Our Interstitial Lung Disease Experiences
Article Information
Suphi Aydin1*, Aydin Balci2
1Departmen of Chest Surgery, Afyonkarahisar Health Sciences University, Afyonkarahisar, Turkey
2Departmen of Pulmonology, Afyonkarahisar Health Sciences University, Afyonkarahisar, Turkey
*Corresponding Author: Suphi Aydin, Departmen of Chest Surgery, Afyonkarahisar Health Sciences University, Afyonkarahisar, Turkey
Received: 12 June 2020; Accepted: 22 June 2020; Published: 03 July 2020
Citation: Suphi Aydin, Aydin Balci. Our Interstitial Lung Disease Experiences. Journal of Surgery and Research 3 (2020): 186-197.
View / Download Pdf Share at FacebookAbstract
Objective: Interstitial lung diseases is the name given to the acute or chronic disease group that affects the lung parenchyma as diffuse, causing inflammation, fibrosis and structural disorders in the parenchyma. Diseases in this group show common characteristics clinically, radiologically, pathologically and functionally. It is a heterogeneous group of diseases whose etiology is not fully clarified. Difficulties are experienced in its diagnosis, treatment and follow-up. In this study, we aimed to evaluate 240 patients diagnosed with interstitial lung diseases retrospectively in terms of diagnosis and treatment.
Material and methods: In this study, patients diagnosed with interstitial lung diseases in our clinic between January 2017 and January 2020, age, gender, smoking, diagnostic methods, operation type, radiological appearance, histopathological diagnosis types, immune marker positivity, pulmonary function tests and In terms of treatment methods applied, it was retrospectively analyzed.
Results: 109 (45.4%) of the patients were women, 131 (54.6%) were male and the mean age was 65 ± 14.3 (18-95). 77 (32.1%) patients were diagnosed by performing transbronchial needle aspiration biopsy with flexible fiberoptic bronchoscopy, transbronchial needle aspiration biopsy was performed in 6 (2.5%) patients by endobronchial ultrasonography and diagnosis was made, 8 (3.3%) patients were diagnosed by mediastinoscopy biopsy, 4 (1.6%) patients by scalene lymph node biopsy, and 10 (4.2%) patients by video assisted thoracoscopic surgery biopsy. Out of 240 patients, 105 (43.75%) pathological definitive diagnosis was reached. The diagnoses were idiopathic pulmonary fibrosis (IPF), sarcoidosis, collogen tissue diseases, cryptogenic organized pneumonia, respectively. The diagnosis of 135 (56.25%) patients who could not be diagnosed pathologically was made with clinica
Keywords
Interstitial lung disease; Idiopathic pulmonary fibrosis; Sarcoidosis; Biopsy
Interstitial lung disease articles, Idiopathic pulmonary fibrosis articles, Sarcoidosis articles, Biopsy articles
Interstitial lung disease articles Interstitial lung disease Research articles Interstitial lung disease review articles Interstitial lung disease PubMed articles Interstitial lung disease PubMed Central articles Interstitial lung disease 2023 articles Interstitial lung disease 2024 articles Interstitial lung disease Scopus articles Interstitial lung disease impact factor journals Interstitial lung disease Scopus journals Interstitial lung disease PubMed journals Interstitial lung disease medical journals Interstitial lung disease free journals Interstitial lung disease best journals Interstitial lung disease top journals Interstitial lung disease free medical journals Interstitial lung disease famous journals Interstitial lung disease Google Scholar indexed journals Idiopathic pulmonary fibrosis articles Idiopathic pulmonary fibrosis Research articles Idiopathic pulmonary fibrosis review articles Idiopathic pulmonary fibrosis PubMed articles Idiopathic pulmonary fibrosis PubMed Central articles Idiopathic pulmonary fibrosis 2023 articles Idiopathic pulmonary fibrosis 2024 articles Idiopathic pulmonary fibrosis Scopus articles Idiopathic pulmonary fibrosis impact factor journals Idiopathic pulmonary fibrosis Scopus journals Idiopathic pulmonary fibrosis PubMed journals Idiopathic pulmonary fibrosis medical journals Idiopathic pulmonary fibrosis free journals Idiopathic pulmonary fibrosis best journals Idiopathic pulmonary fibrosis top journals Idiopathic pulmonary fibrosis free medical journals Idiopathic pulmonary fibrosis famous journals Idiopathic pulmonary fibrosis Google Scholar indexed journals Sarcoidosis articles Sarcoidosis Research articles Sarcoidosis review articles Sarcoidosis PubMed articles Sarcoidosis PubMed Central articles Sarcoidosis 2023 articles Sarcoidosis 2024 articles Sarcoidosis Scopus articles Sarcoidosis impact factor journals Sarcoidosis Scopus journals Sarcoidosis PubMed journals Sarcoidosis medical journals Sarcoidosis free journals Sarcoidosis best journals Sarcoidosis top journals Sarcoidosis free medical journals Sarcoidosis famous journals Sarcoidosis Google Scholar indexed journals Biopsy articles Biopsy Research articles Biopsy review articles Biopsy PubMed articles Biopsy PubMed Central articles Biopsy 2023 articles Biopsy 2024 articles Biopsy Scopus articles Biopsy impact factor journals Biopsy Scopus journals Biopsy