Obstructive Sleep Apnea in Pregnancy – Development, Impact and Potential Mechanisms
Article Information
Hannah Martin1, Kathleen M Antony2, Sathish Kumar1, 2, 3*
1Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin, Madison, USA
2Department of Obstetrics and Gynecology, School of Medicine and Public Health, University of Wisconsin, Madison, USA
3Endocrinology-Reproductive Physiology Program, University of Wisconsin, Madison, USA
*Corresponding author: Dr. Sathish Kumar, Associate Professor, Departments of Comparative Biosciences and Obstetrics and Gynecology, University of Wisconsin, Linden Drive, Madison, WI 53706, USA
Received: 01 November 2020; Accepted: 09 November 2020; Published: 25 November 2020
Citation:
Hannah Martin, Kathleen M Antony, Sathish Kumar. Obstructive Sleep Apnea in Pregnancy – Development, Impact and Potential Mechanisms. Journal of Women’s Health and Development 3 (2020): 446-469.
View / Download Pdf Share at FacebookAbstract
Obstructive sleep apnea (OSA) is a common sleep disturbance shown to lead to significant morbidity in the general population. The development of sleep disturbances during pregnancy is potentially linked to anatomical and physiological changes during gestation, such as increased weight, uterine volume, the elevation of the diaphragm, upper airway remodeling, and changes in hormones such as estrogens and progesterone. During pregnancy, it has been reported that OSA is associated with adverse outcomes for the mother and offspring, though precise underlying mechanisms have not yet been elucidated. OSA during pregnancy has been associated with an increased risk of preeclampsia, gestational hypertension, gestational diabetes mellitus (GDM), perinatal depression, and effects on the fetus, such as impaired growth, congenital abnormalities, and neurobehavioral conditions. Several reports have supported these associations in pregnant women, and further study has revealed potential mechanisms of disease to include oxidative stress, autophagy, inflammation, and endothelial dysfunction. The purpose of this review is to outline the known associations between gestational OSA and adverse peri- and postnatal outcomes, collate evidence supporting potential underlying mechanisms, and highlight gaps in the knowledge to create a comprehensive overview of the effects of OSA in pregnancy and suggest where further research should be aimed.
Keywords
Pregnancy, Sleep apnea, Perinatal outcomes, Fetal outcomes, Gestational diabetes mellitus, Preeclampsia
Pregnancy articles; Sleep apnea articles; Perinatal outcomes articles; Fetal outcomes articles; Gestational diabetes mellitus articles; Preeclampsia articles
Article Details
1. Introduction
All mammalian species and vertebrates like birds [1, 2] and some invertebrates [3], use sleep or sleeplike states. Sleep is defined as a reversible quiescent state during which sensitivity to environmental stimuli is decreased. The conservation of sleep and sleeplike states throughout evolution and across many species indicates its importance in survival. Mammals spend approximately one-third of their lives asleep [4], and extreme sleep deprivation can lead to death in experimental settings [3]. Sleep deprivation (insomnia), sleep fragmentation, and disruption of the sleep circadian rhythm are collectively termed 'sleep deficiencies' [5], and morbidity is greatly affected by these conditions. For example, functional cognitive and neurological impairments can result from sleep deprivation or disruption [6, 7]. 7-9 hours of sleep per 24 hour period is the sleep duration with the lowest morbidity and mortality rates in humans [5, 8-10]; however a recent Sleep in America® poll conducted by the National Sleep Foundation found that most adults have an inconsistent sleep schedule and do not sleep for the same duration every night, suggesting that most are not practicing healthy sleep behaviors and may be at a higher risk of morbidity [11].
It is estimated that sleep-related problems affect 50 to 70 million Americans of all ages and socioeconomic classes. The National Institutes of Health predicts that sleep deprivation is on the rise and that by the middle of the 21st century, more than 100 million Americans will have difficulty falling asleep [12]. A PubMed search using the keywords ‘pregnancy, gestational and obstructive sleep apnea’ generated 339 publications. These were manually screened to identify 118 full-length papers reporting an association between obstructive sleep apnea (OSA) during pregnancy and unfavorable outcomes for either the mother or offspring. This review aims to consolidate and present the current literature describing this link and identify potential underlying mechanisms.
2. Development of OSA in Pregnancy
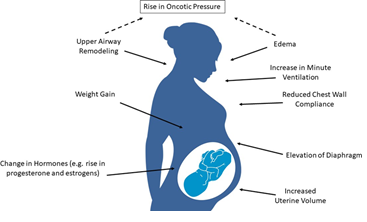
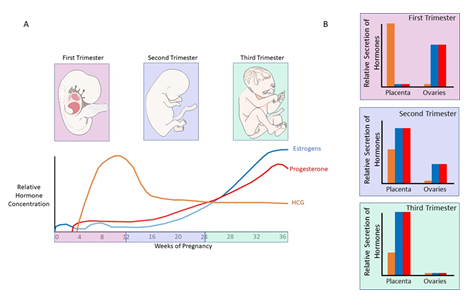
Evidence shows that pregnant women are at a higher risk of developing OSA due to pregnancy- related anatomical and physiological changes (Figure 1). The most significant anatomical changes during pregnancy that may predispose women to OSA include increased weight (up to 20% increase) and increased uterine volume. Elevation of the diaphragm is also affected by the uterine size, leading to impairment of respiration [13]. Similarly, upper airway remodeling and edema during pregnancy are associated with a decrease in oncotic pressure, and this along with reduced functional residual capacity and chest wall compliance may increase the risk of the pregnant woman developing (or the worsening of preexisting) OSA [14]. Hormonal changes during pregnancy such as elevated progesterone and estrogens have also been shown to contribute to sleep disturbances. These hormones are at their peak in the third trimester of pregnancy, suggesting a link between the elevation of estrogens and progesterone and the more severe phenotype of OSA reported in the later stages of pregnancy (Figure 2) [15-18].
