Oral Ketamine as a Premedication in Children Undergoing Day-Case Non-Invasive Procedures
Article Information
Samar Adam MBBCh, Buthaina Bucheery; CABA, Ahmed Maher; MSc
*Corresponding Author: Samar Adam, Salmaniya medical complex, Bahrain
Received: 19 November 2023; Accepted: 01 December 2023; Published: 18 December 2023
Citation: Samar Adam. Oral Ketamine as a Premedication in Children Undergoing Day-Case Non-Invasive Procedures. Anesthesia and Critical Care 5 (2023): 98-107.
View / Download Pdf Share at FacebookAbstract
Ketamine is a dissociative anesthetic related to phencyclidine. The mode of action is disconnection between thalamocortical and limbic systems. The outcome is a sedated patient with amnesia and analgesia. The aim of the study is to assess the minimal effective dose of oral ketamine as a premedication in pediatric patients undergoing day-case non-invasive MRI procedures and assessing any related side effects and complications.
Keywords
Ketamine; Oral; Premidcation; Children; Daycase
Ketamine articles; Oral articles; Premidcation articles; Children articles; Daycase articles.
Ketamine articles Ketamine Research articles Ketamine review articles Ketamine PubMed articles Ketamine PubMed Central articles Ketamine 2023 articles Ketamine 2024 articles Ketamine Scopus articles Ketamine impact factor journals Ketamine Scopus journals Ketamine PubMed journals Ketamine medical journals Ketamine free journals Ketamine best journals Ketamine top journals Ketamine free medical journals Ketamine famous journals Ketamine Google Scholar indexed journals Oral articles Oral Research articles Oral review articles Oral PubMed articles Oral PubMed Central articles Oral 2023 articles Oral 2024 articles Oral Scopus articles Oral impact factor journals Oral Scopus journals Oral PubMed journals Oral medical journals Oral free journals Oral best journals Oral top journals Oral free medical journals Oral famous journals Oral Google Scholar indexed journals Premidcation articles Premidcation Research articles Premidcation review articles Premidcation PubMed articles Premidcation PubMed Central articles Premidcation 2023 articles Premidcation 2024 articles Premidcation Scopus articles Premidcation impact factor journals Premidcation Scopus journals Premidcation PubMed journals Premidcation medical journals Premidcation free journals Premidcation best journals Premidcation top journals Premidcation free medical journals Premidcation famous journals Premidcation Google Scholar indexed journals Children articles Children Research articles Children review articles Children PubMed articles Children PubMed Central articles Children 2023 articles Children 2024 articles Children Scopus articles Children impact factor journals Children Scopus journals Children PubMed journals Children medical journals Children free journals Children best journals Children top journals Children free medical journals Children famous journals Children Google Scholar indexed journals Daycase articles Daycase Research articles Daycase review articles Daycase PubMed articles Daycase PubMed Central articles Daycase 2023 articles Daycase 2024 articles Daycase Scopus articles Daycase impact factor journals Daycase Scopus journals Daycase PubMed journals Daycase medical journals Daycase free journals Daycase best journals Daycase top journals Daycase free medical journals Daycase famous journals Daycase Google Scholar indexed journals analgesia articles analgesia Research articles analgesia review articles analgesia PubMed articles analgesia PubMed Central articles analgesia 2023 articles analgesia 2024 articles analgesia Scopus articles analgesia impact factor journals analgesia Scopus journals analgesia PubMed journals analgesia medical journals analgesia free journals analgesia best journals analgesia top journals analgesia free medical journals analgesia famous journals analgesia Google Scholar indexed journals amnesia articles amnesia Research articles amnesia review articles amnesia PubMed articles amnesia PubMed Central articles amnesia 2023 articles amnesia 2024 articles amnesia Scopus