PubMed journals Biopsy medical journals Biopsy free journals Biopsy best journals Biopsy top journals Biopsy free medical journals Biopsy famous journals Biopsy Google Scholar indexed journals septal interstitium articles septal interstitium Research articles septal interstitium review articles septal interstitium PubMed articles septal interstitium PubMed Central articles septal interstitium 2023 articles septal interstitium 2024 articles septal interstitium Scopus articles septal interstitium impact factor journals septal interstitium Scopus journals septal interstitium PubMed journals septal interstitium medical journals septal interstitium free journals septal interstitium best journals septal interstitium top journals septal interstitium free medical journals septal interstitium famous journals septal interstitium Google Scholar indexed journals reticulonodular pattern articles reticulonodular pattern Research articles reticulonodular pattern review articles reticulonodular pattern PubMed articles reticulonodular pattern PubMed Central articles reticulonodular pattern 2023 articles reticulonodular pattern 2024 articles reticulonodular pattern Scopus articles reticulonodular pattern impact factor journals reticulonodular pattern Scopus journals reticulonodular pattern PubMed journals reticulonodular pattern medical journals reticulonodular pattern free journals reticulonodular pattern best journals reticulonodular pattern top journals reticulonodular pattern free medical journals reticulonodular pattern famous journals reticulonodular pattern Google Scholar indexed journals lymphocytic interstitial pneumonia articles lymphocytic interstitial pneumonia Research articles lymphocytic interstitial pneumonia review articles lymphocytic interstitial pneumonia PubMed articles lymphocytic interstitial pneumonia PubMed Central articles lymphocytic interstitial pneumonia 2023 articles lymphocytic interstitial pneumonia 2024 articles lymphocytic interstitial pneumonia Scopus articles lymphocytic interstitial pneumonia impact factor journals lymphocytic interstitial pneumonia Scopus journals lymphocytic interstitial pneumonia PubMed journals lymphocytic interstitial pneumonia medical journals lymphocytic interstitial pneumonia free journals lymphocytic interstitial pneumonia best journals lymphocytic interstitial pneumonia top journals lymphocytic interstitial pneumonia free medical journals lymphocytic interstitial pneumonia famous journals lymphocytic interstitial pneumonia Google Scholar indexed journals Lung parenchyma biopsy articles Lung parenchyma biopsy Research articles Lung parenchyma biopsy review articles Lung parenchyma biopsy PubMed articles Lung parenchyma biopsy PubMed Central articles Lung parenchyma biopsy 2023 articles Lung parenchyma biopsy 2024 articles Lung parenchyma biopsy Scopus articles Lung parenchyma biopsy impact factor journals Lung parenchyma biopsy Scopus journals Lung parenchyma biopsy PubMed journals Lung parenchyma biopsy medical journals Lung parenchyma biopsy free journals Lung parenchyma biopsy best journals Lung parenchyma biopsy top journals Lung parenchyma biopsy free medical journals Lung parenchyma biopsy famous journals Lung parenchyma biopsy Google Scholar indexed journals lung biopsy articles lung biopsy Research articles lung biopsy review articles lung biopsy PubMed articles lung biopsy PubMed Central articles lung biopsy 2023 articles lung biopsy 2024 articles lung biopsy Scopus articles lung biopsy impact factor journals lung biopsy Scopus journals lung biopsy PubMed journals lung biopsy medical journals lung biopsy free journals lung biopsy best journals lung biopsy top journals lung biopsy free medical journals lung biopsy famous journals lung biopsy Google Scholar indexed journals
Article Details
1. Introduction
The terms interstitial lung disease (ILD) and diffuse parenchymal lung disease are large and heterogeneous diseases that include inflammation and fibrosis of the alveoli, distal respiratory tract, and septal interstitium of the lungs [1]. It affects the gas exchange capacity of the lung either reversibly or irreversibly [2]. It contains more than 150 diseases and most of them are unknown etiology, only 25-30% of them are known. Patients die due to respiratory failure due to pulmonary fibrosis [3]. This disease group shows common clinical, radiological and physiological features. Patients complain of progressive effort dyspnea and dry cough due to pathological changes in the lung parenchyma. Collagen tissue diseases (CTD) increase with the incidence of ILD group such as cryptogenic organized pneumonia (COP), usual interstitial pneumonia (UIP), diffuse alveolar damage (DAD), nonspecific interstitial pneumonia (NSIP) and lymphocytic interstitial pneumonia (LIP) [4]. The monitoring of peripheral cystic, reticular, nodular and linear densities on chest radiographs are findings in favor of ILD and are called "reticulonodular pattern". However, these findings are not specific for ILD [5]. In these patients, a detailed history, physical examination and radiological examination should be performed first.
