One-Year Outcomes in Patients Who Survived COVID-19-Related Acute Respiratory Distress Syndrome (ARDS): Pulmonary Function Tests (PFTs), Oxygen Requirement, and Quality of Life (QoL)
Article Information
Rami Arfoosh*, 1, 2, Rachel Nisbet1, 2, Kimtuyen Nguyen1, 2, Martin Herrera2, Oluseyi Abidoye2, Nathaniel Kim2, Louise Jones2
1Augusta University, Georgia.
2Northeast Georgia Medical Center Graduate Medical Education, Georgia.
*Corresponding author: Rami Arfoosh. Northeast Georgia Medical Center Graduate Medical Education, Georgia.
Received: 12 December 2023; Accepted: 19 December 2023; Published: 28 December 2023
Citation:
Rami Arfoosh, Rachel Nisbet, Kimtuyen Nguyen, Martin Herrera, Oluseyi Abidoye, Nathaniel Kim, Louise Jones. One-Year Outcomes in Patients Who Survived COVID-19-Related Acute Respiratory Distress Syndrome (ARDS): Pulmonary Function Tests (PFTs), Oxygen Requirement, and Quality of Life (QoL). Journal of Pharmacy and Pharmacology Research. 7 (2023): 251-255.
View / Download Pdf Share at FacebookAbstract
Background: The COVID-19 pandemic saw ARDS, caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), emerge as a predominant factor in patient mortality and morbidity. This study sought to gauge the first-year outcomes of survivors of COVID-19-induced ARDS in terms of pulmonary function, liberation from oxygen and QoL.
Methods: We retrospectively screened 492 patients and analyzed 29 patients from our pulmonary clinic who had post-COVID-19 ARDS. We assessed data points around the 3, 6, and 12-month marks, specifically focusing on pulmonary function, oxygen requirement and QoL using the 36-Item Short Form Survey (SF 36) questionnaire.
Results: Our cohort predominantly consisted of middle-aged males, with none having required invasive mechanical ventilation during their hospitalization. By the 3-month mark, a significant portion displayed reduced diffusion and restrictive patterns, with an ongoing oxygen requirement. Notably, all PFT parameters - diffusion, Forced Vital Capacity (FVC), and Forced Expiratory Volume in 1 second (FEV1) - registered improvement over the course of a year, with most of these enhancements becoming evident by the 6-month period. Both FEV1 and FVC approached normative values by the year's end. Diffusion capacity, despite marked enhancement, remained slightly abnormal at the 12-month evaluation. The proportion of patients on supplemental oxygen also dwindled considerably from 3 to 12 months, with significant reductions already observable at 6 months. Interestingly, while the SF 36 assessment for QoL did not evince consistent improvements across the board, social functioning was an exception, improving over the one-year span. The study began with participants holding an average SF 36 general health score of 63, compared to the population norm of 72.
Discussion: Survivors of COVID-19 ARDS, who didn't necessitate invasive mechanical ventilation, demonstrated significant lung function recovery and a decreasing dependence on oxygen supplementation over a year's duration. However, the SF 36 QoL scores remained relatively static, potentially indicating the impact of non-pulmonary factors on QoL.
Keywords
COVID-19-related ARDS, Pulmonary Function Tests (PFTs), Long-term outcomes, Oxygen requirement, Quality of life (SF 36 scores)
Article Details
1. Introduction
Acute respiratory distress syndrome (ARDS) manifests as an inflammatory lung injury that leads to heightened pulmonary vascular permeability, an increase in lung weight, and a reduction in aerated lung tissue [1]. Typical symptoms include hypoxemia, bilateral radiographic opacities, an elevated venous admixture, and a rise in physiological dead space, culminating in decreased lung compliance. The Berlin Criteria, formulated in 2012, offers the most recent consensus for ARDS definition, focusing on acute onset, radiographic findings, PaO2:FiO2 (P: F) ratio, and the absence of cardiac failure or fluid overload as contributing factors [2-4]. At its core, the pathophysiology of ARDS progresses through five phases: injury, exudative, proliferative, fibrotic, and resolution. Direct and indirect causes underpin the injury phase, but they invariably culminate in inflammatory damage to the lung's endothelial and epithelial cells [5, 6]. A defining feature of ARDS at the cellular level is diffuse alveolar damage (DAD), typified by cellular harm at both the endothelial and alveolar lining levels [7]. This results in fluid and cellular exudation, which can evolve into comprehensive interstitial fibrosis [8, 9]. The concurrent existence of DAD and ARDS can aggravate clinical outcomes. Autopsy studies of patients who succumbed to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) reveal a dominant pattern of diffuse alveolar damage, with associated hemorrhage and notable mononuclear responses [10, 11]. For survivors, recovery can be gradual and might not restore the lung architecture to its original state. Herridge et al. have extensively charted the long-term outcomes of ARDS survivors, especially those in younger age brackets. Findings indicate persistent functional limitations primarily due to factors like muscle wasting, intrinsic pulmonary morbidity, and neuropathy, especially up to one year after ARDS [12-14]. A consistent finding across studies is a lingering reduction in diffusion capacity for carbon monoxide (DLCO) [12,14].