articles amnesia impact factor journals amnesia Scopus journals amnesia PubMed journals amnesia medical journals amnesia free journals amnesia best journals amnesia top journals amnesia free medical journals amnesia famous journals amnesia Google Scholar indexed journals American Society of Anesthesiologists articles American Society of Anesthesiologists Research articles American Society of Anesthesiologists review articles American Society of Anesthesiologists PubMed articles American Society of Anesthesiologists PubMed Central articles American Society of Anesthesiologists 2023 articles American Society of Anesthesiologists 2024 articles American Society of Anesthesiologists Scopus articles American Society of Anesthesiologists impact factor journals American Society of Anesthesiologists Scopus journals American Society of Anesthesiologists PubMed journals American Society of Anesthesiologists medical journals American Society of Anesthesiologists free journals American Society of Anesthesiologists best journals American Society of Anesthesiologists top journals American Society of Anesthesiologists free medical journals American Society of Anesthesiologists famous journals American Society of Anesthesiologists Google Scholar indexed journals End-Tidal CO2 articles End-Tidal CO2 Research articles End-Tidal CO2 review articles End-Tidal CO2 PubMed articles End-Tidal CO2 PubMed Central articles End-Tidal CO2 2023 articles End-Tidal CO2 2024 articles End-Tidal CO2 Scopus articles End-Tidal CO2 impact factor journals End-Tidal CO2 Scopus journals End-Tidal CO2 PubMed journals End-Tidal CO2 medical journals End-Tidal CO2 free journals End-Tidal CO2 best journals End-Tidal CO2 top journals End-Tidal CO2 free medical journals End-Tidal CO2 famous journals End-Tidal CO2 Google Scholar indexed journals Day Case Unit articles Day Case Unit Research articles Day Case Unit review articles Day Case Unit PubMed articles Day Case Unit PubMed Central articles Day Case Unit 2023 articles Day Case Unit 2024 articles Day Case Unit Scopus articles Day Case Unit impact factor journals Day Case Unit Scopus journals Day Case Unit PubMed journals Day Case Unit medical journals Day Case Unit free journals Day Case Unit best journals Day Case Unit top journals Day Case Unit free medical journals Day Case Unit famous journals Day Case Unit Google Scholar indexed journals
Article Details
1. Introduction
Over the past 40 years there has been a revolution in surgery. Day case surgery has become increasingly popular due to new surgical techniques, advances in anaesthesia, collection and publication of comparative data, and deliberate policies including financial incentives for hospitals. [1] Statistics show that shorter hospital stays and early mobilization reduce many comorbidities, such asthe risk of venous thromboembolism and hospital-acquired infections. [2] In general, day-case procedures are appropriate for children, since they tend to be healthy and free from systemic disease or degenerative changes. [3] Anesthesia is often the primary specialty involved in the development of selection criteria protocols, postoperative symptom control regimens, and audit coordination in pediatric day-case services. [4] Radiological studies are the most common day-case procedures under anesthesia in the pediatric age group, and given the relatively lengthy magnetic resonance imaging (MRI) study, the best possible images are obtained through sedation and general anesthesia in infants and young children Undergoing such scans, because these children may have difficulty remaining motionless and following breathing commands. [5,6] Ketamine is a dissociative anesthetic related to phencyclidine. The mode of action is disconnection between thalamocortical and limbic systems. The outcome is a sedated patient with amnesia and analgesia. [7]
1.1 Systemic Effects of Ketamine
Ketamine is primarily used to induce and maintain anesthesia. In addition, it is used as a sedative, analgesic, antidepressant, to treat bronchospasm, and to treat complex regional pain syndromes. One of the advantages of ketamine is that respiratory, cardiac function and airway reflexes are protected during drug administration. [8]