However, these findings are not specific for ILD [5]. Firstly, in these patients, a detailed history, physical examination and radiological examination should be performed. As a result of these evaluations, diagnostic laboratory tests, pulmonary function tests (PFT) values, advanced radiological examination, bronchoalveolar lavage and invasive procedures for tissue diagnosis should be planned [6]. Since the etiology of ILD is not clearly known, it is very difficult to diagnose in most of these diseases. Lung parenchyma biopsy is recommended for histopathological examination in order to be able to diagnose ILD. Biopsy should be done in a way to include intact tissue [7]. However, it is not always possible to make a diagnosis with these diagnostic methods even in the most developed centers [8]. In one of the largest series published, it was reported that 502 (40%) of 1234 patients could be diagnosed with open lung biopsy [9]. In this disease group, which is difficult to diagnose, treat and follow-up, a multi-disciplinary approach is required, including chest diseases, thoracic surgery, radiology and pathology specialists.
2. Material and Methods
240 patients diagnosed with ILD in Afyonkarahisar Health Sciences University Chest Diseases Department between January 2017 and January 2020 were included retrospectively. Patients were evaluated in terms of age, gender, smoking, application complaints, radiological imaging methods, PFT, flexible fiberoptic bronchoscopy (FOB) and endobronchial ultrasonography (EBUS) results, histopathological diagnosis, diagnostic methods, immune markers, treatments given. Eye consultation was requested from patients with suspected sarcoidosis. PFT was applied to all patients. As a result of the measurements, obstructive disorder in the first second if the forced expiration volume / forced vital capacity (FEV1 / FVC) is below 70%, while FEV1 / FVC ratio was normal or high, FVC was considered as restrictive type respiratory dysfunction below 80% [10].
As a result of posteroanterior chest radiographs requested from the patients, high resolution computed tomography (HRCT) was requested from the patients in order to better evaluate the parenchyma and mediastinum. In order to make histopathological diagnosis, FOB was performed to patients whose clinical status was appropriate and EBUS according to necessity and transbronchial biopsies were taken during the procedures. Video assisted thoracoscopic surgery (VATS) was applied to patients who could not be diagnosed with these procedures and lung biopsy was taken. If necessary, the lymph nodes in the mediastinum are performed by mediastinoscopy. Lymph nodes in the scalene region were evaluated by scalene lymph node biopsies. In patients who cannot receive biopsy or are taken inadequate, lesions with radiologically compatible noncazified granulomas, multi-organ involvement, other clinical, histological and radiological lesions with similar characteristics were excluded and the diagnosis of sarcoidosis was made. Some of the patients with the diagnosis of CTD were previously diagnosed, and some were made by our clinic. All of them had lung involvement in their radiological examinations. All patients included in our study were evaluated in terms of diagnostic interventions, radiological examinations, biopsy methods, and histopathological diagnoses. The patients were then grouped according to their diagnosis and examined. Clinical and radiological findings, PFT findings were evaluated.