Five years post-ARDS, survivors continue to experience reduced quality of life (QoL), particularly in physical and functional domains. Notably, the etiology of ARDS has an influential role in determining the recovery trajectory and subsequent pulmonary sequelae [14]. The SARS-CoV-2 virus is known to cause lung injury, potentially resulting in ARDS. Preliminary studies have suggested that survivors, especially those with severe illnesses during hospitalization, may experience persistent symptoms and lung function abnormalities, such as fatigue, sleep disturbances, anxiety, and impaired pulmonary diffusion capacities [10,11]. As the world grapples with the COVID-19 pandemic, there's a pressing need to investigate post-COVID-19 ARDS and its recovery course, especially when compared to ARDS from other etiologies.
2. Methods
We conducted a retrospective cohort study on patients aged 18-80 who were admitted from March 2020 to May 2021 due to COVID-19-related Acute Respiratory Distress Syndrome (ARDS). Only those discharged home on supplemental oxygen and who attended follow-up appointments at our designated pulmonary clinic for post-COVID ARDS were included. The diagnosis of ARDS was based on the Berlin definition, with the high Fraction of Inspired Oxygen (FiO2) settings on heated high-flow nasal cannula or required non-invasive mechanical ventilation as a surrogate for the Positive End-Expiratory Pressure/Continuous Positive Airway Pressure (PEEP/CPAP) requirement.
“All methods were approved and performed in accordance with the relevant Institutional Review Board guidelines and regulations at Brenau University. IRB at Brenau University provided exemption waiver on 06/16/2021 for IRB review and informed consent given the retrospective nature of the study”.
2.1 Exclusion Criteria
- Patients with a notable history of pulmonary diseases such as moderate to severe Chronic Obstructive Pulmonary Disease (COPD), interstitial lung diseases, significant emphysema evident with bullae on chest radiographs, prior lung surgeries (whether partial or complete resections), and extrapulmonary restrictive lung disorders.
- Individuals diagnosed with acute pulmonary embolism attributed to COVID-19.
- Patients suffering from post-COVID-19 stroke with lingering impairments.
- Patients with a significant cardiovascular history, especially those diagnosed with Heart Failure with Reduced Ejection Fraction (HFrEF), indicated by a Left Ventricular Ejection Fraction (LVEF) <50%, or moderate to severe pulmonary hypertension, marked by a Right Ventricular Systolic Pressure (RVSP) ≥ 35mmHg.
- Patients who were incapable of undergoing Pulmonary Function Tests (PFTs). However, if such patients were able to complete the quality-of-life questionnaire, their data were incorporated.
- Individuals with a Body Mass Index (BMI) exceeding 45.
- A history of pulmonary cancer necessitating interventions like surgical resection, radiation, or chemotherapy.
- Patients with malignancies in the past year that demanded treatments such as systemic chemotherapy or radiation therapy.
Following the application of these criteria, we meticulously reviewed patient charts to collate data pertaining to oxygen requirements, Pulmonary Function Tests (PFTs), and Rand 36-Item Short Form Survey Instrument (SF 36) scores, when available. These were tracked across three intervals: 1-3 months, 3-6 months, and 6-12 months post-admission. Our study obtained the requisite approval from the institution's research oversight board. The utilized Rand 36-Item Short Form Survey Instrument (SF 36), stands as a rigorously validated tool [15]. It gauges eight health domains, each scored between 0 and 100. The dimensions it assesses encompass general health, physical function, roles influenced by physical health, roles influenced by emotional health, vitality, mental health, social functionality, and bodily pain.