1.2 Pharmacokinetic Characteristics of Ketamine
Ketamine works as a noncompetitive NMDA receptor antagonist. Aimed to be used throughout premedication, analgesia, sedation, induction and maintenance phases of general anesthesia. [9] There is also interest in ketamine for potentially new indications, such as management of chronic pain, treatment of resistant depression, and reversing opioid-induced respiratory depression. [10]
1.3 Administration
Ketamine can be administrated via multiple routes (Table 1) [11]
Table 1: Route of administration, bioavailability, and the starting dose of ketamine
|
Route of administration |
Bioavailability |
Starting dose |
|
Intravenous |
100% |
0.25–1 mg/kg (adults) |
|
0.25–2 mg/kg (children) |
||
|
1–2 mg/kg |
||
|
Intraosseous |
100% |
0.5–1 mg/kg |
|
1–2 mg/kg |
||
|
Intramuscular |
93% |
4–5 mg/kg |
|
8–10 mg/kg |
||
|
Oral |
16–20% |
Children: 3–15 mg/kg |
|
Adults: 500 mg max. |
||
|
Nasal |
45–50 % |
0.25–4 mg/kg |
|
3–9 mg/kg |
||
|
Rectal |
25–30% |
50 mg |
|
8–15 mg/kg |
1.4 Study Procedure
After the approval of the Hospital’s Ethics Committee and parental/ guardian written informed consent, 100 children aged 1 - 6 years with American Society of Anesthesiologists (ASA) physical status I-II underwent day-case non-invasive MRI procedures, assigned randomly and received oral ketamine 20 minutes before the procedure, either in 6 mg.kg-1 (Group A), 7 mg.kg-1 (Group B), or 10 mg.kg-1 (Group C) mixed with clear apple juice with a total volume of 10 ml. All patients, accompanied by their parents/guardians, were taken to the holding area. [12] Vital signs were taken and recorded upon admission to the holding area. Following the administration of the sedative mixture, they were continuously monitored using a multichannel monitor (heart rate, ECG, non-invasive blood pressure, respiratory rate and pulse oximetry) and were recorded at 10 minutes intervals along with the level of sedation using Pediatric Sedation State Scale (PSSS). [13] 20-30 minutes after drinking the mixture, children were transferred to the procedure room. Children’s hemodynamics were continuously monitored and they were recorded at 10, 20, 30, 40, 50 and 60 minutes after drug administration. The response of each child at the time of separation from parents, transfer to procedure room, intravenous access and acceptance of facemask were recorded in accordance with the PSSS. Sevoflurane, administered through a face mask, in a 0.4 MAC along with 50% O2 in air was delivered to all the children to prevent them from moving while the test is being done. A gas analyzer was connected to the mask to monitor inhalational anesthetic and End-Tidal CO2 (ETCO2) concentrations. [14] At the end of the procedure, the time interval between transferring the patient to the recovery area until spontaneous eye opening and response to verbal command was recorded. The occurrence of any side effects related to the procedure during the perioperative period was recorded. Discharge from the Day Case Unit (DCU) was only allowed after full regaining of consciousness, and data collection was stopped at the end of this period.
Exclusion criteria:
- Children below 1 year or older than 6 years.
- ASA > II.
- Body Mass index (BMI) > 30
- Previous negative response to ketamine.
- Emergency procedures.
- Incomplete ingestion of the sedative mixture.
- Failure to commence the procedure > 30 minutes after ingesting the sedative mixture.
- Respiratory tract infection.
2. Materials and Methods
A total of 112 children (aged from 1-6 years) underwent MRI scanning in Salmaniya Medical Complex hospital ( SMC) fulfilling the criteria of the study between March and July 2023. Among them 1 child has reported a history of ketamine allergy , 2 children had a mass body index (MBI) more than 30 , 4 children had respiratory tract infections, and 5 had incomplete ingestion of the sedative mixture . Therefore, these children were excluded from our study. A total of 100 children were enrolled in the study.