3. Results
240 patients, aged between 18-95, were included in the study. The mean age of the patients was 65 ± 14.3. 109 of the patients were female and 131 were male. Considering the age distribution of the patients, it is seen that they peaked in the 6th and 7th Decades. When the patients with definitive diagnosis were evaluated, sarcoidosis (20.2%) was the most common in women and idiopathic pulmonary fibrosis (IPF) (35.1%) was most common in men (Table 1). In general, IPF was the most common in 66 (27.5%) patients, sarcoidosis in 36 (15%) patients and CTD in 23 (9.6%) patients (Table 2). 240 patients were included in the study. In 227 (94.6%), the most common complaint was dyspnea. Dyspnea was present in 93.6% (102 patients) of female patients and 95.4% (125 patients) of male patients. This was followed by a complaint of cough with 159 (66.3%) patients. There were 77 (70.6%) female patients and 82 (62.6%) male patients complained of cough. Of 57 (23.8%) patients with sputum complaints, 23 (21.1%) were female and 34 (26%) were male, and hemoptysis was observed in 7 (2.9%) of patients and only in male patients (5.3%). Hemoptysis was not observed in female patients. Chest pain was detected in a total of 5 (2.1%) patients, 2 (1.8%) female patients, and 3 (2.3%) male patients. PFT values of the patients were compared by sex (Table 3). PFT values of female patients were within normal limits in 58 (53.2%), while 45 (34.4%) of male patients were within normal limits. Mixt type respiratory dysfunction was the most common in both genders. In 35 (32.1%) of the female patients, 53 (40.5%) of the male patients had mixed-type pulmonary dysfunction. In 42 (38.5%) of female patients, in 46 (35.1%) of male patients and overall, 88 (36.6%) patients had smoking history. 135 patients were diagnosed by clinical and radiological methods, 77 patients were diagnosed with bronchoscopy, 10 patients with VATS, 8 patients with mediastinoscopy, 6 patients with EBUS and 4 patients with scalene lymph node biopsy.
Chest radiography was requested from all patients. Then, HRCT was requested for detailed evaluation of the lung parenchyma. In HRCT examinations, 69 (63.3%) of the female patients had ground glass density (Figure 1A, 1B), 54 (49.5%) interstitial thickening, 15 (13.8%) honeycomb appearance (Figure 2A, 2B), mediastinal lymphadenopathy (LAP) in 30 (27.5%), parenchymal or subpleural nodules in 11 (10.1%), bronchiectasis changes in 7 (6.4%), patched infiltrations were observed in 8 (7.3%). In HRCT examinations of male patients, ground glass density in 79 (60.3%), interstitial thickening in 77 (58.8%), honeycomb appearance in 52 (39.7%), mediastinal LAP in 23 (17.6%), parenchymal or subpleural nodules were observed in 13 (9.9%), bronchiectasis changes in 9 (6.9%), and patched infiltrations in 17 (13%). In HRCT examinations, widespread involvement in the lungs was observed in female patients, whereas in men, lung basal segments were more affected (Table 4). In our immune marker examinations, positive results were obtained in 24 (10%) patients. It was positive in 14 (12.8%) of female patients and in 10 (7.6%) of male patients. Anti Scl positivity in 4 of the female patients, RF positivity in 4, P-ANCA positivity in 2, Anti Ds DNA positivity in 1, ANA positivity in 3, P-ANCA positivity in 2 of male patients, C-ANCA was found in 1, Anti SSA positivity in 1 and RF positivity in 6. 36 patients were diagnosed with sarcoidosis. 22 (61.1%) of the patients were female and 14 (38.9%) were male. The average age was 51.08 ± 16.904 (18-84). The average age of women (49.91 ± 13.68) was similar to men (52.93 ± 21.447) (p> 0.05). There was a smoking history in 23 (63.9%) of the patients. All patients had chest pain. Other detected symptoms were dyspnea in 34 (94.4%) patients, cough in 31 (86.1%) patients, sputum in 6 (16.7%) patients and hemoptysis in 1 (2.8%) patient. The most frequent physical examination was erythema nodosum, which was observed in 6 (16.7) patients. When staging according to chest radiographs, 1 patient was 0 stage, 18 patients were 1 stage, 13 patients were stage 2, 4 patients were stage 4 (Figure 3A, 3B). With bronchoscopic transbronchial biopsy, 20 (55.5%) patients, 8 (22.2%) patients with mediastinoscopy 4 (11.1%) patients were diagnosed with scalene lymph node biopsy and 4 (11.1%) patients were diagnosed with clinical and radiological findings.