Figure 2: Hormonal changes during pregnancy. A: Relative hormone concentrations of human chorionic gonadotro-pin (HCG), estrogens and progesterone during pregnancy. HCG begins to rise during the first trimester, peaking as the second trimester begins before falling to around 60% of peak concentration approximately a month later, where its concentration plateaus. Estrogens and progesterone have similar patterns during pregnancy both begin to rise during the first trimester, though estrogens increase earlier and reach a higher peak, and gradually increase in concentration to reach at peak at the end of the third trimester, near the time of birth. B: Relative secretion of hormones from the placenta and ovaries during pregnancy. In the first trimester, HCG is released in high concentrations from the plac-enta, whereas estrogens and progesterone are secreted from the ovaries. During the second trimester, the placenta produces increased levels of estrogens and progesterone, though a proportion of these hormones are still secreted from the ovaries. The initial rise of HCG secretion from the placenta also begins to fall. Lastly, during the third trimester there is little hormone secretion from the ovaries, and the placenta produces high levels of estrogens and progesterone, along with attenuated levels of HCG. Figure created using elements provided by Servier Medical Art.
Many studies indicate that there is commonly an increase in the frequency of awakenings during pregnancy during the night, overall shorter sleep duration and high levels of daytime sleepiness compared to sleep patterns pre-pregnancy [19]. In response to these findings, the American Academy of Sleep Medicine (AASM) has established ‘pregnancy-associated sleep disorder’ as a separate entity [20]. Sleep-disordered breathing ranges from snoring at the mild end of the spectrum to overt OSA at the severe end. OSA is characterized by repetitive episodes of upper airway obstruction during sleep, which results in a reduction of airflow, hypoxemia, sympathetic discharge, and recurrent arousals from sleep. This leads to fluctuations in oxygen saturation levels, which has several downstream effects [21]. Data from the National Sleep Foundation of America in 2014 states that more than 79% of pregnant women reported sleep alterations or sleep disorders that were not present before pregnancy. In contrast, a more recent study estimated that sleep deficiencies occur in only 10-32% of all pregnancies [22]. The discrepancies between these studies highlight the need for standardized protocols for the methods of screening, diagnostic criteria and populations studied, as there is documented evidence of large variations in the prevalence of OSA and other sleep disorders during pregnancy in different populations. In the non-pregnant population, data shows that OSA incidence exceeds 10% in pre-menopausal women, indicating that a proportion of pregnant women enter pregnancy with undiagnosed or unrecognized OSA [23]. This is exacerbated by the fact that in the United States, almost half of women entering pregnancy are either overweight or obese [24]. The presence of obesity itself in pregnancy is associated with several vascular complications such as hypertension, and obstetric complications such as prolonged labor and an increased risk of postpartum hemorrhage in vaginal deliveries [25].
Several studies have also demonstrated that pregnant obese women are at a significantly higher risk of developing OSA than lean pregnant controls. In the general pregnant population, it has been shown that OSA risk increases as pregnancy develop, with up to a third of women experiencing symptoms of OSA in the third trimester [26]. However, in obese women, it has been suggested that OSA development also occurs frequently in the earlier stages of pregnancy. In a pregnant population with a BMI >30, it is reported that the prevalence of mild, moderate and severe cases of OSA in the first trimester were 21%, 6% and 3%, increasing to 35%, 7% and 5% in the third trimester, respectively [27]. As the majority of OSA cases in pregnancy are diagnosed in the third trimester, it is likely that obese pregnant women with early-onset gestational OSA may be overlooked and therefore left untreated until the third trimester. Overall, the prevalence of OSA in obese pregnant women is not clear due to the previously mentioned differences in study design, patient populations and outcome, which make studies difficult to compare. A prospective observational study of 175 obese pregnant women reported that 15.4% of participants had OSA based on AASM diagnostic criteria, noting that the OSA group had higher BMIs than those without OSA [28]. However, studies have reported the prevalence of OSA in obese pregnant women to be as high as 43.3% [25] and 50% [27], and one study found that compared to lean pregnant women (<25 kg/m2), overweight (25-29.9 kg/m2) and obese (>30 kg.m2) pregnant women are at a 4.69- and 13.23-fold higher risk of OSA, respectively [29].
Studies have also reported that OSA disproportionately affects women with low socioeconomic status and women who self-identify as Black [30, 31]. Longitudinal studies indicate that the prevalence of OSA increases through gestation. The prevalence of OSA was found to be 3.6 to 10.5% in early pregnancy and increased to 26% in the third trimester [27, 32, 33]. A recent meta-analysis compared the estimated prevalence of gestational OSA in thirty-three studies. Although the pooled worldwide average of maternal OSA incidence was 15% (95% CI 12-18%), this was found to vary significantly between populations; studies using European populations reported incidences as low as 5%, whereas studies conducted in the Americas estimated a prevalence of up to 20%. One explanation for such variance may be due to the differing methods of assessment and assessment criteria utilized. Liu et al [34] reported differences in gestational age at assessment (between 21.2 and 37.3 weeks), definition of hypopnea used (prevalence estimates using AASM 1997rec and 2007rec definitions were higher than those using the AASM 2007alt definition (22%, 24% and 15%, respectively)) and study design (prospective, prospective/case-control or retrospective) [34]. The most common methods of assessment were attended or unattended polysomnography (PSG), but several studies used alternative methods such as the WatchPat device [35] (Israel), RUSleeping meter [36] (UK) and a pulse oximeter [37, 38] (Japan), [39] (UK). Similarly, diagnostic criteria was also varied; most studies used either AHI or ODI (oxygen desaturation index), whereas others used ICD-9 or -10 classification or respiratory disturbance index (RDI). The meta-study authors also highlight a lack of data from Southeast Asian, African and Eastern Mediterranean regions and sample size for the included studies ranged from just 16 to over 1.5 million subjects [40, 41] (USA). Overall, these data show the need for standardized protocols for studies investigating OSA incidence in pregnancy, as comparisons between many of these studies are impossible due to large variations in almost every facet of study design and implementation.
3. Impact of OSA in Pregnancy
Epidemiological studies have evaluated the effect of OSA during pregnancy on pregnancy outcomes, and several have reported that OSA during pregnancy is associated with increased maternal and fetal morbidities.