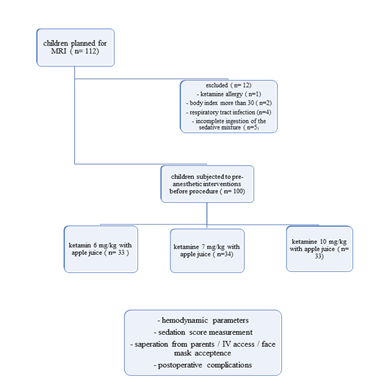
2.1 Statistical analysis
A. Part 1: Participants’ demographic and health characteristics
The sample consisted of 100 participants equally divided into three Ketamine dose groups. These groups are very comparable in terms of their ages, gender, weights, and ASA scores. The mean age across the groups ranged from 3 to 3.5 years with no statistically significant difference detected among them (p=0.486). Both genders were represented in very similar proportions in these dose groups without any statistically significant differences (p=0.814). Although those in 6mg.Kg-1 group weighed less than the other groups, however, this difference did not reach the statistical significance (p=0.170). The three groups did not differ in regard to scores. Each of the 2 included ASA grades were represented in very similar proportions in these in these dose groups without any statistically significant differences (p=0.521). (Table 2):
Table 2: Demographic data of the studied groups
|
Variable |
Total |
6mg.kg-1 |
7mg.kg-1 |
10mg.kg-1 |
p-value |
|||
|
33(33%) |
34(34%) |
33(33%) |
||||||
|
Age (Mean±SD) in years |
3.3±15 |
3 |
±1.5 |
3.4 |
±1.5 |
3.5 |
±1.5 |
0.486* |
|
Gender n(%) |
||||||||
|
Male |
56(56%) |
17 |
30.40% |
20 |
35.70% |
19 |
33.90% |
0.814** |
|
Female |
44(44%) |
16 |
36.40% |
14 |
31.80% |
14 |
31.80% |
|
|
Weight (Mean±SD) in Kg |
15.7±3.9 |
14.7 |
±3.9 |
16.1 |
±4.0 |
16.4 |
±3.8 |
0.170* |
|
ASA |
||||||||
|
1 |
82(82%) |
25 |
30.50% |
29 |
35.40% |
28 |
34.10% |
0.521** |
|
2 |
18(18%) |
8 |
44.40% |
5 |
27.80% |
5 |
27.80% |
|
*Statistically insignificant differences with Kruskal-Wallis test at Alpha 0.05
**Statistically insignificant differences with Chi Square at Alpha 0.05
B. Part 2
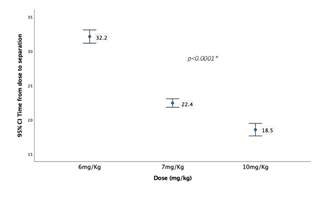
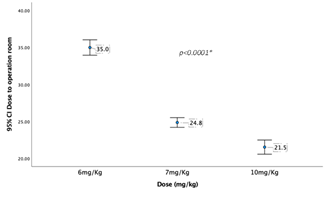
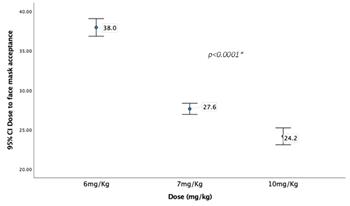
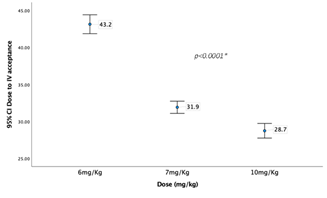
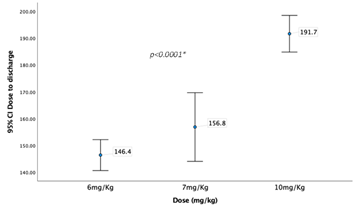
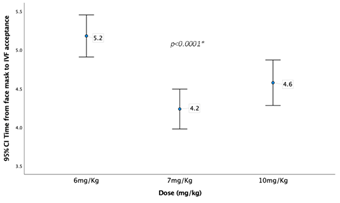
Comparing the three dose groups based on time laps between drug administration and different phases of the study (Table 3) the three groups differed significantly in the time lapsed from dose administration till separation from parents, arriving to operation room, face mask acceptance, acceptance of intravenous (IV) line insertion, and discharge. It required around 32 minutes on average to separate the child after the dose administration in the 6 mg.Kg-1 group. This time was significantly reduced to 22 minutes in the 7 mg.Kg-1 group and even went lower in the 10 mg.Kg-1 group. A very similar results noted in the other intervals except of dose to discharge time. Interestingly, Although the 10 mg.Kg-1 group recorded the lowest time in dose to other steps, this group recorded significantly the highest time to discharge when compared the other groups (191.7±19.3 vs. 165±31.9 and 146.4±16.3, p<0.0001). The three groups showed very similar time required from arrival to the operation room till face mask acceptance without any significant differences. However, they differed significantly in duration of face mask to IV acceptance with the 6 mg.Kg-1 group recoded significantly higher duration compared to the other two groups. All the groups showed no difference in the procedure times as they recorded similar mean times.