3. Results
3.1 Study Population
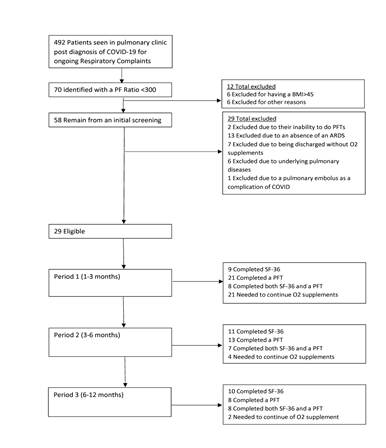
Of the 492 patients initially screened, 70 were diagnosed with Acute Respiratory Distress Syndrome (ARDS). After the application of the established inclusion and exclusion criteria, 29 patients qualified for the study. This cohort comprised mainly of men, with a count of 22, compared to 7 women. It's noteworthy that none of the participants required invasive mechanical ventilation (IMV) during their hospitalization. This observation is likely attributed to the low survival rates and dismal prognosis of those who did necessitate such intervention. The mean age of the participants was 63.1 years, split into 62.1 years for women and 63.6 years for men. The overall mean Body Mass Index (BMI) was recorded at 32.9 (±5.57), with women averaging at 34.8 (±5.9) and men at 32.3 (±6). The average duration of hospital length of stay (LOS) for this group spanned 15.6 days, as detailed in Table 1.
Table 1: Patients demographics.
|
Women |
Men |
Total |
|
|
n |
7 |
22 |
29 |
|
Mean age (SD) |
62.1 (10.9) |
63.6 (12.1) |
63.13 (11.6) |
|
Mean BMI (SD) |
34.8(5.9) |
32.3 (6) |
32.9 (6) |
|
Mean LOS (SD) |
12.9 (10.3) |
16.5 (11.1) |
15.6 (10.9) |
|
Caucasian |
5 |
17 |
22 |
|
Hispanic |
1 |
0 |
1 |
|
Other |
1 |
3 |
4 |
|
Asian |
0 |
2 |
2 |
|
NC |
3 |
1 |
4 |
|
HHFNC |
1 |
11 |
12 |
|
HHFNC and NIPPV |
3 |
10 |
13 |
Abbreviations: BMI = Body Mass Index; LOS = Length of Stay; NC = Nasal Cannula; HHFNC = Heated High-Flow Nasal Cannula; NIPPV = Non-Invasive Positive Pressure Ventilation.
3.2 Follow-up Data Collection
The completeness of collected data varied across the three timeframes: 1-3 months, 3-6 months, and 6-12 months post-discharge. The enrollment chart and specifics of data acquisition at each interval are illustrated in Figure 1.
Abbreviations (Figure 1): COVID-19 - Coronavirus Disease 2019; BMI - Body Mass Index; PFTs - Pulmonary Function Tests; ARDS - Acute Respiratory Distress Syndrome; SF-36 - 36-Item Short Form Survey Instrument.
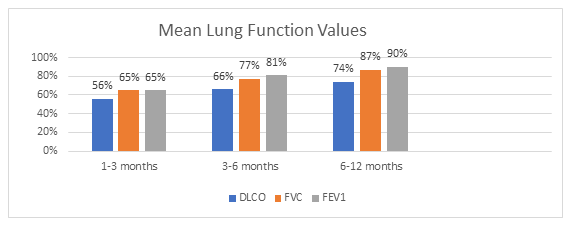
3.3 Pulmonary Function
The primary indicators assessed from the pulmonary function tests were DLCO, FVC, and FEV1. All three parameters manifested improvement over the course of the study. Both FVC and FEV1 readings normalized by the 6–12 month interval (Figure 2). It is crucial to note that this study is retrospective in nature. Patients with normal pulmonary function testing during a specific period were likely not retested in subsequent periods. In such cases, we imputed the normal values into the subsequent periods when calculating the mean values for each parameter applying the known concept in handling missing data, Last Observation Carried Forward (LOCF).
Abbreviations (Figure 2): DLCO - Diffusion Capacity of the Lung for Carbon Monoxide; FVC - Forced Vital Capacity; FEV1 - Forced Expiratory Volume in 1 second.
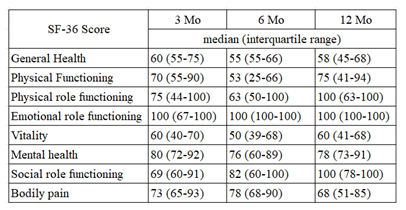
3.4 Quality of Life Assessment
The SF36 scores presented a more nuanced picture. Due to the limited sample size, drawing definitive conclusions was challenging. Nevertheless, a potential uptrend was noted in the social functioning domain (Figure 3).
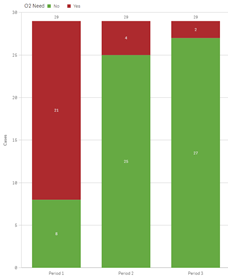
3.5 Oxygen Dependency:
The number of patients reliant on supplemental oxygen showed a consistent decline over time. Initially, only 7 out of the 29 patients were independent of oxygen support during the 1-3 month window. This number increased to 25 in the 3-6 month bracket, and by the final 6-12 month timeframe, a notable 27 out of the 29 patients no longer required oxygen supplementation (Figure 4).
