, interval-time to adjuvant therapy and to establish a predictive score for postoperative complications.
2. Materials and Methods
We included all mastectomies performed from January 2016 to July 2020, with or without IBR from institutional database (study: MAST-C-IPC 2021-024). A retrospective analysis with a prospective data collection was perform in order to determine the immediate surgical results and interval-time to adjuvant treatments.
2.1 Patients
Data were collected regarding patients: age, BMI, ASA (American Society of Anesthesiologists) status, diabetes, tobacco use, treatments received (neo-adjuvant or adjuvant chemotherapy, radiotherapy, endocrine therapy), year of treatment, type of mastectomy (nipple-sparing mastectomy (NSM), skin-sparing mastectomy (SSM), or classic if no reconstruction), modalities of IBR, and complications appeared in 90 days following the operation. Thirteen surgeons performed mastectomies.
Complications were analyzed according to the Clavien-Dindo classification [36]. The operative time was recorded from skin incision to skin closure collected on the anesthetic data. The length of postoperative stay was reported from the surgery day to the discharge day from hospital. A loco regional anesthesia with pectoralis block was systematically perform. Interval-times between surgery and adjuvant chemotherapy (AC) or post-mastectomy radiotherapy (PMRT) were analyze.
2.2 Statistics
Quantitative criteria were analyze with median, mean, 95% CI. Comparisons were determined using the Chi-2 test for qualitative criteria and t-test for quantitative criteria. Factors significantly associated with criteria analyzed were determine by a binary logistic regression adjusted for all significant variables identified by the univariate analysis. We calculated a predictive score for complications using the odds ratio derived from logistic regression. The performance of this score was analyze by calculating the AUC (Area under the Curve) value. Statistical significance was set as p ≤ 0.05. Analyses were perform with SPSS version 16.0 (SPSS Inc., Chicago, Illinois).
3. Results
3.1 Population
During a period of 55 months, 2,112 mastectomies were perform for 1,983 patients: 1,748 mastectomies for primary BC, 219 for local recurrence and 145 for prophylactic mastectomies. Mastectomies were realized after ipsilateral surgery for first non-in-sano conservative surgery in 430 patients (430/1748: 25%). Bilateral mastectomies (258 mastectomies) were perform for 129 patients (12.2%): for primary BC in 56.6%, for local recurrence in 8.9% and for prophylactic mastectomy in 34.5%.
Previous radiotherapy was perform for 287 mastectomies (13.6%): 219 for local recurrences after conservative treatment, 57 for reversals therapeutic sequence (neo-adjuvant chemotherapy [NAC] and neo-adjuvant radiotherapy [N-RTH]) and 11 for a history of irradiation as treatment of Hodgkin's disease. For patients with IBR, a high rate of NSM was achieved (399/851: 46.9%).
3.2 IBR
The IBR rate was 40.5%: 35.4% (618/1,748) for primary BC, 47.9% (105/219) for local recurrences and 91% (132/145) for prophylactic mastectomies. The annual IBR rates according to years of surgery were not significantly different. Number of mastectomies with or without IBR for each surgeon is report in Supplementary Figure 1. Characteristics of patients according to IBR status are report in Table 1. In univariate analysis, several criteria were significantly associated with or without IBR (Tables 1, 2). Tobacco use did not appear as a significant factor to perform an IBR.
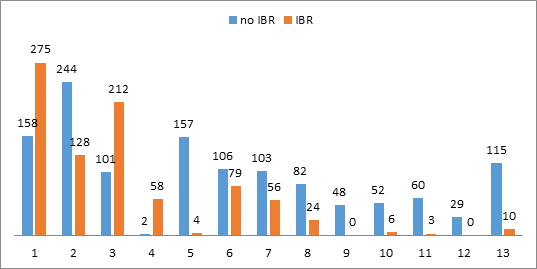
Supplementary Figure 1: Number of mastectomies with or without IBR for each surgeon.
Legend: IBR: immediate breast reconstruction
|
All patients |
no IBR |
IBR |
Chi 2 |
|||||
|
Nb |
% |
Nb |
% |
Nb |
% |
p |
||
|
All patients |
2112 |
1257 |
59.5 |
855 |
40.5 |
|||
|
years |
2016 |
407 |
19.3 |
242 |
19.3 |
165 |
19.3 |
0.076 |
|
2017 |
402 |
19.0 |
231 |
18.4 |
171 |
20.0 |
||
|
2018 |
503 |
23.8 |
279 |
22.2 |
224 |
26.2 |
||
|
2019 |
529 |
25.0 |
335 |
26.7 |
194 |
22.7 |
||
|
2020 |
271 |
12.8 |
170 |
13.5 |
101 |
11.8 |
||
|
age |
<= 40 |
288 |
13.6 |
113 |
9.0 |
175 |
20.5 |
<0.0001 |
|
41-50 |
504 |
23.9 |
215 |
17.1 |
289 |
33.8 |
||
|
51-74 |
991 |
46.9 |
626 |
49.8 |
365 |
42.7 |
||
|
>= 75 |
329 |
15.6 |
303 |
24.1 |
26 |
3.0 |
||
|
ASA |
1 |
616 |
29.2 |
235 |
18.7 |
381 |
44.6 |
<0.0001 |
|
2 |
1246 |
59.0 |
788 |
62.7 |
458 |
53.6 |
||
|
3 |
243 |
11.5 |
227 |
18.0 |
16 |
1.9 |
||
|
4 |
7 |
0.3 |
7 |
0.6 |
0 |
0 |
||
|
Smoker |
No |
1699 |
80.4 |
1017 |
80.9 |
682 |
79.8 |
0.539 |
|
Yes |
413 |
19.6 |
240 |
19.1 |
173 |
20.2 |
||
|
Diabetes |
No |
2002 |
94.8 |
1156 |
92.0 |
846 |
98.9 |
<0.0001 |
|
Yes |
110 |
5.2 |
101 |
8.0 |
9 |
1.1 |
||
|
BMI |
<= 24.9 |
1302 |
61.6 |
640 |
50.9 |
662 |
77.4 |
<0.0001 |
|
25-29.9 |
500 |
23.7 |
359 |
28.6 |
141 |
16.5 |
||
|
>= 30 |
310 |
14.7 |
258 |
20.5 |
52 |
6.1 |
||
|
Mastectomy |
NSM |
399 |
18.9 |
2 |
0.2 |
397 |
46.4 |
<0.0001 |
|
type |
SSM |
452 |
21.4 |
1 |
0.1 |
451 |
52.7 |
|
|
standard |
1261 |
59.7 |
1254 |
99.8 |
7 |
0.8 |
||
|
Indication |
Primary BC |
1748 |
82.8 |
1130 |
89.9 |
618 |
72.3 |
<0.0001 |
|
Local recurrence |
219 |
10.4 |
114 |
9.1 |
105 |
12.3 |
||
|
Prophylactic |
145 |
6.9 |
13 |
1.0 |
132 |
15.4 |
||
|
POLHS |
<= 3 days |
1865 |
88.3 |
1199 |
95.4 |
666 |
77.9 |
<0.0001 |
|
> 3 days |
247 |
11.7 |
58 |
4.6 |
189 |
22.1 |
||
|
Mastectomy |
<= 300 |
721 |
34.1 |
293 |
23.3 |
428 |
50.1 |
<0.0001 |
|
weight |
> 300 |
1391 |
65.9 |
964 |
76.7 |
427 |
49.9 |
|
|
previous ipsilateral |
No |
1432 |
67.8 |
900 |
71.6 |
532 |
62.2 |
<0.0001 |
|
breast surgery |
Yes |
680 |
32.2 |
357 |
28.4 |
323 |
37.8 |
|
|
NAC |
No |
1631 |
77.2 |
900 |
71.6 |
731 |
85.5 |
<0.0001 |
|
Yes |
481 |
22.8 |
357 |
28.4 |
124 |
14.5 |
||
|
previous ipsilateral |
No |
1825 |
86.4 |
1142 |
90.9 |
683 |
79.9 |
<0.0001 |
|
radiotherapy |
Yes |
287 |
13.6 |
115 |
9.1 |
172 |
20.1 |
|
|
NAC and N-RTH |
No |
2055 |
97.3 |
1256 |
99.9 |
799 |
93.5 |
<0.0001 |
|
Yes |
57 |
2.7 |
1 |
0.1 |
56 |
6.5 |
||
|
axillary surgery |
No |
605 |
28.6 |
232 |
18.5 |
373 |
43.6 |
<0.0001 |
|
SLNB |
794 |
37.6 |
410 |
32.6 |
384 |
44.9 |
||
|
ALND |
713 |
33.8 |
615 |
48.9 |
98 |
11.5 |
||
|
Radiotherapy |
No |
986 |
46.7 |
416 |
33.1 |
570 |
66.7 |
<0.0001 |
|
PMRT |
848 |
40.2 |
726 |
57.8 |
122 |
14.3 |
||
|
previous RTH |
221 |
10.5 |
114 |
9.1 |
107 |
12.5 |
||
|
NAC + N-RTH |
57 |
2.7 |
1 |
0.1 |
56 |
6.5 |
||
|
Adjuvant |
No |
1467 |
69.5 |
778 |
61.9 |
689 |
80.6 |
<0.0001 |
|
chemotherapy |
Yes |
645 |
30.5 |
479 |
38.1 |
166 |
19.4 |
|
|
Endocrine |
No |
782 |
37.0 |
372 |
29.6 |
410 |
48.0 |
<0.0001 |
|
therapy |
Yes |
1330 |
63.0 |
885 |
70.4 |
445 |
52.0 |
|
|
Histology |
DCIS |
258 |
12.2 |
84 |
6.7 |
174 |
20.4 |
<0.0001 |
|
NST |
1349 |
63.9 |
920 |
73.2 |
429 |
50.2 |
||
|
Lobular |
332 |
15.7 |
218 |
17.3 |
114 |
13.3 |
||
|
Others |
26 |
1.2 |
21 |
1.7 |
5 |
0.6 |
||
|
Begnin |
147 |
7.0 |
14 |
1.1 |
133 |
15.6 |
||
|
Bilateral |
No |
1854 |
87.8 |
1155 |
91.9 |
699 |
81.8 |
<0.0001 |
|
mastectomy |
Yes |
258 |
12.2 |
102 |
8.1 |
156 |
18.2 |
|
Legend: IBR: immediate breast reconstruction, ASA: American Society of Anesthesiologists, BMI: Body mass index, POHLS: Post-operative hospitalization length, NAC: neo-adjuvant chemotherapy, N-RTH: neo-adjuvant radiotherapy, DCIS: ductal carcinoma in-situ, NST: non-specific invasive carcinoma, BC: breast cancer, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection.