Table 3: Intervals between drug administration and different phases of the study.
|
Time laps (minutes) |
Total |
6mg.Kg-1 |
7mg.Kg-1 |
10mg.Kg-1 |
p-value |
|||
|
Mean±SD |
Mean |
±SD |
Mean |
±SD |
Mean |
±SD |
||
|
Dose to separation |
24.4±6.1 |
32.2 |
±2.7 |
22.4 |
±1.8 |
18.5 |
±2.6 |
<0.0001* |
|
Dose to face mask |
27.1±6.3 |
35 |
±2.9 |
24.8 |
±1.9 |
21.5 |
±2.7 |
<0.0001* |
|
Dose to I. |
34.6±6.9 |
43.2 |
±3.6 |
31.9 |
±2.3 |
28.7 |
±2.8 |
<0.0001* |
|
Dose to discharge |
165±31.9 |
146.4 |
±16.3 |
156.8 |
±36.2 |
191.7 |
±19.3 |
<0.0001* |
|
Procedure time |
30.5±13.7 |
31.2 |
±15.0 |
30.9 |
±13.3 |
29.4 |
±13.2 |
0.851 |
*Statistically significant differences with ANOVA test at Alpha 0.05
Graph 2 shows the change in dose to separation time (minutes) across the three dose groups
Graph 3 shows the time laps between dose administration to transfer to operation room (minutes) across the three dose groups
Graph 4 shows the time laps between dose administration to face mask acceptance (minutes) across the three dose groups
Graph 5 shows the time laps between dose administration to tolerance to intravenous line insertion (minutes) across the three dose groups.
Graph 6 shows the time laps between dose administration to discharge time (minutes) across the three dose groups.
Graph 7 shows the time laps between face mask acceptance and IV acceptance time across the three dose groups.
C. Part 3 : Comparing vital signs and PSSS at 10 minutes intervals across the three dose groups
Despite some variations, the participants in the three groups showed very comparable heart rates, Oxygen saturations, and PSSS scores across the 10 minutes intervals of the procedure without statistically significant differences. For instant, none of the groups differed significantly in HR at 0 minutes (p=0.217) neither in the 60 minutes of the procedure (p=0.744). the same observation is true for the SPO2% and the PSS score. (Table 4)
Table 4: Vital signs and PSSS at 10 minutes intervals
|
Vitals |
Total |
6 mg.Kg-1 |
7mg.Kg-1 |
10mg.Kg-1 |
p-value* |
||||
|
Mean |
±SD |
Mean |
±SD |
Mean |
±SD |
||||
|
Heart Rate at: |
|||||||||
|
0 minutes |
98.6 |
±19.1 |
97 |
±18.6 |
95.5 |
±19.3 |
103.2 |
±19.1 |
0.217 |
|
10 minutes |
114.5 |
±20.7 |
116.3 |
±22.7 |
114.4 |
±22.0 |
112.9 |
±17.4 |
0.8 |
|
20 minutes |
98.3 |
±17.4 |
101.1 |
±17.5 |
95.9 |
±16.9 |
98 |
±17.9 |
0.481 |
|
30 minutes |
96.6 |
±17.9 |
99 |
±18.2 |
94.5 |
±17.8 |
96.4 |
±18.1 |
0.601 |
|
40 minutes |
97.3 |
±18.6 |
99.2 |
±20.3 |
95.3 |
±17.9 |
97.6 |
±17.9 |
0.694 |
|
50 minutes |
96.8 |
±18.5 |
98.4 |
±19.4 |
94.8 |
±18.1 |
97.2 |
±18.3 |
0.718 |
|
60 minutes |
95.7 |
±18.7 |
97.4 |
±19.6 |
93.9 |
±17.3 |
95.8 |
±19.7 |
0.744 |
|
Oxygen saturation (SPO2%) at: |
|||||||||
|
0 minutes |
99.7 |
±0.5 |
99.7 |
±0.7 |
99.4 |
±0.7 |
99.5 |
±0.7 |
0.519 |
|
10 minutes |
99.5 |
±0.9 |
99.4 |
±1.2 |
99.5 |
±0.7 |
99.6 |
±0.7 |
0.594 |
|
20 minutes |
99.6 |
±0.7 |
99.5 |
±0.8 |
99.6 |
±0.7 |
99.7 |
±0.6 |
0.559 |
|
30 minutes |
99.6 |
±0.6 |
99.6 |
±0.7 |
99.6 |
±0.6 |
99.6 |
±0.7 |
0.982 |
|
40 minutes |
99.6 |
±0.7 |
99.6 |
±0.6 |
99.6 |
±0.7 |
99.6 |
±0.7 |
0.983 |
|
50 minutes |
99.6 |
±0.7 |
99.2 |
±0.7 |
99.7 |
±0.6 |
99.7 |
±0.6 |
0.303 |
|
60 minutes |
99.6 |
±0.6 |
99.7 |
±0.5 |
99.6 |
±0.6 |
99.5 |
±0.8 |
0.641 |
|
PSSS at: |
|||||||||
|
0 minutes |
2.3 |
±0.7 |
2.2 |
±0.6 |
2.2 |
±0.6 |
2.4 |
±0.8 |
0.235 |
|
10 minutes |
3.2 |
±1.0 |
3.3 |
±1.0 |
3.3 |
±1.0 |
3.2 |
±1.0 |
0.816 |
|
20 minutes |
2 |
±0.1 |
2 |
±0.2 |
2 |
±0.0 |
2 |
±0.0 |
0.366 |
|
30 minutes |
2 |
±0.1 |
2 |