With bronchoscopic transbronchial biopsy, 20 (55.5%) patients, 8 (22.2%) patients with mediastinoscopy 4 (11.1%) patients were diagnosed with scalene lymph node biopsy and 4 (11.1%) patients were diagnosed with clinical and radiological findings. Steroid treatment was began in 13 (36.1%) of patients diagnosed with sarcoidosis, and 23 (63.9%) were not treated. Six of the untreated patients were stage 0, 12 were stage 1 and 8 were stage 2 patients. PFT was studied in all patients. Mixt type respiratory dysfunction was detected in 5 (13.9%) patients. 20 (30.3%) of 66 patients diagnosed with IPF were women, 46 (66.7%) were male. Average ages were 69.53 ± 8.77 (47-86). 40 (60.6%) of the patients had a smoking history, 26 (39.4%) had no smoking history. In the chest radiographs of IPF patients, reticular appearance was most common, and this appearance was detected in 46 patients. 40 (60.6%) of the patients had honeycomb appearance in HRCT. Application complaints were shortness of breath in 64 (97%) patients, cough in 42 (63.6%) patients, sputum complaint in 15 (22.7%) patients, in 1 (1.5%) patient with hemoptysis, chest pain was 4 (6.1%). PFT was applied to all IPF patients. The test of 21 patients (31.8%) was detected within normal limits. Mixt type was observed in 29 (43.9%) patients, restrictive type in 14 (21.2%) patients, and obstructive pulmonary dysfunction in 2 (3%) patients. In IPF patients, 29 (43.9%) patients were diagnosed with transbronchial lung parenchyma biopsy by bronchoscopy. The patient, who was thought to have 10 IPF, was diagnosed with a VATS biopsy. Steroid therapy was initiated in 17 (25.8%) patients with IPF, pirfenidone therapy was initiated in 18 (27.3%) patients and was followed in 31 (47%) patients. When all patients who were followed up for ILD are evaluated; Steroid treatment was initiated in 70 (29.2%) patients and pirfenidone treatment in 20 (8.3%) patients, while 150 (62.5) patients were followed up. Pirfenidone treatment was started for patients who were diagnosed with IPF in ILD and who met the criteria of pirfenidone treatment of the ministry of health. Steroid therapy was initiated in IPF patients with severe clinical history, patients with stage 2 and above sarcoidosis, and in patients who were followed up with increased symptoms and radiological images. Patients with nonspecific complaints and no dyspnea and cough complaints were followed up.
|
Sex |
Diagnosis |
Number of patients |
||
|
(n) |
% |
|||
|
Woman |
Not Classified |
50 |
45,9 |
|
|
Idiopathic Pulmonary Fibrosis |
20 |
18,3 |
||
|
Cryptogenized Organized Pneumonia |
2 |
1,8 |
||
|
Collagen Tissue Disease |
14 |
12,8 |
||
|
Sarcoidosis |
22 |
20,2 |
||
|
Lenfanjiyoleiyomyomatozis |
1 |
0,9 |
||
|
Total |
109 |
100,0 |
||
|
Men |
Not Classified |
44 |
33,6 |
|
|
Idiopathic Pulmonary Fibrosis |
46 |
35,1 |
||
|
Cryptogenized Organized Pneumonia |
9 |
6,9 |
||
|
Collagen Tissue Disease |
9 |
6,9 |
||
|
Sarcoidosis |
14 |
10,7 |
||
|
Non Specific Interstitial Pneumonia |
1 |
0,8 |
||
|
Lymphocytic Interstitial Pneumonia |
1 |
0,8 |
||
|
Occupational Lung Diseases |
5 |
3,8 |
||
|
Lenfanjiyoleiyomyomatozis |
1 |
0,8 |
||
|
Chronic Eosinophilic Pneumonia |
1 |
0,8 |
||
|
Total |
131 |
100,0 |
||
Table 1: Distribution of diagnoses by gender.
|
Diagnosis |
Number of patients |
|
|
(n) |
% |
|
|
Not Classified |
94 |
39,2 |
|
Idiopathic Pulmonary Fibrosis |
66 |
27,5 |
|
Cryptogenized Organized Pneumonia |
11 |
4,6 |
|
Collagen Tissue Disease |
23 |
9,6 |
|
Sarcoidosis |
36 |
15,0 |
|
Non Specific Interstitial Pneumonia |
1 |
0,4 |
|
Lymphocytic Interstitial Pneumonia |
1 |
0,4 |
|
Occupational Lung Diseases |
5 |
2,1 |
|
Lenfanjiyoleiyomyomatozis |
2 |
0,8 |
|
Chronic Eosinophilic Pneumonia |
1 |
0,4 |
|
Total |
240 |
100,0 |
Table 2: Distribution of general diagnoses.
|
Sex |
Diagnosis |
Number of Patients |
||
|
(n) |
% |
|||
|
Woman |
Obstructive |
2 |
1,8 |
|
|
Restrictive |
14 |
12,8 |
||
|
Mixt |
35 |
32,1 |
||
|
Normal |
58 |
53,2 |
||
|
Total |
109 |
100,0 |
||
|
Male |
Obstructive |
5 |
3,8 |
|
|
Restrictive |
28 |
21,4 |
||
|
Mixt |
53 |
40,5 |
||
|
Normal |
45 |
34,4 |
||
|
Total |
131 |
100,0 |
||
Table 3: PFT results of patients.
