Osteoporosis Management in Patients with Chronic Kidney Disease
Article Information
Mohammad Tinawi*
Adjunct Clinical Assistant Professor of Medicine at Indiana University School of Medicine Northwest-Gary. Nephrology Specialists, Munster, IN, USA
*Corresponding author: Mohammad Tinawi, Nephrology Specialists, P.C., 8840 Calumet Ave, Suite 101, Munster, IN 46321, USA
Received: 08 February 2022; Accepted: 27 April 2022; Published: 3 May 2022
Citation: Mohammad Tinawi. Osteoporosis Management in Patients with Chronic Kidney Disease. Archives of Internal Medicine Research 5 (2022): 161-171.
View / Download Pdf Share at FacebookAbstract
The definition of osteoporosis in the general population according to the World Health Organization (WHO) is a T-score ≤ -2.5, or the presence of a low trauma fracture irrespective of bone mineral density (BMD). Osteopenia is defined by the WHO as T-score between -1 to -2.5. The prevalence of osteoporosis is higher in women with and without chronic kidney disease (CKD). In patients with CKD, osteoporosis falls within the spectrum of chronic kidney disease mineral and bone disorder (CKD-MBD). Kidney Disease Improving Global Outcome (KDIGO) CKD-MBD guidelines recommend measurements of BMD in patients with stage 3 or higher CKD to assess fracture risk. Many pharmacological options exist for the treatment of osteoporosis in the general population. There are no clinical trials in patients with CKD-MBD. Recommendations are based on expert opinion and post hoc analyses of major osteoporosis trials in the general population.
Keywords
Osteoporosis, Osteopenia, Chronic Kidney Disease, CKD-MBD, Renal Osteodystrophy
Osteoporosis articles; Osteopenia articles; Chronic Kidney Disease articles; CKD-MBD articles; Renal Osteodystrophy articles
Article Details
1. Osteoporosis in the General Population
The definition of osteoporosis in the general popul-ation according to the WHO is a T-score ≤-2.5 stand-ard deviations (SDs) based upon BMD measurement by dual-energy x-ray absorptiometry (DXA), or the presence of a low trauma (fragility) fracture irres-pective of BMD. T-score ≤-2.5 SD is BMD that is 2.5 or more SD below the young adult female reference mean. BMD measures the amount of bone mass per unit volume (volumetric density), or bone mass per unit area (areal density) [1]. The National Health and Nutrition Examination Survey (NHANES 2005 - 2008) evaluated bone density of the lumbar spine and hip via DXA in women and men aged ≥ 50 years. It showed that the prevalence of osteopenia and osteo-porosis was 61% and 16% for women, and 38% and 4% for men respectively [2]. In (NHANES 2017–2018) the prevalence of age adjusted osteoporosis at either the hip or lumbar spine or both among adults aged ≥ 50 years was 12.6%. It was higher among women (19.6%) compared with men (4.4%) [3]. The prevalence of osteopenia was 43.1%. It was higher among women (51.5%) compared with men (33.5%). Therefore, Osteoporosis prevalence increased from 2007–2008 through 2017–2018 among women but not men. Osteopenia prevalence was somewhat lower for both men and women.
Compared to men in the same age group, women ≥ 50 years of age have two times higher rate of osteo-penia, four times higher rate of osteoporosis, and a tendency to get fractures 5 - 10 years earlier [2]. Most current guidelines recommend osteoporosis screening with DXA for women > 65 years. Although the diagnosis of osteoporosis is based on BMD, there are three major characteristics of osteoporosis: low bone mass (osteopenia), microarchitectural disruption, and increased skeletal fragility. Osteoporosis increases the risk of fracture due to decreased bone strength. Bone strength is a function of BMD, bone turnover (form-ation and resorption), and bone microarchitecture [4]. Fracture risk is increased in osteoporosis due to com-promised bone strength (bone quality and density). BMD is assessed clinically by dual energy x-ray absorptiometry (DXA) [5]. Bone quality (turnover, mineralization, mass, architecture) can be measured by performing a bone biopsy. Bone biopsies are labor-intensive and are rarely done in clinical practice due to cost, the need for specialized centers, and patients’ discomfort. Primary osteoporosis is common in post-menopausal women, as well as older men and women. Secondary osteoporosis can be due to medications such as corticosteroids [5]. The WHO has developed a clinical risk prediction algorithm that will help physicians determine the risk of a fracture within the subsequent decade: https://www.sheffield.ac.uk/ FRAX/. Lifestyle interventions are recommended in all patients with osteoporosis and are summarized in Table 1 [1, 6]. Pharmacological management of osteoporosis in the general population is summarized in Table 2 [1, 5].