Table 1: Characteristics of all patients and according to immediate breast reconstruction (IBR) or no-IBR.
|
median |
mean |
95%CI |
t-test: p |
||
|
age |
all patients |
56 |
57.74 |
57.1-58.4 |
|
|
no IBR |
64.0 |
62.5 |
61.7-63.4 |
<0.0001 |
|
|
IBR |
49 |
50.7 |
49.9-51.5 |
||
|
BMI |
all patients |
23.38 |
24.54 |
24.3-24.8 |
|
|
no IBR |
24.86 |
25.74 |
25.4-26.1 |
<0.0001 |
|
|
IBR |
22.0 |
22.77 |
22.5-23.0 |
||
|
Weight of |
all patients |
410 |
512 |
494-529 |
|
|
mastectomy |
no IBR |
525 |
622 |
597-648 |
<0.0001 |
|
IBR |
300 |
350 |
335-365 |
||
|
POLHS |
all patients |
1 |
1.87 |
1.80-1.93 |
|
|
no IBR |
1 |
1.4 |
1.33-1.47 |
<0.0001 |
|
|
IBR |
2 |
2.55 |
2.45-2.65 |
||
|
anesthesia |
all patients |
142 |
169.1 |
165-173 |
|
|
duration |
no IBR |
123 |
128.4 |
126-131 |
<0.0001 |
|
IBR |
200 |
228.9 |
222-235 |
||
|
surgery |
all patients |
92 |
115.8 |
113-119 |
|
|
duration |
no IBR |
74 |
80.3 |
79-82 |
<0.0001 |
|
IBR |
141 |
168 |
162-174 |
||
|
implant size |
IBR |
280 |
289 |
282-296 |
Legend: IBR: immediate breast reconstruction, BMI: Body mass index, POHLS: Post-operative hospitalization length.
Table 2: Results (median, mean and 95% confidence interval) of quantitative variables.
|
IBR verus |
p |
OR |
95% CI |
||
|
no IBR |
Inferior |
Superior |
|||
|
age |
<= 40 |
1 |
|||
|
41-50 |
0.924 |
0.982 |
0.686 |
1.407 |
|
|
51-74 |
<0.0001 |
0.530 |
0.374 |
0.755 |
|
|
>= 75 |
<0.0001 |
0.108 |
0.063 |
0.187 |
|
|
ASA |
1 |
1 |
|||
|
2 |
<0.0001 |
0.572 |
0.445 |
0.736 |
|
|
3 |
<0.0001 |
0.160 |
0.087 |
0.295 |
|
|
4 |
0,999 |
NE |
NE |
NE |
|
|
Diabetes |
Yes vs No |
0.020 |
0.388 |
0.175 |
0.859 |
|
BMI |
<= 24,9 |
1 |
|||
|
25-29,99 |
0.001 |
0.606 |
0.455 |
0.807 |
|
|
>= 30 |
<0.0001 |
0.418 |
0.283 |
0.617 |
|
|
Indication |
Primary |
1 |
|||
|
Local recurrence |
<0.0001 |
0.212 |
0.115 |
0.391 |
|
|
Prophylactic |
0.002 |
4.540 |
1.709 |
12.061 |
|
|
Mast weight |
> vs <=300 |
<0.0001 |
0.595 |
0.464 |
0.763 |
|
NAC |
Yes vs No |
<0.0001 |
0.361 |
0.264 |
0.493 |
|
previous RTH |
Yes vs No |
<0.0001 |
12.996 |
7.619 |
22.166 |
|
Histology |
DCIS |
1 |
|||
|
NST |
<0.0001 |
0.276 |
0.197 |
0.385 |
|
|
Lobular |
<0.0001 |
0.312 |
0.210 |
0.464 |
|
|
Others |
<0.0001 |
0.088 |
0.028 |
0.273 |
|
|
Begnin |
0.692 |
0.819 |
0.306 |
2.196 |
|
Legend: IBR: immediate breast reconstruction, ASA: American Society of Anesthesiologists status, NAC: neo-adjuvant chemotherapy, RTH: radiotherapy.
Table 3: Binary logistic regression analysis: Factors associated with immediate breast reconstruction (IBR) in comparison with patients with no-IBR.
In binary logistic regression, IBR were significantly associated with age (less IBR for patients >50 years), ASA status (less IBR for ASA 2-3 versus ASA 1), diabetes (less IBR for diabetic patients), BMI (less IBR for BMI >25), mastectomy weight (less IBR for weight >300gr), neo-adjuvant treatment (less IBR for neo-adjuvant treatment), histological status (more IBR for preoperative diagnosis of in situ carcinomas and prophylaxis, and less IBR for local recurrence), and history of radiotherapy (Table 3).
3.3 Complications
The complication rate was 31.90% (n=675): 29.9% for IBR group and 33.3% for no-IBR group, including 23.2% of grade 3 complications requiring revision surgery. The different grades of 599 breast complications were distributed as 59.6% grade 1 (n=357), 11.0% grade 2 (n=66), 29.2% grade 3 (n=175) and 0.0017% grade 4 (n=1). Others complications were in relation with dorsal complication for latissimus dorsi-flap reconstruction.
Criteria significantly associated with complications in univariate analysis are reported in Table 4, with a significantly higher rate of grade 2 and 3 complications for ASA score ≥2, BMI>= 30, mastectomy weight >300g. In binary logistic regression, complications were significantly associated with year of treatment (fewer complications during the last 2 years), smokers (more complications for smoker patients), age (more complications for patients >50 years), radiotherapy (more complications for patients with a previous radiotherapy), axillary lymph-node dissection associated with mastectomy +/- IBR (more complications than SLNB or no axillary surgery) and mastectomy weight (more complications for weight >300g) (Table 5). In binary logistic regression, grade 2 and 3 complications were significantly more frequent only for a BMI >30 (OR=1.8, p=0.002). When adjusting the regression analysis for IBR or no IBR, there was no significant difference between IBR group and no-IBR group.
Legend: IBR: immediate breast reconstruction, ASA: American Society of Anesthesiologists, BMI: Body mass index, NSM: Nipple-sparing mastectomy, SSM: Skin-sparing mastectomy, BC: breast cancer, DCIS: ductal carcinoma in-situ, NST: non-specific invasive carcinoma NAC: neo-adjuvant chemotherapy, N-RTH: neo-adjuvant radiotherapy, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection.