4. Discussion
Our study undertook an assessment of both pulmonary physiological changes and the self-reported experiences of patients post-Acute Respiratory Distress Syndrome (ARDS) resulting from Coronavirus Disease 2019 (COVID-19) over a span of 12 months. While there was a marked decline in pulmonary function post-infection, our findings indicate a steady improvement in these parameters throughout the year. Interestingly, while physiological metrics improved, the 36-Item Short Form Survey (SF36) scores didn't showcase a consistent trend. Due to the limitations in sample size, some values reached 100%, surpassing expected measurements. One significant physiological observation was the very high rate of liberation from oxygen dependency for most patients by the close of the first year. However, this study does have its limitations. The limited number of participants restricts the scope for in-depth statistical analysis, allowing only for the identification of general trends using descriptive analysis. Additionally, the gaps in the collected data, inherent to the study's observational nature, further compound this challenge. Currently, there's no established guideline on the ideal follow-up regimen for patients recovering from COVID-induced ARDS, which adds another layer of complexity to our analysis.
5. Conclusion
Among patients with Coronavirus Disease 2019 (COVID-19)-induced Acute Respiratory Distress Syndrome (ARDS) who did not require invasive mechanical ventilation (IMV), our one-year follow-up emphasizes a notable recovery in lung function and a considerable rate of independence from oxygen supplementation. Nonetheless, despite these physiological advancements, the 36-Item Short Form Survey (SF36) quality of life questionnaire scores did not exhibit significant improvement. This might highlight the impact of non-pulmonary factors on quality of life, an aspect previously documented in other ARDS studies [11].
Acknowledgements
We would like to acknowledge the assistance provided by Kate Maleki, Paul Takla, Shane Robinson, and Zain Arfoosh in technical support; and data collection and management for this project.
List of Abbreviations
- ARDS - Acute Respiratory Distress Syndrome
- SARS-CoV-2 - Severe Acute Respiratory Syndrome Coronavirus 2
- QoL - Quality of Life
- SF 36 - 36-Item Short Form Survey
- P:F ratio - Ratio of arterial oxygen partial pressure to fractional inspired oxygen
- DAD - Diffuse Alveolar Damage
- IMV - Invasive Mechanical Ventilation
- BMI - Body Mass Index
- LOS - Length Of Stay
- NC - Nasal Cannula
- HHFNC - Heated High-Flow Nasal Cannula
- NIPPV - Non-Invasive Positive Pressure Ventilation
- PFTs - Pulmonary Function Tests
- DLCO - Diffusing Capacity of the Lung for Carbon Monoxide
- FVC - Forced Vital Capacity
- FEV1 - Forced Expiratory Volume in 1 second
- COVID-19 - Coronavirus Disease 2019
Declarations
Ethical Approval
Brenau University IRB waiver, Date 06/16/2021.
Funding
None.
Availability of data and Materials
Any data or material can be requested by contacting the Research and Scholarly GME office at Northeast Georgia Medical Center at GMEResearch@NGHS.com or the corresponding author at rarfoosh@PulmonarySleepMed.com.
References
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults Lancet 2 (1967): 319-23.
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. Report of the American-European Consensus Conference on acute respiratory distress syndrome: Definitions, mechanics, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149 (1994): 818-24.
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 307 (2012): 2526-33.
- Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 38 (2012): 1573-82.
- Capelozzi VL, Allen TC, Beasley MB, Cagle PT, Guinee D, Hariri LP, et al. Molecular and Immune Biomarkers in Acute Respiratory Distress Syndrome: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 141 (2017): 1719-27.
- Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress. Clin Chest Med 21 (2000): 435-66.
- Pablo Cardinal-Fernández, José A Lorente, Aída Ballén-Barragán, Gustavo Matute-Bello. Acute Respiratory Distress Syndrome and Diffuse Alveolar Damage. Ann Am Thorac Soc 14 (2017): 844-850.
- Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6 (2011): 147-63.
- Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage: the role of oxygen, shock, and related factors. Am J Pathol 85 (1976): 209-28.
- Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 8 (2020): 681-6.
- Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matté A, Barr A, et al. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 167 (2003): 690-4.
- Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348 (2003): 683-93.
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364 (2011): 1293-304.
- Wong AW, Ryerson CJ, Guler SA. Prolonged benefit from physiotherapy in patients with severe COVID-19. Respirol Case Rep 8 (2020): e00679.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30 (1992): 473-483.