|
Intervention |
Comments |
|
Calcium from dietary sources |
Use calcium carbonate or citrate if needed |
|
Nutritional Vitamin D: Ergocalciferol (D2) Cholecalciferol (D3) |
Keep 25-hydroxyvitaminD >30 ng/ml (normal range 30-80 ng/ml) |
|
Exercise |
Weight-bearing exercise is paramount |
|
Fall prevention |
Assess gait, vision, and hearing |
|
Tobacco smoking cessation |
|
|
Alcohol intake moderation |
|
|
Balanced general nutrition |
Table 1. Lifestyle recommendation in patients with osteoporosis
|
Supplements |
Bisphosphates |
RANKL inhibitors |
SERMs |
Parathyroid horm-one and parathyroid hormone-related protein analogues |
Sclerostin inhibitors |
|
Calcium & vitamin D were used as co-therapy in all major clinical trials |
Alendronate |
Denosumab |
Raloxifene |
Teriparatide |
Romosozumab |
|
Calcium from dietary sources. Use calcium car-bonate or citrate if needed |
Ibandronate |
Abaloparatide |
|||
|
Nutritional Vitamin D: |
|||||
|
Ergocalciferol (D2) Cholecalciferol (D3) |
Risedronate Zoledronic acid |
||||
Table 2. Medications for treatment of osteoporosis. RANKL: Receptor activator of nuclear factor kappa-Β ligand (NF-κB), SERM: selective estrogen receptor modulator.
2. Chronic Kidney Disease Mineral and Bone Disorder (CKD-MBD)
Chronic kidney disease mineral and bone disorder (CKD-MBD) is a systemic disorder of mineral and bone metabolism due to chronic kidney disease (CKD) characterized by one or more of the following [7]:
- Abnormalities of vitamin D, calcium, phosphorus, or parathyroid hormone (PTH) metabolism [8].
- Abnormalities in bone strength, linear growth, turnover, mineralization, or volume.
- Vascular or other soft tissue calcifications.
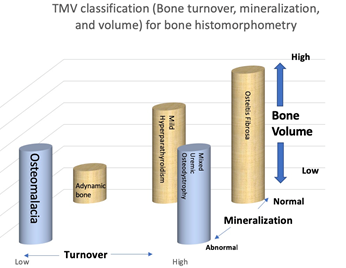
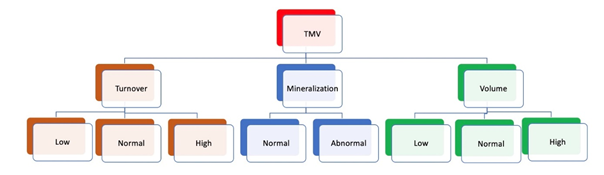
Renal osteodystrophy (ROD) is the bone abnor-malities component of CKD-MBD which increases the fracture risk in patients with CKD [4]. Renal osteodystrophy is a complex disorder of bone density and quality and is a form of osteoporosis. Therefore, the spectrum of CKD-MBD includes osteoporosis, but involves other complex abnormalities as well [9]. Adynamic bone disease is a form of CKD-MBD characterized by low bone turnover and low bone formation [7]. Management strategies for CKD-MBD are summarized in Table 3 [8, 10, 11]. Bone histom-orphometry in renal osteodystrophy is class-ified based on bone turnover, bone mineralization, and bone volume [TMV classification] (Figure 1, Figure 2, Table 4) [7].
The last update of the Kidney Disease Improving Global Outcome (KDIGO) CKD-MBD guidelines published in 2017 recommended measurements of BMD to assess fracture risk [12]. It suggests measure-ment of BMD via DXA in patients with stages 3-5 CKD and those on renal replacement therapy if they have risk factors for osteoporosis or evidence of CKD-MBD provided that outcome of the testing will affect treatment decisions. This recent recommenddation was based on longitudinal studies demonstrating the validity of T scores in patients with and without CKD [13-15].