±0.2 |
2 |
±0.0 |
2 |
±0.0 |
0.366 |
|
40 minutes |
2 |
±0.0 |
2 |
±0.0 |
2 |
±0.0 |
2 |
±0.0 |
1 |
|
50 minutes |
2 |
±0.0 |
2 |
±0.0 |
2 |
±0.0 |
2 |
±0.0 |
1 |
|
60 minutes |
2 |
±0.0 |
2 |
±0.0 |
2 |
±0.0 |
2 |
±0.0 |
1 |
*Statistically insignificant differences with ANOVA test at Alpha 0.05
D. Part 3 Side effects
Around 7(7%) of the sample had side effects like delirium 3(3%), excess salivation 3(3%), and obstructed breathing 1(1%).
Comparing the three groups on the incidence of these side effects, no significant differences were detected among them. (Table 5)
Table 5: Side effects observed during the study:
|
Total |
6mg.Kg-1 |
7mg.Kg-1 |
10mg.Kg-1 |
|||||
|
Delirium |
3 |
3.0% |
0 |
0% |
0 |
0% |
3 |
9.1% |
|
Excess Salivation |
3 |
3.0% |
1 |
3.0% |
1 |
2.9% |
1 |
3.0% |
|
Obstructed Breathing |
1 |
1.0% |
1 |
3.0% |
0 |
0% |
0 |
0% |
3. Discussion
Even the most cooperative child often cannot stay still for an imaging scan of prolonged length, an adequate sedation would be a helpful factor in performing high-quality imaging studies in such children, as Charron et al. stated In their book. [15] Therefore there are many studies that used different medications in order to achieve a proper sedation. There is still no completely accurate way to guarantee a smooth induction of anesthesia for children, nor to reduce the anxiety from those critical moments, mainly at the time of separation from the parents, transfer to the study room, during the intravenous access and induction of anesthesia. Choice of premedication or sedative agent is governed by several factors, which include its ease of administration, rapid action, lack of side-effects, and rapid emergence from anaesthesia. Commonly used pediatric premedication methods can be traumatic to children between 1-6 years old, and some increase the risk of respiratory depression. In our study, oral ketamine was chosen for its ease of administration as a mixture with an apple-flavored juice. The taste of the mixture was palatable for most of the children, and the volume of the mixture was limited to a maximum of 10 ml. In a previous prospective, randomized, double-blind placebo-controlled study conducted by Turhano?lu et al., 80 children (ASA 1-11) aged 2-8 years, scheduled for surgery, were given an oral mixture of ketamine (4, 6 or 8 mg.kg-1) and cherry juice, 30 minutes before induction of anesthesia, they stated that it’s safe to administer 10 ml of syrup orally without increasing risk of intraoperative aspiration. [16] In addition, a study by Kaviani et al., found that it is convenient to use 20 ml orange juice containing 0.5 mg.kg-1of oral midazolam, as a premedication 20 minutes before starting the anesthesia, on 62 healthy non-cooperative children, candidate for dental procedures under general anesthesia. [17]
The authors used the Pediatric Sedation State scale (PSSS), that was recorded in the holding area after administration of the sedative mixture and then repeatedly to assess the efficacy of the sedation during different phases of the study. This scale was adopted in a study by Cravero et al. to help measuring the quality and safety of 25 invasive and non-invasive pediatric procedural sedation in different hospital settings. [18] In our study, 6 mg.kg-1 showed the poorest outcomes in comparison to the other two study groups. the poorest outcomes in comparison to the other two study groups. And although 7 mg.kg-1 dose was comparable to the 10 mg.kg-1 dose, the latter group recorded the lowest time lapse during different phases of the study, comparable incidence of