Table 4: Significant criteria associated with complications in univariate analysis.
|
Complication: Yes vs No |
p |
OR |
95% CI |
||
|
Inferior |
Superior |
||||
|
Years |
2016 |
1 |
|||
|
2017 |
0.823 |
1.034 |
0.769 |
1.392 |
|
|
2018 |
0.667 |
1.064 |
0.802 |
1.410 |
|
|
2019 |
0.027 |
0.723 |
0.541 |
0.964 |
|
|
2020 |
<0.0001 |
0.495 |
0.343 |
0.714 |
|
|
ASA |
1 |
1 |
|||
|
2 |
0.517 |
1.082 |
0.853 |
1.373 |
|
|
3 |
0.492 |
1.140 |
0.785 |
1.655 |
|
|
4 |
0.157 |
3.051 |
0.650 |
14.322 |
|
|
Smoker |
Yes vs No |
<0.0001 |
1.707 |
1.348 |
2.163 |
|
Diabetes |
Yes vs No |
0.333 |
1.230 |
0.809 |
1.869 |
|
Age |
<= 40 |
1 |
|||
|
41-50 |
0.211 |
1.245 |
0.883 |
1.754 |
|
|
51-74 |
0.015 |
1.493 |
1.080 |
2.064 |
|
|
>= 75 |
0.002 |
1.849 |
1.244 |
2.749 |
|
|
BMI |
<= 24,9 |
1 |
|||
|
25-29,99 |
0.504 |
1.087 |
0.852 |
1.386 |
|
|
>= 30 |
0.339 |
1.156 |
0.859 |
1.556 |
|
|
previous RTH |
Yes vs No |
<0.0001 |
1.6660 |
1.248 |
2.207 |
|
axillary surgery |
No |
1 |
|||
|
SLNB |
0.262 |
1.157 |
0.897 |
1.492 |
|
|
ALND |
0.028 |
1.334 |
1.032 |
1.724 |
|
|
Mastect weight |
> vs <= 300 |
0.001 |
1.496 |
1.185 |
1.888 |
|
Surgeon |
0.978 |
1.000 |
0.980 |
1.021 |
|
|
Grade 2-3 breast |
p |
OR |
95% CI |
||
|
complication |
Inferior |
Superior |
|||
|
ASA |
1 |
1 |
|||
|
2 |
0.243 |
1.218 |
0.874 |
1.697 |
|
|
3 |
0.295 |
1.289 |
0.802 |
2.072 |
|
|
4 |
0.216 |
2.903 |
0.536 |
15.736 |
|
|
BMI |
<= 24,9 |
1 |
|||
|
25-29,99 |
0.789 |
1.050 |
0.734 |
1.502 |
|
|
>= 30 |
0.002 |
1.836 |
1.255 |
2.686 |
|
|
Mast weight |
> vs <= 300 |
0.168 |
1.270 |
0.904 |
1.784 |
Legend: ASA: American Society of Anesthesiologists, BMI: Body mass index, POHLS: Post-operative hospitalization length, RTH: radiotherapy.
Table 5: Factors associated to complications and grade 2-3 breast complications in binary logistic regression.
|
Type of complication |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Total |
% |
|
|
All |
cutaneous |
108 |
20 |
29 |
0 |
157 |
26.2 |
|
patients |
hemetoma |
37 |
7 |
88 |
0 |
132 |
22.0 |
|
infection |
3 |
19 |
45 |
1 |
68 |
11.4 |
|
|
seroma |
152 |
11 |
12 |
0 |
175 |
29.2 |
|
|
others |
57 |
9 |
1 |
0 |
67 |
11.2 |
|
|
Total (%) |
357 (59.6) |
66 (11.0) |
175 (29.2) |
1 (0.2) |
599 |
100 |
|
|
no-IBR |
cutaneous |
39 |
9 |
13 |
0 |
61 |
14.9 |
|
hematoma |
29 |
6 |
55 |
0 |
90 |
21.9 |
|
|
infection |
3 |
17 |
18 |
0 |
38 |
9.3 |
|
|
seroma |
152 |
10 |
12 |
0 |
174 |
42.4 |
|
|
others |
40 |
6 |
1 |
0 |
47 |
11.5 |
|
|
Total (%) |
263 (64.1) |
48 (11.7) |
99 (24.1) |
0 |
410 |
||
|
IBR |
cutaneous |
69 |
11 |
16 |
0 |
96 |
50.8 |
|
hematoma |
8 |
1 |
33 |
0 |
42 |
22.2 |
|
|
infection |
0 |
2 |
27 |
1 |
30 |
15.9 |
|
|
seroma |
0 |
1 |
0 |
0 |
1 |
0.5 |
|
|
others |
17 |
3 |
0 |
0 |
20 |
10.6 |
|
|
Total (%) |
94 (49.7) |
18 (9.5) |
76 (40.2) |
1 (0.5) |
189 |
100 |
Legend: IBR: immediate breast reconstruction.
Table 6: Breast complications according to grading for all patients and for patients with or without IBR.
Breast complications according to grading is report in Table 6: Seroma was the most frequent complication with 86.9% of grade 1, while there was 66.7% of grade 3 hematomas. There were 68.8% of grade 1 cutaneous complications. Cutaneous complications and infections were more frequent for IBR-patients and seromas were more frequent for no-IBR patients. Implant loss rate was 7.2% (44/609): 6.1% (34/560) for implant-based IBR and 20.4% (10/49) for latissimus dorsi-flap (LDF) IBR with implant (p <0.001).
Legend: 543 patients score 0 (25.7%), 621 score 1 (29.4%), 914 score 2 (43.3%) and 34 score 3 (1.6%).
For example, for a simplified C-score G1-2-3 value of 3, the postoperative complication rate was 64.7% with 26.5% of grade 2-3 complications versus 20.8% of complications for a simplified score of 0 with only 8.1% of grade 2 and 3 complications.
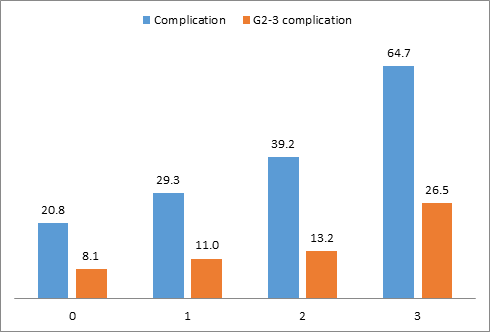
Figure 1: Complication rate and grade 2-3 complications according to the simplified score (C-score G1-2-3).
3.4 Complication score
The following equation to calculate a complication risk score was: smoker + age + previous radiotherapy + axillary surgery + mastectomy weight. Odds ratios described above were used to determine the value of each criterion: smoker (0 or 2), age (0 for age ≤50 years, 1.5 for age between 61 and 74, 2 for age ≥75 years), previous radiotherapy (0 or 2), axillary surgery (0 for SLNB or no axillary surgery and 1 for axillary lymph-node dissection), mastectomy weight (0 if ≤ 300g or 1.5 if > 300g). A simplified score (C-score-G1-2-3) was determined according to the result of the equation: 0 for values ≤ 1.5, 1 for values between 2 and 3, 2 for values between 3.5 and 6 and 3 for values ≥6.5. A significantly increasing rate of complications (p<0.0001) and grade 2-3 complications was observe for higher values of this simplified score (Figure 1) with 0.603 AUC value (95% CI: 0.577-0.628) for all complications.
A simplified score for Grade 2-3 breast complications (C-score-G2-3) was determined [simplified C-score-G1-2-3 value (0 to 3) + BMI (>=30=2, <30=0)]. A significant increase of Grade 2-3 breast complications rates was observed (p<0.0001) with 0.591 AUC value (95%CI: 0.552-0.630) (Figure 2).
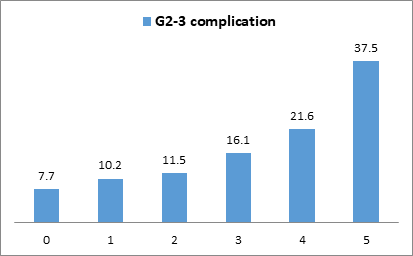
Figure 2: Grade 2-3 complications rates according to the simplified score.
Legend: 519 patients score 0 (24.6%), 510 score 1 (24.1%), 771 score 2 (36.5%), 137 score 3 (6.5%), 167 score 4 (7.9%) and 8 score 5 (0.4%).