2.1 Mechanisms of Osteoporosis in Chronic Kidney Disease
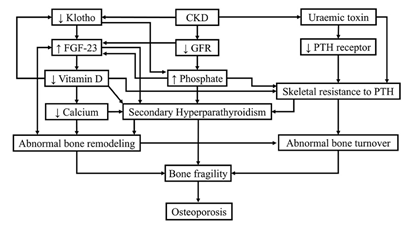
Different mechanisms are at play in patients with CKD and osteoporosis [8, 10, 16]. These mechanisms are summarized in Figure 3.
2.2 Prevalence of Osteopenia and Osteoporosis in Chronic Kidney Disease
A systematic review by Tariq et al. included 8 studies in patients with CKD [17]. It reported a prevalence of osteopenia between 33.3% and 81% (average of 45.91%), and a prevalence of osteoporosis between 2.24% and 31.3% (average of 23.29%). Females and CKD patients with low Body Mass Index had a higher prevalence. Therefore, the prevalence of osteoporosis in CKD patients is higher than the general population [16]. In the majority of studies, lumbar spine was the most susceptible site for osteoporosis.
2.3 Fracture Risk in Chronic Kidney Disease Patients
Alem et al. used data from the United States Renal Data System (USRDS) and concluded that the risk of hip fracture in Caucasian patients with ESRD was about four times higher than the general population irrespective of age or gender. The report was in Cau-casians because ESRD patients were compared to the general population of Olmstead County in Minnesota, USA, which is >90% Caucasians [18]. Nickolas et al. studied hip fracture incidence in CKD patients [19]. They obtained data from the The Third National Health and Nutrition Examination Survey (NHANES) which was done between 1988-1994. Subjects with eGFR <60 ml/min had an increased risk of hip fracture (odds ratio [OR] 2.12; 95% confidence interval [CI] 1.18 to 3.80). Conversely, subjects aged 50-74 years with hip fracture were three times more likely to have CKD. The association of moderate to severe CKD with hip fracture was independent of hip fracture traditional risk factors.
|
Phosphate binders |
Active vitamin D sterols |
Calcium sensing receptor activators (calcimimetics) |
Correction of metabolic abnormalities |
|
Calcium carbonate |
Calcitriol (D3) |
Cinacalcet |
Alkylating agents such as sodium bicarbonate in metabolic acidosis |
|
Calcium acetate |
Doxercalciferol (D2) |
Etelcalcetide |
Correction of hyponatremia |
|
Sevelamer carbonate |
Paricalcitol (D2) |
||
|
Ferric citrate |
1-alpha-calcidiol (D3) |
||
|
Lanthanum carbonate |
Table 3. Medications for the treatment of CKD-MBD.
|
Condition |
Bone turnover |
Mineralization |
Bone volume |
|
Osteomalacia |
Low |
Abnormal |
Low to medium |
|
Adynamic bone disease |
Low |
Normal |
Low to normal |
|
Mild hyperparathyroidism |
Medium |
Normal |
Variable |
|
Advanced hyperparathyroidism (Osteitis fibrosa) |
High |
Normal |
Variable |
|
Mixed uremic osteodystrophy |
High |
Abnormal |
Normal |
Table 4: The TMV classification system allows accurate characterization of bone and mineral disorders in CKD patients.
Figure 3. Different mechanisms in CKD lead to osteoporosis. FGF-23: fibroblast growth factor, GFR: glomerular filtration rate, PTH: parathyroid hormone. Diagram is courtesy of: Tasnim et al. Cureus 13(10): e18488. 2021. DOI 10.7759/cureus.18488, under the terms of the Creative Commons Attribution License CC-BY 4.0.
3. Managing Osteoporosis as Part of CKD-MBD
Table 5. proposes a step-by-step approach to the management of osteoporosis in CKD-MBD [4].
3.1 Antiresorptive Agents
Antiresorptive agents include bisphosphonates, raloxi-fene, and denosumab [1]. BMD increases in osteop-orosis with antiresorptive agents due to increased bone mineralization. Antiresorptive agents also decrease bone turnover [20]. Overall fracture risk decreases by approximately 50%.These agents may prevent bone loss in CKD patients with normal to high-turnover bone disease. There are no clinical trials in patients with CKD-MBD [4]. Recommendations are based on expert opinion and post hoc analyses of major osteo-porosis trials in the general population. Note that post hoc analyses included patients with CKD, but it is unknown how many of them had CKD-MBD.