3.1 Placental Development
The placenta is crucial for a successful pregnancy and is an important determinant of birth weight [42-44]. As the critical organ for fetal growth, the placenta is responsible for important functions during gestation, such as transport of nutrients and oxygen, inflammatory mediator release, hormone and growth factor release, and any adaptive responses resulting from environmental cues stressors [45]. Maternal OSA has been found to be associated with increased placental weight, which is correlated with OSA severity and neonatal adiposity (body fat) of the offspring independent of the BMI of the mother [46]. A greater placental weight often results in a decreased fetal-placental ratio, which is used as a proxy measure for placental efficiency; thus, a reduction suggests an impaired nutrient transport ability of the placenta, which can influence the development of negative obstetric outcomes [46-48].
3.2 Gestational Hypertension and Preeclampsia
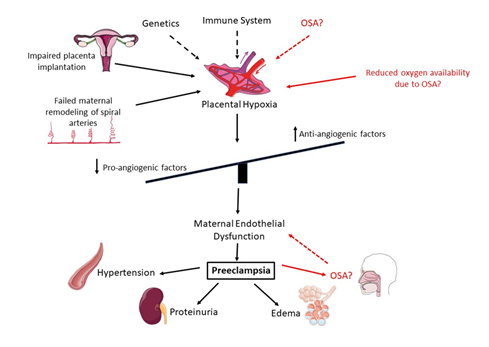
OSA during pregnancy has been linked with the development of preeclampsia (PE) and gestational hypertension [49]. PE is a serious complication of pregnancy, characterized by hypertension and proteinuria (Figure 3), and treatments for PE can help manage the symptoms and reduce the risk of serious complications, but there is no cure except delivery of the fetus and placenta.
Figure 3: Development of preeclampsia (PE). Genetic factors and heightened inflammation are thought to predispose pregnant women to PE. These factors increase the risk of failed maternal remodeling of spiral arteries important for oxygen and nutrient exchange to the fetus and defective placental implantation, resulting in reduced oxygen availability and placental hypoxia. This initiates an imbalance of pro- and anti-angiogenic factors, which results in endothelial dysfunction of maternal cells. Endothelial dysfunction can result in several adverse effects, in this case the most common being hypertension, proteinuria and edema. In red is the potential role for OSA in the development of PE. OSA may result in reduced oxygen availability, which would lead to placental hypoxia and the downstream effects. However, PE itself may also increase the risk of developing OSA due to edema. The precise interactions between OSA and PE are currently unknown. Figure created using elements provided by Servier Medical Art.
Snoring and daytime sleepiness (both symptoms of OSA) are more common in pregnant women with PE than normotensive pregnant and nonpregnant controls [32] and several studies have reported an increase in PE and gestational hypertension among pregnant women with OSA after adjusting for BMI and diabetes [50]. In one study, pregnant women with diagnosed OSA were found to have a higher incidence of PE (19%), compared to both obese (11%) and normal weight (7%) controls [51]. Olivarez et al. [52] confirmed this association in a large study of over 1000 participants, reporting that OSA is a significant independent risk factor for PE [52] and a following 2014 retrospective review of five small cohort studies reported that PE is twofold higher in women with OSA (adjusted OR 2.34, 95% CI 1.60-3.09) [53]. A large prospective multicenter cohort of the Nulliparous Pregnancy Outcomes Study (nuMoM2b) that included 3306 pregnant women reported an increased risk for PE among women found to have sleep apnea in either early- pregnancy (odds ratio (OR) 1.94, 95% confidence interval (CI) 1.07-3.51) or mid-pregnancy (OR 1.95, 95% CI 1.18-3.23) [54]. This study also reported that women with severe OSA (apnea hypopnea index (AHI) > 15/h) showed a greater risk of PE adjusted (aOR) = 4.27 (95% CI, 1.74 – 10.45) than pregnancies had mild-moderate OSA (AHI 5–14.9/hour) (aOR 1.94 (95% CI 1.07–3.51)) indicating a severity-dependent impact [54]. These reports were consistent with other smaller retrospective and prospective cohort studies that found a 2-fold increased adjusted odds of PE in association with OSA [53, 55]. The largest cohort study of women with OSA (n = 791) found that women with preexisting OSA had an increased risk of PE (OR 1.6, 95% CI 2.16-11.26) compared with women without an OSA diagnosis [56]. Another retrospective cohort study of all women who gave birth at a military treatment facility (n=305,001) reported that women with an OSA diagnosis were more likely to have gestational hypertension (aOR, 2.46; 95% CI, 1.30–4.68), PE (aOR, 2.42; 95% CI, 1.43–4.09), and/or preterm delivery (aOR, 1.90; 95% CI, 1.09–3.30) [57]. Gestational OSA has also been shown to lead to more severe maternal morbidity. A large inpatient database study reported that pregnant women who have OSA at the time of delivery had a higher risk of cardiomyopathy (aOR: 9.0), congestive heart failure (aOR: 8.94), and pulmonary embolism (aOR: 4.5). These findings were found to overlap significantly with the incidence of obesity in these patients, yet still found significant results when controlling for BMI, suggesting that OSA is associated with an increased risk of cardiovascular complications independent of obesity [49]. Interestingly, a small study has reported that the use of nasal continuous positive airway pressure (CPAP), the first-line therapy for OSA, successfully reduced mean nocturnal blood pressure in 11 women with severe PE [58]. However, there have been no large-scale studies determining CPAP's effect on maternal and fetal outcomes following gestational OSA.
3.3 Perinatal Depression
It is widely known that sleep disturbances are a common symptom of depression, but more recent studies have also shown that self-reported sleep disturbances or inadequate sleep can contribute to the development of mood disturbances [59]. Patients with OSA, in particular, have been found to have increased levels of depression, and conventional OSA treatments such as CPAP can reduce both the symptoms of depression and the need for antidepressants [60, 61]. Depressive symptoms are also common in women during pregnancy; it has been reported that between 6.5% and 11% of third-trimester pregnancies in the developed world are affected by depressive disorders, and this rises to between 7% and 15% in the 6 months following birth [62]. Depression itself is linked to several adverse pregnancy outcomes similar to those we see in sleep apnea during pregnancy, including the risk of preterm birth and low birth weight of offspring [63, 64]. OSA during pregnancy has been linked to a personal history of depression and in the third- trimester, the odds of depressive symptoms are eight times higher than in controls [65]. Women with no history of depression were also found to have an increase in depressive symptoms during pregnancy when they presented with OSA, and those with a history of depression were observed to have more severe depressive symptoms when OSA was present [65]. A meta-analysis of pregnant women found a significant relationship between OSA and perinatal depressive symptoms (point estimate, 0.366; 95% CI), 0.205-0.508) [66].