3.5 IBR types
Implant-based IBR were performed on 649 patients (76%), excluding one patient with exclusive lipofilling IBR. LDF-IBR was performing for 205 patients (24%) and was associated with an implant-based for 52 patients (22.2%). Factors significantly associated with IBR-LDF in univariate analysis are report in Supplementary Table 1. In binary logistic regression, higher rate of IBR by LDF was significantly associated with BMI ≥25, neo-adjuvant treatments, diabetes, radiotherapy, lobular histology, age between 51-74 years old, and lesser rate was significantly associated with SSM, bilateral mastectomies and prophylactic mastectomies (Supplementary Table 2).
|
IBR |
Implant |
LDF |
Chi 2 |
|||
|
(1 patient: lipofilling-IBR excluded) |
Nb |
% |
Nb |
% |
p |
|
|
All patients |
649 |
76.0 |
205 |
24.0 |
||
|
NAC |
No |
597 |
92.0 |
133 |
64.9 |
<0.0001 |
|
Yes |
52 |
8.0 |
72 |
35.1 |
||
|
years |
2016 |
124 |
19.1 |
41 |
20.0 |
<0.0001 |
|
2017 |
112 |
17.3 |
59 |
28.8 |
||
|
2018 |
153 |
23.6 |
71 |
34.6 |
||
|
2019 |
167 |
825.7 |
26 |
12.7 |
||
|
2020 |
93 |
14.3 |
8 |
3.9 |
||
|
Indication |
Primary |
459 |
70.7 |
159 |
77.6 |
<0.0001 |
|
Local recurrence |
60 |
9.2 |
44 |
21.5 |
||
|
Prophylactic |
130 |
20.0 |
2 |
1.0 |
||
|
Mast type |
NSM |
315 |
48.5 |
81 |
39.5 |
0.013 |
|
SSM |
331 |
51.0 |
120 |
58.5 |
||
|
standard |
3 |
0.5 |
4 |
2.0 |
||
|
Bilateral |
No |
494 |
76.1 |
204 |
99.5 |
<0.0001 |
|
mastectomy |
Yes |
155 |
23.9 |
1 |
0.5 |
|
|
ASA |
1 |
311 |
47.9 |
70 |
34.1 |
0.002 |
|
2 |
327 |
50.4 |
131 |
63.9 |
||
|
3 |
11 |
1.7 |
4 |
2.0 |
||
|
Smoker |
No |
524 |
80.7 |
157 |
76.6 |
0.197 |
|
Yes |
125 |
19.3 |
48 |
23.4 |
||
|
Diabetes |
No |
646 |
99.5 |
199 |
97.1 |
0.008 |
|
Yes |
3 |
0.5 |
6 |
2.9 |
||
|
Previous |
No |
428 |
65.9 |
104 |
50.7 |
<0.0001 |
|
breast surgery |
Yes |
221 |
34.1 |
101 |
49.3 |
|
|
Previous |
No |
581 |
89.5 |
102 |
49.8 |
<0.0001 |
|
radiotherapy |
Yes |
68 |
10.5 |
103 |
50.2 |
|
|
NAC+N-RTH |
No |
648 |
99.8 |
150 |
73.2 |
<0.0001 |
|
Yes |
1 |
0.2 |
55 |
26.8 |
||
|
Cup size |
A-B |
377 |
58.1 |
92 |
44.9 |
0.002 |
|
C |
182 |
28.0 |
68 |
33.2 |
||
|
> C |
90 |
13.9 |
45 |
22.0 |
||
|
Axillary |
No |
277 |
42.7 |
95 |
46.3 |
<0.0001 |
|
surgery |
SLNB |
329 |
50.7 |
55 |
26.8 |
|
|
ALND |
43 |
6.6 |
55 |
26.8 |
||
|
Histology |
DCIS |
140 |
21.6 |
34 |
16.6 |
<0.0001 |
|
NST |
303 |
46.7 |
126 |
61.5 |
||
|
Lobular |
76 |
11.7 |
37 |
18.0 |
||
|
Others |
1 |
0.2 |
4 |
2.0 |
||
|
Begnin |
129 |
19.9 |
4 |
2.0 |
||
|
Age |
<= 40 |
143 |
22.0 |
32 |
15.6 |
0.002 |
|
41-50 |
233 |
35.9 |
56 |
27.3 |
||
|
51-74 |
254 |
39.1 |
110 |
53.7 |
||
|
>= 75 |
19 |
2.9 |
7 |
3.4 |
||
|
BMI |
<= 24.9 |
529 |
81.5 |
132 |
64.4 |
<0.0001 |
|
25-29.9 |
90 |
13.9 |
51 |
24.9 |
||
|
>= 30 |
30 |
4.6 |
22 |
10.7 |
||
|
Mastectomy |
<= 300 |
347 |
53.5 |
80 |
39.0 |
<0.0001 |
|
weight |
> 300 |
302 |
46.5 |
125 |
61.0 |
|
Legend: IBR: immediate breast reconstruction, LDF: latissimus dorsi-flap, ASA: American Society of Anesthesiologists, BMI: Body mass index, RTH: radiotherapy, NSM: Nipple-sparing mastectomy, SSM: Skin-sparing mastectomy, BC: breast cancer, DCIS: ductal carcinoma in-situ, NST: non-specific invasive carcinoma NAC: neo-adjuvant chemotherapy, N-RTH: neo-adjuvant radiotherapy, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection.
Supplementary Table 1: Factors associated with LDF-IBR versus implant-IBR.
|
LDF-IBR versus implant-IBR |
p |
OR |
95% CI |
||
|
Inferior |
Superior |
||||
|
ASA |
1 |
1 |
|||
|
2 |
0.115 |
0.696 |
0.443 |
1.093 |
|
|
3 |
0.231 |
0.380 |
0.078 |
1.855 |
|
|
BMI |
<= 24.9 |
1 |
|||
|
25-29.9 |
<0.0001 |
3.002 |
1.758 |
5.125 |
|
|
>= 30 |
<0.0001 |
2.867 |
1.272 |
6.462 |
|
|
Mastectomy |
<= 300 |
1 |
|||
|
weight |
> 300 |
0.381 |
0.791 |
0.468 |
1.336 |
|
Mast type |
NSM |
1 |
|||
|
SSM |
0.043 |
0.643 |
0.420 |
0.985 |
|
|
standard |
0.163 |
4.005 |
0.571 |
28.078 |
|
|
Indication |
Primary |
1 |
|||
|
Local recurrence |
<0.0001 |
0.025 |
0.005 |
0.128 |
|
|
Prophylactic |
0.036 |
0.040 |
0.002 |
0.806 |
|
|
NAC |
No |
1 |
|||
|
Yes |
0.035 |
2.214 |
1.057 |
4.637 |
|
|
Bilateral |
No |
1 |
|||
|
mastectomy |
Yes |
<0.0001 |
0.003 |
0.000 |
0.046 |
|
Diabetes |
No |
1 |
|||
|
Yes |
0.035 |
5.446 |
1.122 |
26.431 |
|
|
Previous |
No |
1 |
|||
|
radiotherapy |
Yes |
<0.0001 |
140.95 |
29.33 |
677.5 |
|
Cup size |
A-B |
1 |
|||
|
C |
0.164 |
1.443 |
0.860 |
2.422 |
|
|
> C |
0.439 |
1.308 |
0.662 |
2.585 |
|
|
Histology |
DCIS |
1 |
|||
|
NST |
0.678 |
1.119 |
0.659 |
1.900 |
|
|
Lobular |
0.030 |
1.991 |
1.068 |
3.710 |
|
|
Others |
0.187 |
5.359 |
0.444 |
64.70 |
|
|
Begnin |
0.170 |
6.712 |
0.442 |
101.86 |
|
|
Age |
<= 40 |
1 |
|||
|
41-50 |
0.261 |
1.545 |
0.724 |
3.297 |
|
|
51-74 |
0.012 |
2.603 |
1.236 |
5.485 |
|
|
>= 75 |
0.322 |
1.899 |
0.533 |
6.763 |
|
Legend: IBR: immediate breast reconstruction, LDF: latissimus dorsi-flap, ASA: American Society of Anesthesiologists, BMI: Body mass index, NAC: neo-adjuvant chemotherapy, NSM: Nipple-sparing mastectomy, SSM: Skin-sparing mastectomy, DCIS: ductal carcinoma in-situ, NST: non-specific invasive carcinoma NAC: neo-adjuvant chemotherapy.
Supplementary Table 2: Factors associated with LDF-IBR versus implant IBR in binary logistic regression.
3.6 Post-operative hospitalization length (POHL)
The median POHL was 1 day (mean 1.87): 1 day without IBR and 2 days with IBR (Table 2) and 247 patients (11.7%) were hospitalize more than 3 days. The POHL was ≤1 day for 54.3% of mastectomies (1,146/2,112): 73.5% (924/1,257) in the no-IBR group and 25.96% (222/855) in the IBR-group (222/650: 34.2% of IBR-implants).