side effect and insignificant higher time to discharge statistically, when compared to the rest of groups.This is similar to the finding of Oyedepo et al. in which two different dosages of 5 mg.kg-1 and 10 mg.kg-1 of oral ketamine were compared. Adequate sedation was observed in 52% of children who received 5 mg.kg-1, 68% of those who received 10 mg.kg-1 of ketamine. Anxiolysis was better with 10 mg.kg-1 of ketamine and no emergence phenomenon was noted despite the high dose. [19] The result of Turhano?lu et al. study, showed that the group received oral ketamine 8 mg.kg-1, were significantly calmer than other groups with 4 or 6 mg.kg-1, and anesthesia induction was easier. [20] Similar to other studies, Side effects that we noticed were of short duration and minor significance, in a way that doesn’t affect the stability of the patient. Both delirium and excessive salivation were reported in 3% of the patients and obstructed breathing happened in 1%. Side effects were noted more in the 10 mg.kg-1 group. In a randomized controlled trial on 78 children of ASA I or II scheduled for elective ophthalmic surgery, that was designed by Darlong et al. to evaluate whether the combination of low dose oral midazolam (0.25 mg.kg-1) and low dose oral ketamine (3 mg.kg-1) provides better premedication than oral midazolam (0.5 mg.kg-1) or oral ketamine (6 mg.kg-1), the incidence of excessive salivation was significantly higher in the ketamine alone group (P<0.05). [21] S. ?ekerci et al. who evaluated Ketamine 3 - 6 mg.kg−1 given by mouth to paediatric patients for anaesthetic premedication on 43 children, aged between 2–9 years, found that 3 mg.kg−1 has a decreased incidence of side effects such as nystagmus and vomiting. [22]
4. Conclusion
In conclusion, the results showed the premedication of children may be less sufficient with the lower doses of ketamine (6 mg.kg-1 – 7 mg.kg-1). We found that oral administration of ketamine with 10 mg.kg-1 provides a more rapid onset of satisfactory sedation, with less anxiety in response to separation from parents, face mask application and intravenous line insertion. We suggest that if prolonged recovery period is not an obstacle to the day care unit, oral administration of ketamine 10 mg.kg-1 may be a good choice to use as a single drug for premedication of children, since in practice the mean time to discharge from DCU was only +/- 35 minutes longer than the 7 mg.kg-11 group.
Acknowledgments
The authors are thankful to the MRI and anesthesiology staff of Salmaniya Medical Complex Hospital.
Recommendations for future studies
- Building upon findings of our research, we recommend the use of oral ketamine in a dose of 10 mg.kg-1 as a single sedative agent for non-invasive day-case radiological procedures.
- We also recommend that further studies should be conducted on the use of ketamine orally in other non-invasive and invasive procedures, including the effect of using ketamine orally on the consumption of analgesic and opioid drugs until the discharge of pediatric patients of Salmaniya medical complex (SMC).
Conflict of interest
The authors declare no conflict of interest.
Data Availability
The datasets supporting the finding of this study are available from the corresponding author upon reasonable request.
References
- John Appleby. Day case surgery: a good news story for the NHS. BMJ ; 351 (2015):4060.
- C R Bailey, M Ahuja, K Bartholomew et al. Guidelines for day-case surgery 2019: guidelines from the Association of Anaesthetists and the British Association of Day Surgery. 74 (2019): 778-792.