3.4 Gestational Diabetes Mellitus (GDM)
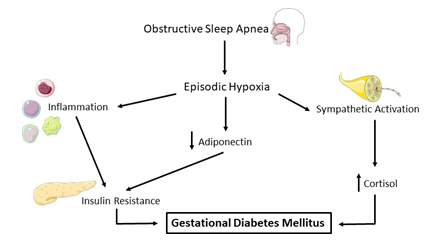
In the nonpregnant population, OSA has been found to be associated with a high frequency of type-2 diabetes and impaired glucose tolerance. Meslier et al [67]. found that in a cross-sectional study, fasting and post-load blood glucose increased with the severity of sleep apnea, and insulin sensitivity decreased with increasing OSA severity. These findings were independent of both BMI and age [67]. OSA involves increases in both inflammation and sympathetic activation, which have been linked to an increased risk of GDM (Figure 4) [68, 69]. A study examining the link between GDM and OSA reported that OSA was significantly more prevalent in GDM patients, compared to controls (22% vs. 9%, and in individual models found that women with OSA are at higher risk of GDM (OR:4.71) based on variables such as high AHI, higher AHI during REM sleep and higher oxygen desaturation index [70]. A 2014 systematic review observed that pregnant women with OSA were at increased risk of developing GDM ((aOR) 1.86, 95% CI 1.30-2.42; five studies) [50, 71, 72]. Prospective data from the large nuMoM2b cohort confirmed that GDM was associated with OSA in early pregnancy (OR 3.47, 95% CI 1.95-6.19) or mid-pregnancy (OR 2.79, 95% CI 1.63-4.77) [54]. More recently, Izci Balserak [70] has shown that in a small population of women with newly-diagnosed GDM, OSA was present in significantly more cases than controls. In individual models, GDM risk was found to be significantly higher in women with higher AHI, AHI during rapid eye movement (REM) sleep, and an oxygen desaturation index of ≥4%. Additionally, not only was GDM specifically associated with AHI during REM sleep, the percentage of non-REM sleep was significantly associated with a decreased risk of GDM, suggesting that the relationship between glucose metabolism and OSA may be dependent on sleep state [70]. It has been reported that in a cohort of diet-controlled women with GDM, severe OSA is significantly associated with higher fasting glucose (correlated with increased oxygen desaturation), insulin resistance, and β-cell dysfunction [73]. Independent of other risk factors, individuals with OSA have an increased risk of developing GDM and hyperinsulinemia. Although the causal link has not been proven, improved glucose control has been observed in nonpregnant patients after initiating OSA treatment with CPAP [74, 75].
Figure 4: OSA and GDM. Obstructive sleep apnea (OSA) is characterized by episodic hypoxia, which has downstream effects including immune system activation and sympathetic activation. Heightened inflammation can directly affect insulin resistance, leading to gestational diabetes mellitus (GDM). Further, hypoxia induces a decrease in adiponectin and an increase in the stress hormone cortisol, both of which have been linked to increased risk of GDM. Figure created using elements provided by Servier Medical Art.
4. Adverse Fetal Effects
4.1 Congenital abnormalities
A recent study was the first to examine the effect of OSA and other sleep-disordered breathing on fetal congenital anomalies. Bourjeily et al [76] have reported that OSA was associated with a higher risk for several congenital anomalies in offspring, including musculoskeletal (OR: 1.89), ophthalmologic (OR: 1.84), gastrointestinal (OR: 1.80) and circulatory (OR: 1.37) abnormalities. These associations were significant even after adjustment for obesity, diabetes, alcohol, tobacco, and drug use [76]. Interestingly, after adjusting for covariates, this study did not report a significant association between OSA and small for gestational age offspring. Another recent study showed maternal OSA was associated with shorter telomere lengths in the DNA collected from cord blood [77]. Although shorter telomere length has been observed in the DNA of adults with OSA, no study has yet investigated the link between OSA during pregnancy-mediated short telomere length and infant health.
4.2 Fetal growth and birth weight
Adverse neonatal outcomes linked to gestational OSA include prematurity and growth restriction [49, 56], and offspring are also more likely to require admission to an intensive care unit [78]. An association between OSA and impaired fetal growth have been reported in case reports and retrospective studies, although confounders such as PE were generally present [56, 79-81]. After controlling for conditions that predispose fetal growth restriction such as hypertension, the association persisted with maternal OSA associated with a 1.5 to 2-fold increased frequency of low birth weight and small for gestational age infants [53, 55, 83]. In a prospective cohort study of women who underwent a home sleep test, OSA was found to be associated with small-for-gestational-age babies (OR 2.65, 95% CI 1.15-6.10) and a systematic review of sleep-disordered breathing in pregnancy agreed but specified that OSA was associated with only a modest increase in small for gestational age babies (<2500 g, OR 1.67, 95% CI 1.00-2.78) [83]. This study also reported an increased risk of stillbirth or perinatal death in sleep-disordered pregnancies (OR 2.02, 95% CI 1.25- 3.28) [83]. Conversely, Telerant et al. [84] reported an association between OSA during the third trimester and accelerated fetal growth [84]. A 2016 study complemented this with the reported association between sleep-disordered breathing and development of large for gestational age babies in a Chinese population [85]. This association may be partially explained by the high rates of maternal obesity and diabetes in this cohort, two risk factors for large for gestational age infants. Therefore, more stringent studies with strict inclusion criteria of different forms of sleep-related breathing disorders, and controls on study cofounders, such as maternal obesity and other complications of pregnancy, are needed to fully elucidate these associations.