The POHL rate ≤1 day has increased over the past 2 years compared to 2016-2018 for patients without IBR (69.9% vs. 78.8%) and for patients with IBR (19.8% vs. 37.6%). In univariate analysis, factors significantly associated with a POHL >3 days were the year of treatment, age, type of mastectomy, indication for mastectomy, histology, bilateral mastectomy, IBR or no-IBR, axillary surgery, previous ipsilateral surgery, previous ipsilateral radiotherapy and the surgeon (Table 7). In binary logistic regression, POHL >3 days was significantly increased by NST or lobular histological status, bilateral mastectomies, IBR, previous radiotherapy, and decreased during the last 2 years of treatment, and by age between 41-50 years (Table 8).
|
POHL |
<= 3 days |
> 3 days |
Chi2 |
|||
|
Nb |
% |
Nb |
% |
p |
||
|
all patients |
1865 |
88.3 |
247 |
11.7 |
||
|
years |
2016 |
345 |
18.5 |
62 |
25.1 |
<0.0001 |
|
2017 |
352 |
18.9 |
50 |
20.2 |
||
|
2018 |
423 |
22.7 |
80 |
32.4 |
||
|
2019 |
485 |
26.0 |
44 |
17.8 |
||
|
2020 |
260 |
13.9 |
11 |
4.5 |
||
|
age |
<= 40 |
242 |
13.0 |
46 |
18.6 |
0.019 |
|
41-50 |
453 |
24.3 |
51 |
20.6 |
||
|
51-74 |
869 |
46.6 |
122 |
49.4 |
||
|
>= 75 |
301 |
16.1 |
28 |
11.3 |
||
|
ASA |
1 |
529 |
28.4 |
87 |
35.2 |
0.060 |
|
2 |
1120 |
60.1 |
126 |
51.0 |
||
|
3 |
210 |
11.3 |
33 |
13.4 |
||
|
4 |
6 |
0.3 |
1 |
0.4 |
||
|
Smoker |
No |
1507 |
80.8 |
192 |
77.7 |
0.267 |
|
Yes |
358 |
19.2 |
55 |
22.3 |
||
|
Diabetes |
No |
1769 |
94.9 |
233 |
94.3 |
0.760 |
|
Yes |
96 |
5.1 |
14 |
5.7 |
||
|
BMI |
<= 24.9 |
1143 |
61.3 |
159 |
64.4 |
0.285 |
|
25-29.9 |
440 |
23.6 |
60 |
24.3 |
||
|
>= 30 |
282 |
15.1 |
28 |
11.3 |
||
|
Mastectomy type |
NSM |
306 |
16.4 |
93 |
37.7 |
<0.0001 |
|
SSM |
358 |
19.2 |
94 |
38.1 |
||
|
standard |
1201 |
64.4 |
60 |
24.3 |
||
|
Indication |
Primary BC |
1573 |
84.3 |
175 |
70.9 |
<0.0001 |
|
Local recurrence |
173 |
9.3 |
46 |
18.6 |
||
|
Prophylactic |
119 |
6.4 |
26 |
10.5 |
||
|
Histology |
DCIS |
229 |
12.3 |
29 |
11.7 |
0.024 |
|
NST |
1208 |
64.8 |
141 |
57.1 |
||
|
Lobular |
287 |
15.4 |
45 |
18.2 |
||
|
Others |
22 |
1.2 |
4 |
1.6 |
||
|
Begnin |
119 |
6.4 |
28 |
11.3 |
||
|
Bilateral mastectomy |
No |
1657 |
88.8 |
197 |
79.8 |
<0.0001 |
|
Yes |
208 |
11.2 |
50 |
20.2 |
||
|
IBR |
No |
1199 |
64.3 |
58 |
23.5 |
<0.0001 |
|
Yes |
666 |
35.7 |
189 |
76.5 |
||
|
Surgeon |
<0.0001 |
|||||
|
NAC |
No |
1443 |
77.4 |
188 |
76.1 |
0.686 |
|
Yes |
422 |
22.6 |
59 |
23.9 |
||
|
previous ipsilateral |
No |
1290 |
69.2 |
142 |
57.5 |
<0.0001 |
|
surgery |
Yes |
575 |
30.8 |
105 |
42.5 |
|
|
previous ipsilateral |
No |
1657 |
88.8 |
168 |
68.0 |
<0.0001 |
|
radiotherapy |
Yes |
208 |
11.2 |
79 |
32.0 |
|
|
NAC + N-RTH |
No |
1836 |
98.4 |
219 |
88.7 |
<0.0001 |
|
Yes |
29 |
1.6 |
28 |
11.3 |
||
|
axillary surgery |
No |
500 |
26.8 |
105 |
42.5 |
<0.0001 |
|
SLNB |
713 |
38.2 |
81 |
32.8 |
||
|
ALND |
652 |
35.0 |
61 |
24.7 |
||
|
Mastectomy weight |
<= 300 |
626 |
33.6 |
95 |
38.5 |
0.134 |
|
> 300 |
1239 |
66.4 |
152 |
61.5 |
Legend: POHL: postoperative hospitalization length, IBR: immediate breast reconstruction, ASA: American Society of Anesthesiologists, BMI: Body mass index, NAC: neo-adjuvant chemotherapy, N-RTH: neo-adjuvant radiotherapy, NSM: Nipple-sparing mastectomy, SSM: Skin-sparing mastectomy, BC: breast cancer, DCIS: ductal carcinoma in situ, NST: non-specific tumor (invasive ductal carcinoma), SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection.
Table 7: Factors associated with a POHL >3 days in univariate analysis.
|
POHL > 3 days versus |
p |
OR |
95% CI |
||
|
< 3 days |
Inferior |
Superior |
|||
|
Years |
2016 |
1 |
|||
|
2017 |
0.214 |
0.755 |
0.485 |
1.176 |
|
|
2018 |
0.764 |
0.941 |
0.633 |
1.399 |
|
|
2019 |
0.001 |
0.454 |
0.290 |
0.711 |
|
|
2020 |
<0.0001 |
0.194 |
0.096 |
0.391 |
|
|
Age |
<= 40 |
1 |
|||
|
41-50 |
0.033 |
0.598 |
0.373 |
0.960 |
|
|
51-74 |
0.929 |
1.020 |
0.662 |
1.570 |
|
|
>= 75 |
0.202 |
1.487 |
0.809 |
2.732 |
|
|
Mast type |
NSM |
1 |
|||
|
SSM |
0.120 |
0.743 |
0.511 |
1.080 |
|
|
standard |
0.403 |
2.182 |
0.351 |
13.575 |
|
|
Indication |
Primary |
1 |
|||
|
Local recurrence |
0.209 |
0.654 |
0.337 |
1.269 |
|
|
Prophylactic |
0.334 |
0.572 |
0.184 |
1.777 |
|
|
Histology |
DCIS |
1 |
|||
|
NST |
0.044 |
1.629 |
1.013 |
2.619 |
|
|
Lobular |
0.002 |
2.391 |
1.378 |
4.147 |
|
|
Others |
0.140 |
2.893 |
0.705 |
11.878 |
|
|
Begnin |
0.278 |
1.940 |
0.586 |
6.421 |
|
|
Bilateral Mast |
Yes vs No |
0.003 |
1.895 |
1.242 |
2.890 |
|
IBR |
Yes vs No |
0.002 |
17.643 |
2.829 |
110.022 |
|
previous surgery |
Yes vs No |
0.257 |
1.262 |
0.844 |
1.887 |
|
previous RTH |
Yes vs No |
<0.0001 |
3.481 |
2.050 |
5.909 |
|
axillary surgery |
No |
1 |
|||
|
SLNB |
0.580 |
0.884 |
0.571 |
1.369 |
|
|
ALND |
0.439 |
1.210 |
0.746 |
1.964 |
|
Legend: POHL: postoperative hospitalization length, IBR: immediate breast reconstruction, RTH: radiotherapy, NSM: Nipple-sparing mastectomy, SSM: Skin-sparing mastectomy, DCIS: ductal carcinoma in situ, NST: non-specific tumor (invasive ductal carcinoma), SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection.
Table 8: Factors associated with a POHL >3 days in binary logistic regression
3.7 Operative time and anesthesia time
The median operative time was 92 minutes (mean 115.8). The median duration of anesthesia was 142 minutes (mean 169.1) with a significant longer duration for IBR versus no-IBR (Table 2).