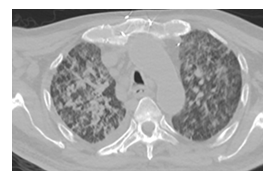
Figure 1A: Areas showing increased density in ground-glass appearance in both lungs.
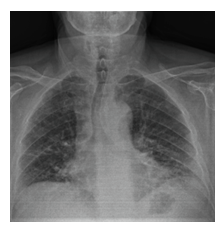
Figure 1B: Subpleural reticular density increments and the appearance of ground-glass areas on posteroanterior chest x-ray.
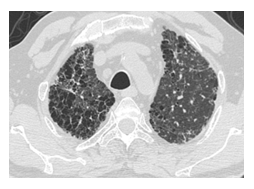
Figure 2A: Common honeycomb image in both lungs.
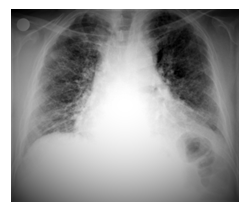
Figure 2B: Image of honeycomb in posteroanterior chest x-ray.
|
Sex |
Number of Patients |
||
|
(n) |
% |
||
|
Woman |
No |
35 |
32,1 |
|
Increased Involvement in Basal |
22 |
20,2 |
|
|
Common Involvement |
48 |
44,0 |
|
|
upper lobes |
3 |
2,8 |
|
|
Peripheral |
1 |
0,9 |
|
|
Total |
109 |
100,0 |
|
|
Male |
No |
15 |
11,5 |
|
Increased Involvement in Basal |
56 |
42,7 |
|
|
Common Involvement |
55 |
42,0 |
|
|
upper lobes |
5 |
3,8 |
|
|
Total |
131 |
100,0 |
|
Table 4: HRCT findings.
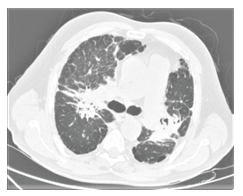
Figure 3A: Computed tomography image of stage 4 sarcoidosis with tubular bronchiectasis that causes hesitation in the parenchymal structures, increased subpleural reticular density, subpleural paraseptal emphysema.
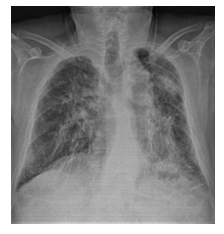
Figure 3B: Posteroanterior chest x-ray image of stage 4 sarcoidosis.
4. Discussion
IPF and sarcoidosis are the most common ILD [11]. In our study, the majority of ILD cases, which could be diagnosed, were IPF with 66 (27.5%) patients and sarcoidosis with 36 (15%) patients. When analyzed by sex, female patients had 22 (20.2%) patients with sarcoidosis in the first rank and second (20.3%) patients with IPF. In male patients, IPF was the first in 46 (35.1%) patients and sarcoidosis was the second in 14 (10.7%) patients. CTD with 23 (9.6%) patients was in the third place in both sexes. CTD was detected in 14 (12.8%) female patients and 9 (6.9%) male patients. In addition, COP was found in 9 (6.9%) male patients. IPF was detected in 35% of cases with ILD in the literature [8]. In our study, the rate of patients with IPF was consistent with the literatüre. The average age of our patients was 65 ± 14.3. When looking at the distribution of age, it was observed that the 6th and 7th decades peaked. The reason for this is that most of the interstitial lung patients that cannot be classified are seen in the 6th decade and most of the patients with IPF are in the 7th decade. The findings we found in our study were consistent with the literature. Sarcoidosis is seen mostly in middle ages, IPF is more common in advanced ages [12]. Smoking has a place in the development of ILD. In the study of De Cremoux, 66% of patients with IPF have a smoking history [13]. There was a smoking history in 152 (63.3%) of our patients diagnosed with ILD. Smoking was available in 67 (61.5%) of female patients and 85 (64.9%) of male patients. Smoking was available in 61 (64.9%) of our patients with IPF and in 23 (63.9%) of our patients with sarcoidosis. In the radiological stages of our patients with sarcoidosis, condensation was detected in 18 patients with stage 1 and 13 patients with stage 2 and was consistent with the literature [14]. Çilekar et al. They examined 402 patients with ILD and performed respiratory function tests in 386 (96%) of the patients. They achieved pulmonary function test results in 3.5% of patients in obstructive type, 17.9% in restrictive type, 29.9% in mixed type, and 44.8% in normal limits [15]. In our study, mixt type respiratory dysfunction was observed most frequently in both sexes. There were mixed respiratory dysfunction in 35 (32.1%) of female patients and 53 (40.5%) of male patients.