4.3 Fetal cardiovascular effects
Apneas are thought to have a direct effect on fetal heart rate. In general, late fetal decelerations (heart rate falling below the fetal baseline) are known to occur when there is a fall in fetal blood oxygen level, triggering chemoreceptor in the fetus to divert blood flow to vital organs such as the brain, heart and adrenal glands. Blood flow is diverted via the reflex constriction of blood vessels serving non-vital peripheral organs. However, this vasoconstriction contributes to the development of hypertension, which in turn stimulates a baroceptor mediated vagal nerve response, slowing heart rate [86, 87]. If the hypoxia is sustained, acidosis can develop in fetal tissues due to increased anaerobic metabolism. A significant increase in acidemia (acid in the blood) can depress the fetal nervous system and myocardium, causing adverse effects on the development of the heart, abnormal fetal heart rate, and reduced cardiac performance [88]. Several reports suggest the presence of these acute fetal decelerations in response to maternal OSA episodes [80, 89, 90]. However, these results were collected from clinical observations rather than via polysomnographic data and may not be reliable. Using polysomnography, a small study of four pregnant women with OSA reported late fetal decelerations in relation to apneic episodes [91], but the findings were not adjusted for several comorbidities such as GDM and cardiovascular disease, again making clear associations difficult. Indeed, a 2010 study investigating OSA during pregnancy and fetal heart rate found no relationship between OSA-related hypoxic episodes and fetal heart decelerations [92].
4.4 Fetal respiratory effects
Fetal breathing movements are detected as early as 11 weeks of gestation in humans [93], and continue to increase until late pregnancy when the fetus performs breathing motions up to 30% of the time [94]. This suggests that a wide time period of development is susceptible to acute changes in maternal oxygen levels [93, 95]. In rat models, there is evidence to suggest that the timing of intermittent hypoxia (IH) is an important determinant of offspring outcome. Offspring from pregnancies where IH was present from embryonic day (ED) 0.5 to birth did not show alterations in the diaphragm [96]. In contrast, those exposed to IH from ED 5.0 to birth had higher ventilatory responses. Furthermore, these effects were still present into adulthood (tested at 4 months of age) [89]. More evidence of the effect of OSA on respiratory neural activity was presented by Johnson et al [97], who examined brainstem-spinal cord preparations from rat offspring exposed to gestational IH and reported increases in burst rhythm and a trend towards a lower burst rate compared to controls [97]. This suggests that offspring exposed to gestational IH may have disruptions in respiratory rhythm and pattern-generating neural circuitry.
4.5 Fetal neurobehavioral effects
Recent evidence has suggested that the infants of pregnant women who report increased daytime sleepiness have decreased executive function and increased neuropsychiatric problems in early childhood [98]. Additionally, offspring exposed prenatally to PE have a significantly higher risk of developing a neuropsychiatric condition during childhood, such as cerebral palsy [99]. Given the previously described associations between OSA and PE, it is possible that gestational OSA may also lead to the development of neuropsychiatric conditions in offspring; however, a more in-depth study of these links is needed to determine causality. A small study followed the offspring of seventy-four mothers who experienced OSA during pregnancy found no difference in general motor scores, but there was an increased frequency of low social development scores at 1 year of age [100]. Further, a 2017 meta-analysis reported associations between maternal apnea and low test reading scores in 6-9 years of age children, based on Year 3 exam educational records obtained from public schools in Australia [101]. Interestingly, this study also reported that offspring had increased hospital admissions partly due to the development of pediatric sleep apnea, suggesting that maternal OSA is also associated with the development of OSA in offspring. [101].
5. Potential Underlying Mechanisms
Although many clear associations have been made between OSA and adverse outcomes for both mothers and their offspring, there is still limited knowledge of the pathophysiological mechanisms that link OSA to adverse pregnancy outcomes. Understanding the risk, commencement, and development of OSA on a biochemical, endocrine, and physiological level is important to interpret symptoms and outcomes further. Identifying processes and pathways novel to OSA may serve as targets for future treatments or risk-managing strategies for both pregnant women with OSA and their offspring.
5.1 Autophagy
The cardinal feature of OSA is frequent bouts of short-period hypoxia followed by a return to normal oxygen levels (normoxia). IH is present in several other physiological and pathophysiological conditions, including intense exercise and asthma, and has been shown to induce autophagy in response to protein aggregates present in cardiac disease [102]. Autophagy is the metabolic process where the body consumes its own cells in times of stress or other cellular damage, to rid the body of damaged cells and make way for new healthy cells. In the case of IH, autophagy is thought to be protective by avoiding the accumulation of misfolded proteins induced by ischemia. By inducing IH in rat primary cultured cardiomyocytes and in a normotensive mouse model, Chang et al [103] have shown that autophagy-related proteins are upregulated following IH, followed by a reduction in the endoplasmic reticulum (ER)-stress related proteins. With the addition of an autophagy inhibitor, the level of ER stress proteins was restored in both models, suggesting that IH may induce autophagy in cardiomyocytes as an adaptive response to protect the cells or tissues from ER stress and downstream apoptosis, which can result in further damage to surrounding cells and tissue [103] Extrapolating this data to OSA, it is possible that autophagy is also being initiated following the hypoxic environment during bouts of sleep apnea; further investigation into the role of autophagy in OSA is needed to ascertain what effect, if any, is has in the condition, and how manipulating it could lead to the reduction of adverse maternal or fetal outcomes.
5.2 Oxidative stress
The intermittent hypoxic environment in OSA creates oxidative stress, which has been strongly implicated in several diseases such as cardiovascular disease, diabetes, and pregnancy complications and it is suggested that it is oxidative stress that links the intermittent hypoxia of OSA with the development of PE and adverse pregnancy outcome [104, 105]. In turn, oxidative stress can lead to endothelial dysfunction, which is associated with both OSA and cardiovascular disease [106]. Further, it has been shown that antioxidant use is beneficial in OSA, confirming the importance of a balance between pro and antioxidants [107]. Despite this, there are conflicting reports regarding the role of oxidative stress in gestational OSA. Contrary to their hypothesis, Khan et al. [108] found that women with gestational OSA had significantly lower oxidative and carbonyl stress markers and an accompanying higher antioxidant capacity than controls [108]. This suggests that the role of oxidative stress in gestational OSA is more complex and requires more study, ensuring the standardization of factors such as study sample timing and presence or absence of therapy.