3.8 Adjuvant treatments
AC was administered in 28.6% of mastectomies (603/2112): 35.5% (463/1306) for primary invasive BC without neo-adjuvant chemotherapy (NAC) [25.6% (127/497) and 41.5% (336/809) for mastectomies with and without IBR, respectively] and 17.0% (31/182) of patients for invasive ipsilateral breast local recurrence [14.6% (15/103) and 20.3% (16/79) for mastectomies with and without IBR, respectively]. NAC was administered in 482 patients (22.8%: 482/2112): 21.5% (95/442) for primary invasive BC [15.7% (19/121) and 23.7% (76/321) for mastectomies with and without IBR, respectively] and 24.3% (9/37) of patients for invasive ipsilateral breast local recurrence (2 patients with IBR and 35 without IBR). PMRT was delivered in 38.2% of mastectomies (806/2112): 48.3% (802/1661) for primary invasive BC without previous radiotherapy: 21.7% (120/553) and 61.6% (682/1108) for mastectomies with and without IBR, respectively.
Endocrine therapy was delivered in 76.9% (1166/1517) of mastectomies for primary invasive BC [79.7% (372/467) and 75.6% (794/1050) for mastectomies with and without IBR, respectively] and 70.9% (127/179) of patients with invasive ipsilateral breast local recurrence [76.3% (58/76) and 67% (69/103) for mastectomies with and without IBR, respectively]. Median interval-time between surgery and adjuvant therapy was 46 days: 42 days for AC and 51 days for PMRT (Table 9). Median interval-times between surgery and adjuvant therapy were not significantly different for mastectomies with and without IBR (p=0.536), for mastectomies with and without complications (p=0.057) and significant (50 and 45 days) for mastectomies with and without Grade 2-3 complications (p<0.0001), and for patients 51-74 years old (p=0.048) and >=75 years old (p=0.033). Interval-times were >60-days in 20.5% of patients (184/896) (712 patients <= 60-days): in 16.1% for AC and 26.8% for PMRT (p<0.0001). Results were non-significant according to IBR or no-IBR (p=0.294), according to complications or not (p=0.138). Higher rates of interval-times >60-days were observed for mastectomies with Grade 2-3 complications (p=0.026) and according to age groups (p<0.0001).
|
median |
mean |
95%CI |
t-test: p |
|
|
interval-time before adjuvant therapy |
||||
|
all patients |
46 |
50 |
48.6-51.5 |
|
|
no IBR |
45 |
49.8 |
48.1-51.5 |
0.536 |
|
IBR |
47 |
50.9 |
47.8-54.1 |
|
|
AC |
42 |
46.2 |
44.3-48.0 |
<0.0001 |
|
PMRT |
51 |
55.6 |
53.2-58.0 |
|
|
Complication |
48 |
52.8 |
49.9-55.6 |
0.057 |
|
no complication |
44 |
48.7 |
47.0-50.4 |
|
|
G 2-3 complication |
50 |
57.9 |
52.3-63.5 |
<0.0001 |
|
no G 2-3 complication |
45 |
48.9 |
47.4-50.4 |
|
|
<= 40-years |
41 |
43.6 |
40.6-46.7 |
|
|
41-50 |
44 |
46.6 |
44.0-49.2 |
0.727 |
|
51-74 |
46 |
51.7 |
49.3-54.1 |
0.048 |
|
>= 75-years |
54 |
57.0 |
53.3-60.7 |
0.033 |
|
interval-time >60 days |
Nb |
% |
||
|
all patients (n=896) |
184 |
20.5 |
||
|
AC (n=527) |
85 |
16.1 |
<0.0001 |
|
|
PMRT (n=369) |
99 |
26.8 |
||
|
no IBR (702) |
141 |
20.1 |
0.294 |
|
|
IBR (n=194) |
43 |
22.2 |
||
|
Complication (n=289) |
66 |
22.8 |
0.138 |
|
|
no complication (n=607) |
118 |
19.4 |
||
|
G 2-3 complication (n=110) |
31 |
28.2 |
0.026 |
|
|
no G 2-3 complication (n=786) |
153 |
19.5 |
||
|
<= 40-years (n=130) |
17 |
13.1 |
<0.0001 |
|
|
41-50 years (n=219) |
36 |
16.4 |
||
|
51-74 years (n=422) |
86 |
20.4 |
||
|
>= 75-years (n=125) |
45 |
36.0 |
||
Legend: IBR: immediate breast reconstruction, AC: adjuvant chemotherapy, PMRT: post-mastectomy radiotherapy.
Table 9: Interval time between surgery and adjuvant therapy.
In binary logistic regression, significant factor associated with interval-time >60-days for AC was age >=75-years old (OR=3.718, p=0.008, CI95%=1.42-9.74) in comparison with patients <=40-years old. Others age groups (41-50 and 51-74), grade 2-3 complications and IBR versus no IBR were non-significant. Significant factors associated with interval-time >60-days for PMRT were age >=75-years old (OR=4.146, p=0.002, CI95%=1.66-10.34) in comparison with patients <=40-years old, Grade 2-3 complications (OR=2.059, p=0.040, CI95%=1.03-4.10) in comparison with no-Grade 2-3 complications and IBR (OR=3.000, p=0.002, CI95%=1.49-6.02) versus no-IBR. Others age groups (41-50 and 51-74) were non-significant.
4. Discussion
We reported a large cohort of patients treated by mastectomy in recent years with a high rate of IBR. IBR was significantly associated with several factors in multivariate analysis corresponding to a selection of patients in whom an IBR was perform. Consequently, complication rate was analyze in regression analysis adjusted on confounding factors and there was no significant impact of IBR or no-IBR. High BMI (> 30) was the only independent factor associated with Grade 2-3 complications. A predictive score of complication risk was calculate in order to evaluate for each patient the level of complication rate for all grades and grade 2-3. Performing IBR was not significantly associated with higher rate of interval time >60-days for AC but interval time >60-days rate for PMRT was increased by IBR and complications Grade 2-3.
4.1 IBR
IBR rate from 2016 to 2020 was estimate at 40.5%, which is relatively high and much higher than reported in the literature. It also marks a significant increase compared to 2014. Indeed, Negre et al. [5] carried out an observational study in France using the database of the Information Systems Medicalization Program (PMSI) between 2008 and 2014 making it possible to identify 140,904 women who had undergone a total mastectomy for BC. The IBR rate in France was therefore assess at 16.1%. In England, the number or implant-IBR have increased since 2009: 10.0% until 2005 and 23.3% by 2013-2014 [10]. In Chinese, IBR rate was 9.6% (1,554/16,187) in year 2018, with implant or expander in 76.6% of these IBR [12]. However, the average rate of reconstruction in the United States in 2010 was 45%, surging to 54% in 2015 [37].
There is a great disparity in IBR rates among surgeons in the same center, linked to surgical habits, their training, and the average age of their patient. However, some of the surgeons not performing IBR either referred their patients to other surgeons on the team, or performed the procedure in collaboration with another surgeon on the team. In the UK multicenter prospective cohort study [7], 2108 patients had 2655 mastectomies with implant-IBR in 81 units during 28 months: 11 patients’ per-year per unit in comparison with 144 implant-IBR patients per-year in our unit. We reported a high rate of NSM, 46.9% among patients with IBR. NSM rate was 17.7% (287/1625) for implant reconstructions in the MROC study [6] and 23% (486/2108) in the UK multicenter prospective cohort study [7].
4.2 Complications
There was no significant difference of complication rate between the IBR group and the no-IBR group: 29.9% for the IBR group vs. 33.3% for the no-IBR group. The surgical revision rate was 23.2%. However, we have shown patient’s selection for IBR in comparison with no-IBR in regression analysis. Consequently, factors associated with complications were analyze with adjustment on these confounding factors and IBR was not associated with complications or Grade 2-3 complications. Only BMI >=30 was significantly associated with more Grade 2-3 complications (OR=1.836).
In a prospective multicenter American study, the surgical revision rate was 19.3% (453/2343) [38] and in the NMBRA cohort, which included 3,389 patients with IBR, this rate was 15.8%. [39]. Although the comparison of complication rates between different studies is difficult due to a large disparity of IBR types, reported complications, indications for mastectomies, and monitoring time, we reported a complication rate similar to those reported in other studies [6, 40-45]. However, complications rates reported in recent studies of NSM were lower (5.1% to 20%) with 20.5% average overall complication rate in a recent review of 3716 NSM-IBR only for prophylactic indications [34].
We reported a 6.1% rate of implant loss for 560 implant-based IBR, which is lesser than rates reported by others [6, 40, 41], mainly in relation with infectious and cutaneous complications, but with use of pre-operative antimicrobial therapy for patients with nasal germs and per-operative antimicrobial prophylaxis that is not performed systematically by others. However, implant loss rate was higher for LDF-IBR associated with breast implant, 20.4% in our study, frequently realized for patients with previous ipsilateral radiotherapy.