- Stephen Akau Kache, Danjuma Sale, Jonathan Luka Ajah et al. Paediatric day-case surgery in a new pediatric surgical unit in Northwestern Nigeria. Afr J Paediatr Surg. 15(2018): 97-99.
- Brennan Liam J, Prabhu Atul J et al. Paediatric day-case anaesthesia. BJA CEPD Reviews 3 (2003) 134-138.
- Maddy Artunduaga, C. Amber Liu, Cara E. Morin et al. Safety challenges related to the use of sedation and general anesthesia in pediatric patients undergoing magnetic resonance imaging examinations. Pediatr Radiol.; 51(2021): 724–735.
- Garrett S. Pacheco MD, Angelique Ferayorni DO et al. Pediatric Procedural Sedation and Analgesia. Emergency Medicine Clinics of North America 31: 831-852.
- Ricardo Jorge Dinis-Oliveira. Metabolism and metabolomics of ketamine: a toxicological approach. Forensic Sci Res. 2 (2017): 2–10.
- Mohamed A Hendaus, Fatima A Jomha Ahmed H Alhammadi et al. Is ketamine a lifesaving agent in childhood acute severe asthma? Ther Clin Risk Manag. 12 (2016): 273–279.
- Behrooz Zaman, Siavash Hojjati Ashrafi , Seyedalireza Seyed Siamdoust. The Effect of Ketamine and Dexamethasone in Combination with Lidocaine on the Onset and Duration of Axillary Block in Hand and Forearm Soft Tissue Surgery. Anesth Pain Med 7 (2017): 15570.
- Jasper Kamp, Erik Olofsen, Thomas K. Henthorn. Ketamine Pharmacokinetics: A Systematic Review of the Literature, Meta-analysis, and Population Analysis. Anesthesiology. 133 (2020): 1192-1213.
- Susan Marland, John Ellerton, Gary Andolfatto, et al. Ketamine: Use in Anesthesia. CNS Neuroscience & Therapeutics. 19 (2013). 381-389.
- W Funk, W Jakob, T Riedl, et al. Oral preanaesthetic medication for children: double-blind randomized study of a combination of midazolam and ketamine vs midazolam or ketamine alone. BJA. 84 (2000): 335-340
- Joseph P. Cravero, Nissa Askins, Patcharee Sriswasdi, et al. Validation of the pediatric sedation state scale. Pediatrics. 13 (2017): e20162897
- Joseph P. Cravero, Nissa Askins, et al. MAC-awake of sevoflurane in children. Andrew J Davidson, Aaron Wong, Graham Knottenbelt, et al. Anaesth. 2008 Aug;18 (2008): 702-707
- Kaye, R. Sedation of the pediatric patient. In: Charron, M. (eds) Pediatric PET Imaging. Springer, New York, NY; 2006: 21-29
- Turhanoilu, A. Kararmaz, M. A. Ozyilmaz, et al. Effects of different doses of oral ketamine for premedication of children. European Journal of Anaesthesiology. 20 (2003): 56-60.
- Nasser Kaviani, Mina Shahtusi, Maryam Haj Norousali Tehrani, et al. Effect of oral midazolam premedication on children’s co-operation before general anesthesia in pediatric dentistry. J Dent (Shiraz). 15 (2014): 123-128.
- Joseph P Cravero , Nissa Askins , Patcharee Sriswasdi , et al. Validation of the pediatric sedation state scalePediatrics. 139 (2017): 20162897.
- Oo Oyedepo, Aa Nasir, Lo Abdur-Rahman, et al. Efficacy and safety of oral ketamine premedication in children undergoing day case surgery. J West Afr Coll Surg. 6 (2016): 1-15.
- S Turhano?lu , A Kararmaz, Ozyilmaz, et al. Effects of different doses of oral ketamine for premedication of children. Eur J Anaesthesiol. 20 (2003): 56-60.
- Darlong, D. Shende, M. S. Subramanyam, et al. Oral Ketamine or Midazolam or Low Dose Combination for Premedication in Children.. Anaesth Intensive Care 32 (2004): 246-249.
- ?ekerci, A. Dönmez, Y. Ate?, et al. Oral ketamine premedication in children (placebo controlled double-blind study). European Journal of Anaesthesiology, 13 (1996): 606-611.