5.3 Inflammation
In non-pregnant OSA, frequent recurrent bouts of hypoxia and reoxygenation reportedly result in activations of neutrophils, which are able to adhere to the endothelium and generate reactive oxygen species. This leads to endothelial dysfunction and may contribute to the development of atherosclerosis [109, 110]. In PE, intravascular inflammation leads to endothelial cell dysfunction and is a common pathway for several PE mechanisms of disease [111, 112]. Although no studies have yet reported this effect in pregnant mothers with OSA, there are several similarities in the inflammatory response in OSA with and without pregnancy, which could suggest that neutrophils have a similar role in gestational OSA. For example, pro-inflammatory cytokines that are increased in gestational OSA, such as IL-6, TNF-α and C-reactive protein have also been linked to OSA in the absence of pregnancy, and have been associated with the development of atherosclerosis, endothelial dysfunction, insulin resistance and type 2 diabetes [113, 114]. If these associations persist in gestational OSA, links could be made between the inflammatory response of gestational OSA and the increased risk of cardiovascular disease development in mothers postnatally, however further studies are needed to confirm this hypothesis.
5.4 Antiangiogenic process
In both OSA and PE there is up-regulation of antiangiogenic proteins, which are also thought to lead to endothelial dysfunction [115]. Endothelial dysfunction can lead to vasoconstriction and proteinuria (seen in PE) and hypertension and related cardiovascular diseases seen in OSA [116, 117]. Bourjeily et al [118] conducted a small retrospective study that provided preliminary data showing that an imbalance of placental-secreted glycoproteins and markers of angiogenesis in pregnant women with OSA, compared to pregnant controls with no OSA [118]. Intermittent hypoxia in the brain is known to result in changes in the microvasculature, similar to those seen in patients with depression [119]. Therefore, OSA may cause similar changes in pregnant women, particularly because frontal lobe centers responsible for modulating emotions are sensitive to disturbed sleep [120].
5.5 Sympathetic activation
As discussed earlier, OSA in pregnancy leads to an increase in sympathetic activation, which has been proposed to increase the risk of GDM. However, sympathetic activation may also contribute to other adverse pregnancy outcomes due to its systemic effect on the vasculature. In pregnancy, it is reported that vasoconstriction occurs as a direct consequence of increased sympathetic tone, and that sympathetic neural activity is significantly higher in women with PE, compared to both women with normotensive pregnancies and nonpregnant normotensive women [121]. As OSA is characterized by bouts of intermittent hypoxia, which has been shown to increase sympathetic activation, it is possible then that the presence of OSA during pregnancy may result in vasoconstriction via increased sympathetic activation, suggesting a link between OSA and hypertensive disorders of pregnancy, including PE. Increased sympathetic activation can also result in an increase in the stress hormone cortisol. Hypoxia is considered a stress condition, and both hypoxia and sleep fragmentation have been reported to increase cortisol secretion, which may lead to abnormalities in insulin and glucose metabolism via activation of the hypothalamic-pituitary- adrenal (HPA) axis [122-124]. Although several studies have reported this association, there are conflicting reports describing the link between OSA and HPA axis activation via increased cortisol. Some studies have found no significant evidence of a link between OSA and increased cortisol/HPA axis activation [125, 126], highlighting the complex nature of the interaction and the importance of standard sampling protocols, especially in the case of cortisol which functions on a diurnal rhythm and is, therefore, variable depending on the time of sampling. Additionally, continuous cortisol secretion also reduces the sensitivity of the main receptor for cortisol, the glucocorticoid receptor (GR). The GR has an important role in the HPA axis, and reduced sensitivity of the GR to cortisol may lead to chronic activation of the HPA axis and downstream adverse effects, potentially leading to the development of GDM [127].
5.6 Alterations in the endocrine environment
Hormonal changes of pregnancy, specifically elevated progesterone and estrogens, have been shown to contribute to sleep disturbances [15-17]. Additionally, OSA has also been shown to affect hormone levels independently of pregnancy. Sleep deprivation alone is shown to affect both estrogen and luteinizing hormone (LH) [128]. A milder form of sleep-disordered breathing, upper airway resistance syndrome (UARS), does not involve significant hypoxemia [129], yet 50% of women with UARS have either amenorrhea (lack of menses) or dysmenorrhea (menstrual cramps), suggesting hormone imbalances are present [130]. Further, both amenorrhea and dysmenorrhea disappeared following CPAP treatment. The hormonal imbalance is reversed, clarifying that sleep disturbances alone may have the ability to affect reproductive health via the regulation of hormones. Pregnant women with OSA have been shown to have lower progesterone levels than controls [131]. It is suggested that lower progesterone accompanied by reduced functional residual lung capacity towards the end of pregnancy (due in part to diaphragm elevation) results in poor maternal oxygenation and may be a significant contributing factor to the development of OSA in pregnancy [13]. Lastly, adiponectin is a hormone and adipokine involved in the regulation of glucose levels and fatty acid breakdown. Adiponectin is affected by increases in pro-inflammatory cytokines similar to those we see in OSA [132, 133] and along with the energy balance control hormone leptin, is found to be altered in OSA. Alterations in hormones regulating metabolism during OSA is an important discovery and may lead to a deeper understanding of the development of diabetes or GDM [134].
5.7 Placental pathology
Although the presence of OSA is clearly linked to several adverse maternal and fetal outcomes, there is evidence to suggest that during hypoxia, both the placenta and fetus adapt to the low oxygen environment by using compensatory mechanisms. For example, it has been reported that hypoxia early in rat placental development (from GD 8.5 to GD 9.5), results in enlargement of the junctional zone of the placenta, which produces invasive trophoblast cell progenitors. It encourages endovascular trophoblast invasion and uterine vascular remodeling [135]. It is known that oxygen is an important regulator of placental development so when oxygen is restricted, such as in OSA, low oxygen may lead to pathologic placental development. It has been shown in humans that hypoxia alters differentiation of cytotrophoblast differentiation [136] and in rats, the fate of placental cells is regulated by hypoxia-inducible factor 1 (HIF1)- related processes [137]. HIF-1, a transcription factor that is activated by hypoxia, is upregulated in the placentas of pregnant women living at a high altitude (and therefore lower oxygen environments) with hypertensive disorders of pregnancy and rodent models of growth-restricted offspring [138-140]. In nonpregnant adults with OSA with hypertension, HIF1, or the sister transcription factor HIF2, has been shown to mediate processes such as hypoxemia, sympathetic nervous system activation, oxidative stress, and endothelial dysfunction [141].