3.1.1 Bisphosphonates: Bisphosphonates are not FDA-approved for patients with eGFR < 30 ml/min [4]. These medications induce apoptosis of osteoclasts and are retained in bone for several years. The concern in patients with advanced CKD (eGFR < 30 ml/min including CKD-4, CKD-5, and patients on dialysis) is the development of adynamic bone disease due to suppression of bone remodeling. Miller et al. conducted a pooled analysis of nine randomized, double-blind, placebo-controlled phase III risedronate trials [21]. The goal was to study the effect of age-related reduction in renal function on the safety and effectiveness of risendronate in osteoporotic women. Renal function was measured via the Cockcroft and Gault equation. Adverse events, preservation of BMD, and reduction of vertebral fractures were similar irrespective of renal function. A limited number of subjects underwent transiliac bone biopsies. None of those biopsies showed adynamic bone disease.
3.1.2 Denosumab: Denosumab is a monoclonal antibody against RANKL (receptor activator of nuclear factor kappa-Β ligand [NF-κB]) [1]. Like bisphosphonate it is an anti-resorptive agent that inhibits osteoclasts proliferation. However, unlike bisphosphonate, it is not renally cleared. Jamal et al. in a post hoc analysis of the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) Trial showed that the adverse effects and treatment benefit of denosumab were similar in patients with stage 3 and 4 CKD [22]. Creatinine clearance was estimated using the Cock-croft and Gault method. Patients should be monitored for hypocalcemia [23]. Two small studies (each had 12 patients) demonstrated the efficacy of denosumab for osteoporosis in end-stage renal disease patients on dialysis. Hypocalcemia was the most significant adverse event [24, 25].
3.2 Anabolic Agents
Anabolic agents should not be used in CKD patients with high bone turnover state such as secondary hyperparathyroidism [4]. Their use is a consideration in patients with low bone turnover state such as adynamic bone disease. In such patients they enhance bone turnover and improve BMD [6, 20]. As with antiresorptive agents more data from trials in CKD-MBD patients are needed.
3.2.1 Teriparatide: Miller et al. used data from the Fracture Prevention Trial to determine the safety and efficacy of teriparatide [rhPTH(1-34)] in postmeno-pausal women with osteoporosis and CKD [26]. GFR was calculated using the Cockcroft-Gault equation. Patients were randomized to receive daily subcuta-neous injections of teriparatide 20 or 40 mcg/day or placebo. All patients had normal PTH. Therefore, as with the above-mentioned studies with antiresorptive agents, this is not an analysis in patients with CKD-MBD. All patients had creatinine ≤ 2.0 mg/dl. teriparatide-mediated reduction in vertebral and nonvertebral fracture was essentially the same independent of renal function. The main adverse events were hypercalcemia and hyperuricemia, both were more common in patients with lower creatinine clearance. Sumida et al. administered 56.5-μg terip-aratide once-weekly for 48 weeks to 22 adult hemodialysis patients with hypoparathyroidism and low bone mass [27]. BMD did not change in the femoral neck and distal one-third radius but increased at the lumbar spine by 3.3 ± 1.9 % (mean ± SEM) and 3.0 ± 1.8 % at 24 and 48 weeks respectively. The most common adverse event was transient hypotension.
3.2.2 Abaloparatide
Abaloparatide is a new anabolic agents, it is an analog of PTH-related protein [20]. It is associated with a lower risk of hypercalcemia compared to teriparatide. The ACTIVE phase 3 study was an 18-month, rand-omized, double-blind, placebo-controlled trial of postmenopausal women with osteoporosis who received subcutaneous abaloparatide 80 µg, placebo, or open-label teriparatide 20 µg daily [28]. The trial excluded patients with serum creatinine >2.0 mg/dL or eGFR <37 mL/min based on Cockcroft-Gault formula. In patients with mild to moderate CKD, abaloparatide and teriparatide had similar efficacy and safety. Abaloparatide caused significantly less hypercalcemia compared to teriparatide in patients with CKD.