Different types of complications were observe for patients with IBR in comparison with patients without IBR in our study: a small difference of infection rate (30/855: 3.5%) was observe for IBR in comparison with patients without IBR (38/1257: 3.0%) and higher cutaneous complication rate was observe after IBR (96/855: 11.2%) in comparison with patients without IBR (61/1257: 4.9%). In literature studies, the more frequent complication for mastectomy with implant-IBR was infection, 0% to 17.8%, and obesity was associated with more complications [7, 46] as we reported. In contrast, hematomas and seromas were more frequent for patients without IBR: 90/1257: 7.2% versus 42/855: 4.9% hematomas, 174/1257: 13.8% versus 1/855: 0.1% seromas for no-IBR-group versus IBR-group, respectively. However, hematomas were more severe complications for IBR versus no-IBR, 78.6% (33/42) grade 3 for IBR and 61.1% (55/90) for no-IBR. The most frequent complication that we reported was seromas (29.2%), with 87.4% (152/174) of grade 1 seromas and then, cutaneous complications (26.2%), with 18.5% of grade 3 as extensive necrosis requiring surgical revision. The 3rd most frequent complication is hematoma (22%), of which 67% were grade 3.
We reported an implant loss rate of 7.2%, concordant with literature rates from 1 to 9.9%. Potter et al [7] reported an implant loss rate of 8.75% (182/2081: 95%CI=8-10), infection rate of 25% (522 patients, 95%CI=23-27) and 370 patients (18%, 95%CI=16-20) required return to theatre for complications within 3 months of their initial surgery. Bennet et al. reported a skin infection rate of 9.8% and a reconstruction failure rate of 7.1% [38]. In addition, as our results show, obesity is significantly associated with a high complication risk rate, as reported in a recent study by Srinivasa et al. with an increased high-grade complication rate for implant-IBR: (OR 1.71) and for autologous flap (OR 2.72) in the BMI > 30 group [46]. This significant BMI impact was also reported by Potter et al [7] for implant loss, infection and reoperation. The Simplified Predictive Complication Score is a decision aid to inform patients of complications risk, especially for high scores. It can be use very simply during the preoperative consultation, the main goal being to avoid delaying adjuvant treatments and to more precisely identify patients eligible for IBR, and the risk of failure. Moreover, this Score can help to compare complications rates between several studies, several techniques of IBR and several periods of treatment. For example, implant-IBR with or without meshes, and pre-pectoral or retro-pectoral implant-IBR. Predictive score of complication include several factors known before surgery but also mastectomy weight that is not known before surgery. Breast cup size was not recorded in our database for patients without IBR. However, a strong correlation is well established between mastectomy weight >300 gr and breast cup size >=C. An external validation of this score is necessary with, if possible, optimized accuracy.
4.3 NSM
We reported 46.4% NSM that is particularly high. In the MROC study, the NSM rate was 17.7% (287/1,625) for IBR by implants [6]. The limits to performing NSM were the risk of local recurrence linked to a retro-areolar glandular residue [24] or, conversely, the management of the skin casing (and therefore the risk of nipple areola complex (NACx) necrosis) [47]. Nevertheless, in the literature, there are many data contradicting, since the reported local recurrence rate on NAC is very low and the rate of NAC necrosis was less than 11% [21, 24, 48, 49, 50]. NSM can therefore be proposed safely and after information on the risk of NACx necrosis when it’s indicated. The remaining question, which still generates considerable questioning and requires validating prospective series, is regarding the limits of the therapeutic indication of NSM, particularly tumor-nipple distance of 2 centimeters or 1 centimeter [51].
4.4 Treatment
Regarding radiotherapy, a recent review by Ho et al [52] evaluated the possibility of IBR in combination with radiotherapy without increasing the risk of complications, and without compromising the oncological or esthetic outcome. It seems that PMRT and IBR are compatible, but PMRT may adversely affect patient-reported outcome [53].
4.5 POHL
54.3% of mastectomies had a POHL ≤1 day. The mean POHL was 1.8 days with a median of 1 day for mastectomies without IBR, and 2 days for mastectomies with IBR. Ambulatory or semi-ambulatory hospitalization is tending to increase, since studies have shown that it allows, on the one hand, reducing postoperative complications and, on the other hand, to help socio-professional reintegration. In addition, in a study carried out by the American College of Surgeons [54] including 40,000 patients, 8,365 (20.6%) were operated in ambulatory surgery, 23,252 (57.2%) spent one night in hospital and 8,958 (22.2%) stayed more than one night in hospital. Patients operated in ambulatory had a morbidity of 2.4% vs. 3.9% after 1 night hospitalization vs. 8.8% for prolonged hospitalization (p <0.0001).
This is the largest study on the safety of ambulatory surgery for breast cancer, showing a significantly higher complication rate in hospitalized patients. These data suggest that mastectomy for breast cancer can be perform safely as ambulatory surgery with patients who have been well informed.
4.6 Interval time between mastectomy and adjuvant treatment
Interval time >60-days between mastectomy and AC was not impacted by IBR and complications. However, interval time >60-days between mastectomy and PMRT was significantly impacted by IBR and complications Grade 2-3. Age >=75-years was also significantly associated with interval time >60-days between mastectomy and AC or PMRT. These results suggest that IBR is not a contra-indication in order to start adequately AC. However, O’Connell et al reported that major complications were significantly associated with treatment delays [35].
4.7 Bilateral mastectomies
A study of the American College of Surgeons [55] between 2007 and 2010 compared the morbidity of unilateral (n=3,722) versus bilateral mastectomies (n=497).
The surgical complication rate was significantly higher in the bilateral versus unilateral group: 5.8% versus 2.9% [unadjusted odds ratio (OR) 2.1, 95% confidence interval (CI) 1.3–3.3, p <0.01]. The data observed in our series differ from those observed by the American College of Surgeons. Indeed, we have observed a complication rate similar in the bilateral mastectomies group and the unilateral mastectomies group: 31% vs 32% with p = 0.143.
5. Limitations
The main limitation is the retrospective design of this study but with a prospective data collection.
6. Conclusion
Treatment by mastectomy with or without IBR is a technique with an acceptable overall complication rate. Performing IBR was not significantly associated with complications and higher rate of interval time >60-days for AC but rate of interval time >60-days for PMRT was increased by IBR and complications Grade 2-3. The preoperative complication predictive score can be a tool to orient the therapeutic strategy by informing patients at risks of complications. The aim of this score is also to not delay adjuvant treatments and to compared results with others studies and or with more recent techniques like pre-pectoral implant-IBR with or without use of acellular or synthetic matrices and tumescent or non-tumescent techniques.
Acknowledgements
Not applicable.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
No funding
References
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11 (2010): 927-933.
- Houvenaeghel G, Cohen M, Raro P, et al. Overview of the pathological results and treatment characteristics in the first 1000 patients randomized in the SERC trial: axillary dissection versus no axillary dissection in patients with involved sentinel node. BMC Cancer 18 (2018): 1153.
- Matala CM, McIntosh SA, Purushotham AD. Immediate breast reconstruction after mastectomy for cancer. Br J Surg 87 (2000): 1455-1472.
- Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 150 (2015): 9-16.
- Houvenaeghel G, Lambaudie E, Cohen M, et al. Therapeutic escalation - De-escalation: Data from 15.508 early breast cancer treated with upfront surgery and sentinel lymph node biopsy (SLNB). Breast 34 (2017): 24-33.
- Wilkins EG, Hamill JB, Kim HM, et al. Complications in postmastectomy breast reconstruction: one-year outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) study. Ann Surg 267 (2018): 164-170.
- Potter S, Conroy EJ, Cutress RI, et al. iBRA Steering Group; Breast Reconstruction Research Collaborative. Short-term safety outcomes of mastectomy and immediate implant-based breast reconstruction with and without mesh (iBRA): a multicentre, prospective cohort study. Lancet Oncol 20 (2019): 254-266.
- Nègre G, Balcaen T, Dast S, et al. Breast reconstruction in France, observational study of 140,904 cases of mastectomy for breast cancer. Ann Chir Plast Esthet. févr 65 (2020): 36-43.
- Dauplat J, Kwiatkowski F, Rouanet P, et al. Quality of life after mastectomy with or without immediate breast reconstruction. Br J Surg. août 104 (2017): 1197-11206.
- Jeevan R, Mennie JC, Mohanna PN, et al. National trends and regional variation in immediate breast reconstruction rates. Br J Surg 103 (2016): 1147-1156.
- Mylvaganam S, Conroy E, Williamson PR, et al. Variation in the provision and practice of implant-based breast reconstruction in the UK: results from the iBRA national practice questionnaire. Breast 35 (2017): 182-190.