Therefore, it is likely that HIF1 is induced in the hypoxic environment created by OSA and may be involved in the pathogenesis of the adverse outcomes following OSA in pregnancy. Fetal growth restriction in non-OSA pregnancy is further identified via histological changes in the placenta such as reduced villous size, a quickening of villous maturation, and decreased total number of villi [142, 143]. A cardinal feature of chronic low-grade hypoxia in the placenta is choranagiosis – increased numbers of villous capillarization [142, 144], the presence of which suggests that hypoxia, similar to what is experienced during OSA, is able to alter placental vasculature. In pregnancy with OSA, histopathological signs of hypoxia in the placenta have also been reported. Specifically, placentas from women with sleep-disordered breathing were found to have increased levels of fetal normoblastemia (immature nucleated red blood cells), and significantly higher expression of the tissue hypoxia marker carbonic anhydrase IX (CAIX), compared to on normal pregnancy placentas. This provides evidence to support the link between OSA in pregnancy and fetoplacental hypoxia, though underlying mechanisms are not yet reported [21].
6. Conclusions
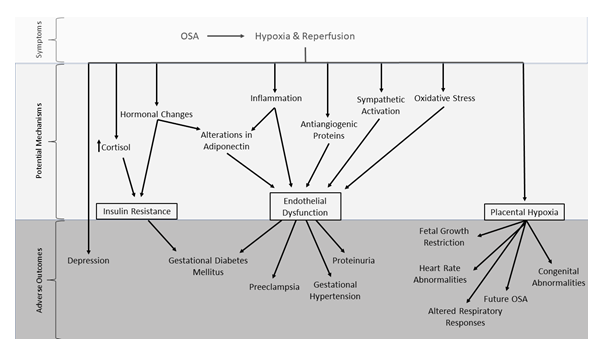
The evidence presented here highlights the associations between OSA and adverse pregnancy outcomes, the main points of which are summarized in Figure 5. The episodic hypoxia induced by OSA results in the initiation of several downstream pathways, many of which relate to three main potential effects – insulin resistance, endothelial dysfunction and placental hypoxia. These, in turn, lead to several of the adverse outcomes that have been previously associated with OSA during pregnancy, such as GDM, PE, gestational hypertension and effects on offspring both peri- and postnatally. As expected, the pathways that lead to these outcomes are complex and may overlap or contradict other possible mechanisms. This emphasizes the importance of continued clinical and basic research into the associations between gestational OSA and adverse pregnancy outcomes and the potential mechanisms underlying the disorder's pathophysiology.
Figure 5: Proposed summary of the mechanisms leading to adverse pregnancy outcomes related to gestational OSA. Symptoms of OSA are related to episodic hypoxia and reperfusion during transient upper airway obstruction. Evidence suggests that hypoxia and reoxygenation leads to several physiological changes which may inform the identification of potential mechanisms of disease such as hormonal changes, inflammation, sympathetic activation and oxidative stress. These proposed mechanisms result in insulin resistance, endothelial dysfunction and placental hypoxia, which are directly associated to the development of adverse pregnancy outcomes including gestational diabetes mellitus, preeclampsia and effects on the developing fetus. Other adverse pregnancy outcomes associated with OSA, such as depression, are less well understood.
It is important to note that data is very limited on the effectiveness of current OSA therapies when used during pregnancy. There is no clear evidence that gestational OSA is a fully modifiable risk factor in pregnancy. Further, studies in the non-pregnant population have produced mixed results on the effectiveness of treatments such as CPAP on general health improvement, potentially due to issues with compliance. It is clear from the available data that more attention should be paid to OSA during pregnancy, standardized protocols for identification, diagnosis and treatment of OSA specifically in pregnancy are needed, and systematic screening should be employed to identify expectant mothers at greater risk of OSA. This would reduce serious adverse obstetric and perinatal outcomes, which pose significant health issues for both the mother and offspring, even postnatally.
References
- Zepelin H, Rechtschaffen A. Mammalian sleep, longevity, and energy metabolism. Brain Behav Evol 10 (1974): 425- 470.
- Campbell S S, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev 8 (1984): 269-300.
- Shaw P J, Cirelli C, Greenspan R J, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science 287 (2000): 1834-1837.
- Faith S Luyster, Patrick J Strollo, Phyllis C Zee, James K Walsh. Sleep: a health imperative. Sleep 35 (2012): 727-734.
- Daniel F Kripke, Lawrence Garfinkel, Deborah L Wingard, Melville R Klauber, Matthew R Marler. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 59 (2002): 131-136.
- Michele L Okun, James M Roberts, Anna L Marsland, Martica Hall. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstet Gynecol Surv 64 (2009): 273-280.
- Romero R, Badr M S. A role for sleep disorders in pregnancy complications: challenges and opportunities. Am J Obstet Gynecol 210 (2014): 3-11.
- Jane E Ferrie, Martin J Shipley, Francesco P Cappuccio, Eric Brunner, Michelle A Miller, Meena Kumari, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep 30 (2007): 1659-1666.
- Christer Hublin, Markku Partinen, Markku Koskenvuo, Jaakko Kaprio. Sleep and mortality: a population-based 22-year follow-up study. Sleep 30 (2007): 1245-1253.
- Michael A Grandner, Lauren Hale, Melisa Moore, Nirav P Patel. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev 14 (2010): 191-203.
- National Sleep Foundation. Sleep Health & Scheduling. Sleep in America Poll (2019).
- National Center on Sleep Disorders Research. National Institutes Of Health Sleep Disorders Research Plan, Nih (2011).
- Silvestri R, Arico I. Sleep disorders in pregnancy. Sleep Sci 12 (2019): 232-239.
- Bourjeily G, Ankner G, Mohsenin V, et al. Sleep-disordered breathing in pregnancy. Clin Chest Med 32 (2011): 175-189.
- Archer G W, Marx G F. Arterial oxygen tension during apnoea in parturient women. Br J Anaesth 46 (1974): 358-360.