In recent years, there is the idea that treatment and evaluation can be performed for lung diseases with specific radiological images without biopsy. It has been reported clinically and radiologically with HRCT that sarcoidosis, silicosis, hypersensitivity pneumonia, lymphangitis carcinomatosis, eosinophilic granuloma, asbestosis, and constructive bronchiolitis, lymphangioleiomyomatosis and scleroderma can be diagnosed [16]. In a study by Swenson et al., They showed that 93% of 85 patients can be diagnosed correctly with HRCT [17]. It has been reported that in patients with sarcoidosis, transbronchial lung biopsy can be diagnosed at a rate of 50% even if radiological parenchymal involvement is not observed [18]. In our study, 58.3% of patients with sarcoidosis were diagnosed with transbronchial biopsy. Symptoms and signs that require treatment in sarcoidosis are still controversial. In our study, steroid treatment was initiated in 13 (36.1%) of 36 patients diagnosed with sarcoidosis. 23 (63.9%) patients were followed up. When studies in the literature are examined, 60-70% of patients with sarcoidosis improve without treatment [19]. In our study, the rate of patients we followed without treatment was compatible with the literature. In our study, the majority of patients with IPF were male patients, in line with the literature. The ratio of male patients to female patients was 2.3 [20]. In the literature, more than 70% of patients with IPF have a smoking history [21]. The smoking rate of IPF patients in our study was 64.9% and was slightly lower than the literature. In our study, 29 (43.9%) of IPF patients were diagnosed with transbronchial lung biopsy and 10 patients were diagnosed with VATS biopsy. The diagnosis of other patients was made with clinical and radiological findings. In the 588 disease studies of Johnston et al., They performed transbronchial lung biopsy 28% and open lung biopsy 12.4% [22]. In another study, Ertürk et al. reported that 20% of 15 IPF patients were diagnosed with open lung biopsy and 13% with transbronchial lung biopsy [14].
Consequently, open lung biopsy rate is low in IPF patients. The reason for this may be the difficulty of tolerating the operation due to the impaired lung parenchymal structure. Our study is compatible with the literature. Prognosis is generally poor in IPF patients. Even in studies involving many patients, the average 5-year survival is around 50% [22].
In the literatures including many published series, no difference was observed between the treated and untreated patients in terms of survival [23]. Pirfenidone treatment was started for our patients who were diagnosed with IPF and complied with the pirfenidone treatment criteria of the Ministry of Health. In addition to the patients with bad clinics, steroid treatment was started. Considering the overall of our study, 147 (61.2%) of 240 patients were diagnosed by clinical and radiological methods, 77 (32%) patients were bronchoscopic biopsy, 6 (2.5%) patients were biopsies accompanied by EBUS, and 10 (4.1%) patients were biopsied by VATS. The reason for the high rate of our clinical and radiological diagnosis is due to the high number of IPF patients in our study. The reason for the low rate of mediastinoscopy and scalene lymph node biopsies in our clinic is that the diagnosis rates are higher in biopsy procedures with less invasive methods, bronchoscopy and EBUS. In some studies, it is advocated that 88% of IPF can be diagnosed with clinical findings and HRCT findings [24]. As a result; ILD is a common disease group and as determined in our study, sarcoidosis and IPF are the most common. No specific diagnosis can be made in a significant number of patients. In our study, this rate is 39.2%. Since these patients have common clinical, radiological and physiological features, it is difficult to make a specific diagnosis. Sometimes, even an open lung biopsy may not be sufficient to make a diagnosis. Therefore, clinical, radiological and physiological data of patients should be carefully examined. With the development of radiological diagnostic methods, mediastinoscopy, bronchoscopy, EBUS and thoracoscopic surgery methods, specific diagnosis rates increase in this disease. However, it may not always be possible to make a diagnosis even with open surgery. Transbronchial lung biopsy should be applied to patients who cannot be diagnosed clinically and radiologically. The treatment to be given varies according to the diseases. Treatment response is low in IPF patients. In some patients, it can be seen in serious complications related to treatment. Treatment should be planned considering possible complications.