- Feng Xu1, Chuqi Lei1, Heng Cao1, et al. Chinese Society of Breast Surgery, Multi-center investigation of breast reconstruction after mastectomy from Chinese Society of Breast Surgery: A survey based on 31 tertiary hospitals (CSBrS-004). Chinese Journal of Cancer Research 33 (2021).
- Lai H-W, Toesca A, Sarfati B, et al. Consensus Statement on Robotic Mastectomy—Expert Panel from International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019: Annals of Surgery. janv 1 (2020).
- Lai HW, Chen ST, Mok CW, et al. Robotic versus conventional nipple sparing mastectomy and immediate gel implant breast reconstruction in the management of breast cancer- A case control comparison study with analysis of clinical outcome, medical cost, and patient-reported cosmetic results. J Plast Reconstr Aesthet Surg 73 (2020):1514-1525.
- Houvenaeghel G, Barrou J, Jauffret C, et al. Robotic Versus Conventional Nipple-Sparing Mastectomy with Immediate Breast Reconstruction. Front Oncol 11 (2021): 637049.
- Toesca A, Sangalli C, Maisonneuve P, et al. A Randomized Trial of RoboticMastectomy versus Open Surgery in Women with Breast Cancer or BRCA Mutation. Ann Surg (2021).
- Sewart E, Turner NL, Conroy EJ, et al. Implant Breast Reconstruction Evaluation (iBRA) Steering Group and the Breast Reconstruction Research Collaborative. Patient-reported outcomes of immediate implant-based breast reconstruction with and without biological or synthetic mesh. BJS Open 5 (2021): zraa063.
- Sorkin M, Qi J, Kim HM, et al. Acellular dermal matrix in immediate expander/implant breast reconstruction: a multicenter assessment of risks and benefits. Plast Reconstr Surg 140 (2017): 1091-1100.
- Gschwantler-Kaulich D, Schrenk P, Bjelic-Radisic V, et al. Mesh versus acellular dermal matrix in immediate implant-based breast reconstruction—a prospective randomized trial. Eur J Surg Oncol 42 (2016): 665-671.
- McCarthy C, Lee C, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter blinded randomised controlled trial. Plast Reconstr Surg 130 (2012): 57-66.
- Houvenaeghel G, Cohen M, Dammacco MA, et al. MAPAMc trial group. Prophylactic nipple-sparing mastectomy with immediate breast reconstruction: results of a French prospective trial. Br J Surg 108 (2021): 296-301.
- Simon P, Barrou J, Cohen M, et al. Types of Mastectomies and Immediate Reconstructions for Ipsilateral Breast Local Recurrences. Front Oncol 10 (2020): 567298.
- Quilichini O, Barrou J, Bannier M, et al. Mastectomy with immediate breast reconstruction: Results of a mono-centric 4-years cohort. Ann Med Surg (Lond) 61 (2020): 172-179.
- Wu ZY, Kim HJ, Lee JW, et al. Breast Cancer Recurrence in the Nipple-Areola Complex after Nipple-Sparing Mastectomy with Immediate Breast Reconstruction for Invasive Breast Cancer. JAMA Surg 154 (2019): 1030-1037.
- Wei CH, Scott AM, Price AN, et al. Psychosocial and sexual well-being following nipple-sparing mastectomy and reconstruction. Breast J 22 (2016): 10-17.
- Moyer HR, Ghazi B, Daniel JR, et al. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg 68 (2012): 446-450.
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple–areola complex and autologous reconstruction: an extended follow-up study. Ann Surg 249 (2009): 461-468.
- Agha RA, Al Omran Y, Wellstead G, et al. Systematic review of therapeutic nipple-sparing versus skin-sparing mastectomy. BJS Open 3 (2018): 135-145.
- Weber WP, Haug M, Kurzeder C, et al. Oncoplastic Breast Consortium consensus conference on nipple sparing Mastectomy. Breast Cancer Res Treat 172 (2018): 523-537.
- Manning AT, Wood C, Eaton A, et al Nipple-sparing mastectomy in patients with BRCA1/2 mutations and variants of uncertain significance. Br J Surg 102 (2015): 1354-1359.
- Jakub JW, Peled AW, Gray RJ, et al Oncologic safety of prophylactic nipple-sparing mastectomy in a population with BRCA mutations: a multi-institutional study. JAMA Surg 153 (2018): 123-129.
- Smith BL, Tang R, Rai U, et al. Oncologic safety of nipple-sparing mastectomy in women with breast cancer. J Am Coll Surg 225 (2017): 361-365.
- Li M, Chen K, Liu F, et al. Nipple sparing mastectomy in breast cancer patients and long-term survival outcomes: an analysis of the SEER database. PLoS One 12 (2017): e0183448.
- Muller T, Baratte A, Bruant-Rodier C, et al. Oncological safety of nipple-sparing prophylactic mastectomy: a review of the literature on 3716 cases. Ann Chir Plast Esthet 63 (2018): e6-e13.
- O'Connell RL, Rattay T, Dave RV, et al. iBRA-2 Steering Group; Breast Reconstruction Research Collaborative. The impact of immediate breast reconstruction on the time to delivery of adjuvant therapy: the iBRA-2 study. Br J Cancer 120 (2019): 883-895.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann Surg [Internet]. août 2009 [cité 23 mars 2021] 250(2009): 187-1
- Panchal H, Matros E. Current trends in postmastectomy breast reconstruction. Plast Reconstr Surg 140 (2017): 7S-13S
- Bennett KG, Qi J, Kim HM, et al. Comparison of 2-Year Complication Rates among Common Techniques for Postmastectomy Breast Reconstruction. JAMA Surg 153 (2018): 901-90
- National mastectomy and breast reconstruction audit – third annual report (2010).
- Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, et al. Complications analysis of 266 immediate breast reconstructions. J Plast Reconstr Aesthet Surg 59 (2006): 1017-10
- Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. juin 109 (2002): 2265-22
- Berry T, Brooks S, Sydow N, et al. Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol 17 (2010): 202-2
- Contant CM, van Geel AN, van der Holt B, et al. Morbidity of immediate breast reconstruction (IBR) after mastectomy by subpectorally placed silicone prosthesis: the adverse effect of radiotherapy. Eur J Surg Oncol. juin 26 (2000): 344-3
- Ducic I, Spear SL, Cuoco F, et al. Safety and risk factors for breast reconstruction with pedicled transverse rectus abdominis musculocutaneous flaps: a 10-year analysis. Ann Plast Surg. déc 55 (2005): 559-5
- Headon HL, Kasem A, Mokbel K. The Oncological Safety of Nipple-Sparing Mastectomy: A Systematic Review of the Literature with a Pooled Analysis of 12,358 Procedures. Arch Plast Surg. juill 43 (2016): 328-3
- Srinivasa DR, Clemens MW, Qi J, et al. Obesity and Breast Reconstruction: Complications and Patient-Reported Outcomes in a Multicenter, Prospective Study. Plast Reconstr Surg 145 (2020): 481e-4
- Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 345 (2001):159-1
- Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years’ experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat [Internet]. janv 2009 [cité 14 mars 2021] 117(2021): 333-33
- Lago V, Maisto V, Gimenez-Climent J, et al. Nipple-sparing mastectomy as treatment for patients with ductal carcinoma in situ: A 10-year follow-up study. Breast J 24 (2018): 298-
- Metere A, Fabiani E, Lonardo MT, et al. Nipple-Sparing Mastectomy Long-Term Outcomes: Early and Late Complications. Medicina (Kaunas) 56 (2020): 166.
- Moo TA, Saccarelli CR, Sutton EJ, et al. Tumor-Nipple Distance of ≥ 1 cm Predicts Negative Nipple Pathology After Neoadjuvant Chemotherapy. Ann Surg Oncol 28 (2021): 6024-6029.
- Ho AY, Hu ZI, Mehrara BJ, et al. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol [Internet]. 1 déc 2017 [cité 10 mars 2021] 18(2021):e742-e7
- Sewart E, Turner NL, Conroy EJ, et al. iBRA Steering Group and the Breast Reconstruction Research Collaborative. The Impact of Radiotherapy on Patient-reported Outcomes of Immediate Implant-based Breast Reconstruction With and Without Mesh. Ann Surg (2020).
- Same-Day Major Breast Cancer Surgery is Safe: An Analysis of Short-Term Outcomes Using NSQIP Data | SpringerLink [Internet] (2021).
- Osman F, Saleh F, Jackson TD, et al. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: an analysis of the NSQIP database. Ann Surg Oncol 20 (2013): 3212-321