3.3 Romosozumab
Sclerostin is a protein secreted by osteocytes. It inhibits bone formation. Romosozumab targets scler-ostin to support new bone formation. Romosozumab is a monoclonal antibody with a dual effect of increasing bone formation and decreasing bone resorption [20]. FRAME was a double-blind, randomized, placebo-controlled study involving 7,180 postmenopausal women with osteoporosis [29]. Patients were injected subcutaneously on a monthly basis with either placebo or 210 mg romosozumab. At baseline, most subjects (88%) had mild or moderate renal insufficiency (stages 1,2, and 3); 0.3% had severe CKD (stage 4). No patients had stage 5 CKD [30]. At the end of study, the improvement in BMD, reduction in new vertebral fractures, cardiovascular events, and adverse events, were the same irrespective of baseline eGFR. These findings do not apply to patients with stages 4, and 5 CKD.
|
Step |
Comments |
|
Confirm the diagnosis of CKD |
evidence of kidney damage or eGFR <60 ml/min/1.73 m2 for 3 months or more. |
|
Establish the diagnosis of CKD-MBD |
Abnormal calcium, phosphorus, vitamin D, PTH, bone abnormalities, or tissue calcifications (see above) |
|
Screen for osteoporosis with DXA scan every 1-2 years |
If T-score ≤ -2.5, or if the patient has low trauma fracture irrespective of T-score, move to step 4 |
|
Obtain a bone biopsy to assess bone turnover if feasible to guide management decisions |
Bone biopsies are not commonly done due to multiple logistical issues |
|
Evidence of low bone turnover such as low intact PTH (e.g., below 100 pg/ml) in a dialysis patient and a low bone-specific alkaline phosphatase |
Consider starting an anabolic agent |
|
Evidence of normal or high bone turnover such as high intact PTH (e.g., above 600 pg/ml) in a dialysis patient and an above mid-range bone-specific alkaline phos-phatase |
Consider starting an anti-resorptive agent |
Table 5: Bone-specific alkaline phosphatase (BAP) is synthesized by the osteoblasts, reference range is approximately 7-27mcg/L. Intact parathyroid hormone (PTH) reference range is 10-65 pg/ml. KDIGO guidelines recommend maintaining intact PTH in the range of 2-9 times the upper range of normal in dialysis patients. A low intact PTH (e.g., below 100 pg/ml) in a dialysis patient is consistent with low bone turnover state such as adynamic bone disease, while a high intact PTH (e.g., above 600 pg/ml) is consistent with high bone turnover state such as secondary hyperparathyroidism. Bone biopsy studies have shown significant overlap. African Americans dialysis patients tend to have higher intact PTH level. Oversuppression of intact PTH is undesirable as it may lead to adynamic bone disease.
4. Conclusions
Compared to men in the same age group, women ≥ 50 years of age have two times higher rate of osteopenia, four times higher rate of osteoporosis and, and a tendency to get fractures 5 - 10 years earlier.
- Renal osteodystrophy (ROD) is the bone abnormalities component of CKD-MBD which increases the fracture risk in patients with CKD. Renal osteodystrophy is a complex disorder of bone density and quality and is a form of osteoporosis.
- The prevalence of osteoporosis in CKD patients is higher than the general population.
- There are no osteoporosis clinical trials in patients with CKD-MBD. In case of osteo-porosis and low bone turnover in a CKD-MBD patient consider starting an anabolic agent, and in case of normal or high bone turnover consider an anti-resorptive agent.
- Many major osteoporosis trials in the general population included patients with CKD. The results of post hoc analyses of these trials are encouraging and permit individualized treat-ment of osteoporosis in CKD patients.
References
- Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmen-opausal women. Osteoporosis International 30 (2019): 3-44.
- Alswat KA. Gender Disparities in Osteo-porosis. Journal of Clinical Medicine Research 9 (2017): 382-387.
- Sarafrazi N, Wambogo E, Shepherd J. Osteoporosis or Low Bone Mass in Older Adults: United States, 2017–2018. NCHS Data Brief Number 405 (2021): 1-7.
- Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clinical Journal of the American Society of Nephrology 13 (2018): 962-969.
- Ensrud KE, Crandall CJ. In the Clinic Osteoporosis. Annals of Internal Medicine 167 (2017): ITC17-ITC33.
- Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteo-porosis to prevent fractures in men and women: A clinical practice guideline update from the American college of physicians. Annals of Internal Medicine 166 (2017): 818-839.
- Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney International 69 (2006): 1945-1953.
- Tinawi M. Disorders of Calcium Meta-bolism: Hypocalcemia and Hypercalcemia. Cureus 13 (2021): e12420.
- Damasiewicz MJ, Nickolas TL. Rethinking Bone Disease in Kidney Disease. JBMR Plus; 2. Epub ahead of print (2018).
- Tinawi M. Disorders of Phosphate Meta-bolism: Hypophosphatemia and Hyperphosp-hatemia. Archives of Clinical and Biomedical Research 5 (2021): 538-555.
- Tinawi M. Pathophysiology, Evaluation and Management of Metabolic Acidosis. Archi-ves of Clinical and Biomedical Research 5 (2021): 85-109.
- Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney International 92 (2017): 26-36.
- Iimori S, Mori Y, Akita W, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients-a single-center cohort study. Nephrology Dialysis Transplantation 27 (2012): 345-351.
- Naylor KL, Garg AX, Zou G, et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clinical Journal of the American Society of Nephrology 10 (2015): 646-653.
- Yenchek RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clinical Journal of the American Society of Nephrology 7 (2012): 1130-1136.
- Tasnim N, Dutta P, Nayeem J, et al. Osteopo-rosis, an Inevitable Circumstance of Chronic Kidney Disease: A Systematic Review. Cureus 13 (2021): e18488.
- Tariq MH, Sulaiman SAS. Prevalence of Osteopenia and Osteoporosis among Chronic Kidney Disease Patients: A Systematic Review. The Open Urology & Nephrology Journal 13 (2020): 5-12.
- Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Inter-national 58 (2000): 396-399.
- Nickolas TL, McMahon DJ, Shane E. Relationship between Moderate to Severe Kidney Disease and Hip Fracture in the United States. Journal of the American Soci-ety of Nephrology 17 (2006): 3223-3232.
- Shoback D, Rosen CJ, Black DM, et al. Pharmacological Management of Osteo-porosis in Postmenopausal Women: An Endocrine Society Guideline Update. Journal of Clinical Endocrinology and Metabolism 105 (2020): 587-594.
- Miller PD, Roux C, Boonen S, et al. Safety and Efficacy of Risedronate in Patients With Age-Related Reduced Renal Function as Estimated by the Cockcroft and Gault Method: A Pooled Analysis of Nine Clinical Trials. Journal of Bone and Mineral Research 20 (2005): 2105-2115.
- Jamal SA, Ljunggren Ö, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. Journal of Bone and Mineral Research 26 (2011): 1829-1835.
- Block GA, Bone HG, Fang L, et al. A single-dose study of denosumab in patients with various degrees of renal impairment. Journal of Bone and Mineral Research 27 (2012): 1471-1479.
- Chen CL, Chen NC, Hsu CY, et al. An open-label, prospective pilot clinical study of denosumab for severe hyperparathyroidism in patients with low bone mass undergoing dialysis. Journal of Clinical Endocrinology and Metabolism 99 (2014): 2426-2432.
- Festuccia F, Jafari MT, Moioli A, et al. Safety and efficacy of denosumab in osteoporotic hemodialysed patients. Journal of Nephrology 30 (2017): 271-279.
- Miller PD, Schwartz E, Chen P, et al. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 18 (2007): 59-68.
- Sumida K, Ubara Y, Hoshino J, et al. Once-weekly teriparatide in hemodialysis patients with hypoparathyroidism and low bone mass: a prospective study. Osteoporos Int 27 (2016): 1441-1450.
- Bilezikian JP, Hattersley G, Mitlak BH, et al. Abaloparatide in patients with mild or moderate renal impairment: results from the ACTIVE phase 3 trial. Current Medical Research and Opinion 35 (2019): 2097-2102.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. New England Journal of Medicine 375 (2016): 1532-1543.
- Miller P, Chines A, Ben-Hur A, et al. Efficacy and Safety of Romosozumab vs Placebo Among Patients with Mild-to-Moderate Chronic Kidney Disease. New Orleans: American Society of Rheumatology (2019): 2727-2732.