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- DuBois TM, Costabel U. Diffuse lung disease: classification and diagnostic approach. In: Gibson GJ, Geddes DM, Costabel U, et al. Respiratory medicine. London: Elsevier Science (2003): 1545-1556.
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188 (2013): 733-748.
- Xaubet A, Ancochea J, Morell F, et al. Report on the incidence of interstitial lung diseases in Sarcoidosis, vasculitis, and diffuse lung diseases; official journal of WASOG / World Association of Sarcoidosis and Other Granulomatous Disorders 21 (2004): 64-70.
- Capobianco J, Grimberg A, Thompson BM, et al. Thoracic Manifestations of Collagen Vascular Diseases. RadioGraphics 32 (2012): 33-50.
- Basaran Demirkaz?k F. Interstisyel Akciger Hastal?klar?n?n Tan? ve Takibinde Radyolojinin Rolü. Güncel Gögüs Hastal?klar? Serisi 2 (2014): 291-312.
- Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med 150 (1994): 1423-1428.
- Aydogdu K, F?nd?k G, Kaya S, et al. Interstisyel Akciger Hastal?klar?n?n Tan?s? Için Akciger Biyopsisi Almada Videotorakoskopi ve Torakotominin Kars?last?r?lmas?. Turk Toraks Derg 14 (2013): 59-63.
- Raghu G. Interstitial lung disease: A clinical overview and general approach. In: Fishman AP ed. Fishman's Pulmonary Diseases and Disorders. Third edition. New York: McGraw-Hill (1998): 1037-1057.
- Gaensler EA, Carrington CB. Open biopsy for chronic diffuse infiltrative lung diseases: clinical, roentgenographic, and physiological correlations in 502 patients. Ann Thorac Surg 30 (1980): 411-427.
- Global Obstructive Lung Disease Initiative, updated (2006).
- Coultas DB, Zumwalt RE, Black WC, et al. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 150 (1994): 967-972.
- Arbak P, Karacan Ö, Idilman R ve ark. Idiopatik pulmoner fibrozis (12 olgu nedeni ile). Tuberk Toraks 48 (2000): 57-62.
- De Cremoux H, Bernaudin JF, Laurent P, et al. Interactions between cigarette smoking and natural history of idiopathic pulmonary fibrosis. Chest 98 (1990): 71-76.
- sengül B, Uzun O, F?nd?k S, et al. Klinigimizde interstisyel akciger hastal?g? tan?s? alan 92 hastan?n incelenmesi. Tüberküloz ve Toraks Dergisi 57 (2009): 314-326.
- Çilekar s, Dumanl? A, Gürhan Öz G, et al. Klinigimizde Son Bes Y?ll?k Interstisyel Akciger Hastal?g? Tan? ve Tedavisi, Bozok Med J10 (2020): 1-5.
- Franquet T, Gimenes A, Monill JM, et al. Primary Sjögren's Syndrom and associated lung disease: CT findings in 50 patients. AJR 169 (1997): 655-658.
- Swensen SJ, Aughenbaugh GL, Myers JL. Diffuse lung disease: Diagnostic accuracy of CT in patients undergoing surgical biopsy of the lung. Radiology 205 (1997): 22934.
- Hunninghake GW, Gilbert S, Pueringer R. Outcome of the treatment of sarcoidosis. Am J Respir Crit Care Med 149 (1994): 893-898.
- Johns JC, Michele TM. The clinical management of sarcoidosis. A 50 year experience at the Johns Hopkins Hospital. Medicine 78 (1999): 65-111.
- Mapel DW, Hunt WC, Utton R, et al. Idiopathic pulmonary fibrosis: Survival in population based and hospital based cohorts. Thorax x 53 (1998): 469-476.
- Baughman RP, Teirstein AS, Judson MA, et al. A case control etiologic study of sarcoidosis (Access) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 164 (2001): 1885-1889.
- Johnston ID, Prescott RJ, Chalmers JC, et al. British Thoracic Society study of cryptogenic fibrosing alveolitis: Current presentation and initial management. Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. Thorax 52 (1997): 38-44.
- Douglas W, Jay H, Schroeder DR. Idiopathic pulmonary fibrosis. Impact of oxygen and colchicine, prednisone, or no therapy on survival. Am J Respir Crit Care Med 161 (2000): 1172-1178.
- Müller NL. Clinical value of high-resolution CT in chronic diffuse lung disease. AJR 157 (1991): 1163-1170.