- Cheun J K, Choi K T. Arterial oxygen desaturation rate following obstructive apnea in parturients. J Korean Med Sci 7 (1992): 6-10.
- Bobrowski R A. Pulmonary physiology in pregnancy. Clin Obstet Gynecol 53 (2010): 285-300.
- Morong, S, Hermsen B, de Vries N. Sleep-disordered breathing in pregnancy: a review of the physiology and potential role for positional therapy. Sleep Breath 18 (2014): 31-37.
- Robertson N, Turner JM, Kumar S. Pathophysiological changes associated with sleep disordered breathing and supine sleep position in pregnancy. Sleep Med Rev 46 (2019): 1-8.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders (2000): 114-115.
- Sanjita Ravishankar, Ghada Bourjeily, Geralyn Lambert-Messerlian, Mai He, Monique E De Paepe, Füsun Gündogan. Evidence of Placental Hypoxia in Maternal Sleep Disordered Breathing. Pediatr Dev Pathol 18 (2015): 380-386.
- Judette M Louis, Matthew A Koch, Uma M Reddy, Robert M Silver, Corette B Parker, Francesca L Facco, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol 218 (2018): 521 e521- 521 e512.
- Terry Young, Laurel Finn, Diane Austin, Andrea Peterson. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 167 (2003): 1181-1185.
- Amy M Branum, Sharon E Kirmeyer, Elizabeth C W Gregory. Prepregnancy Body Mass Index by Maternal Characteristics and State: Data From the Birth Certificate, 2014. Natl Vital Stat Rep 65 (2016): 1-11.
- Ghesquière L, Deruelle P, Ramdane Y, Garabedian C, Charley-Monaca C, Dalmas A F. Obstructive sleep apnea in obese pregnant women: A prospective study. PLoS One 15 (2020): e0238733.
- Jennifer E Dominguez, Andrew D Krystal, Ashraf S Habib. Obstructive Sleep Apnea in Pregnant Women: A Review of Pregnancy Outcomes and an Approach to Management." Anesth Analg 127 (2018): 1167-1177.
- Francesca L Facco, David W Ouyang, Phyllis C Zee, William A Grobman. Sleep disordered breathing in a high-risk cohort prevalence and severity across pregnancy. Am J Perinatol 31 (2014): 899-904.
- Judette Louis, Dennis Auckley, Branko Miladinovic, Anna Shepherd, Patricia Mencin, Deepak Kumar, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women." Obstet Gynecol 120 (2012): 1085-1092.
- Jayne R Rice, Gloria T Larrabure-Torrealva, Miguel Angel Luque Fernandez, Mirtha Grande, Vicky Motta, Yasmin V Barrios, et al. High risk for obstructive sleep apnea and other sleep disorders among overweight and obese pregnant women. BMC Pregnancy Childbirth 15 (2015): 198.
- Jodi A Mindell, Rae Ann Cook, Janeta Nikolovski. Sleep patterns and sleep disturbances across pregnancy. Sleep Med 16 (2015): 483-488.
- David A Kalmbach, Philip Cheng, Roopina Sangha, Louise M O’Brien, Leslie M Swanson, Laura Palagini, et al. Insomnia, Short Sleep, And Snoring In Mid-To-Late Pregnancy: Disparities Related To Poverty, Race, And Obesity. Nat Sci Sleep 11 (2019): 301-315.
- Bilgay Izci, Sascha E Martin, Kirsty C Dundas, Wang A Liston, Andrew A Calder, Neil J Douglas. Sleep complaints: snoring and daytime sleepiness in pregnant and pre-eclamptic women. Sleep Med 6 (2005): 163-169.
- Grace W Pien, Daniel Fife, Allan I Pack, Emeka Nkwuo J, Richard J Schwab. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep 28 (2005): 1299-1305.
- Lina Liu, Guang Su, Shuling Wang, Bingqian Zhu. The prevalence of obstructive sleep apnea and its association with pregnancy-related health outcomes: a systematic review and meta-analysis. Sleep Breath 23 (2019): 399-412.
- Haim Bassan, Shimrit Uliel-Sibony, Shlomit Katsav, Mira Farber, Riva Tauman. Maternal Sleep Disordered Breathing and Neonatal Outcome. Isr Med Assoc J 18 (2016): 45-48.
- Longworth H, McCallin K, Narayanan R P, Turner M A, Quenby S, Rycroft D, et al. Screening methods for obstructive sleep apnoea in severely obese pregnant women." Clin Obes 7 (2017): 239-244.
- Miyagawa S, Emori Yoko, Kawano A, Sakurai S, Tanigawa T. Relationship between sleep-disordered breathing and perinatal outcome in pregnant women. J Jpn Acad Midwif (2011): 5-12.
- Minako Watanabe Hitomi Shinohara Hideya Kodama. Impact of overnight oximetry findings on cardiac autonomic modulation in women during second trimester of uncomplicated pregnancy." J Obstet Gynaecol Res 41 (2015): 689-696.
- Yin TT, Williams N, Burton C, Ong SS, Loughna P, Britton JR, et al. Hypertension, fetal growth restriction and obstructive sleep apnoea in pregnancy." Eur J Obstet Gynecol Reprod Biol 141 (2008): 35-38.
- Katherine M Sharkey, Kelly Waters, Richard P Millman, Robin Moore, Susan M Martin, Ghada Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnography in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med 10(2014): 497-502.
- Ghada Bourjeily, Valery A Danilack, Margaret H Bublitz, Heather Lipkind, Janet Muri, Donna Caldwell, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort." Sleep Med 38 (2017): 50-57.
- Murphy V E, Clifton V L, Gibson P G. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax 61 (2006): 169-176.
- Jansson T, Powell T L. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 113 (2007): 1-13.
- Jansson T. Placenta plays a critical role in maternal-fetal resource allocation. Proc Natl Acad Sci U S A 113 (2016): 11066-11068.
- Longtine M S, Nelson D M. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med 29 (2011): 187-196.
- Debora Kidron, Yamit Bar-Lev, Ilan Tsarfaty, Ariel Many, Riva Tauman. The effect of maternal obstructive sleep apnea on the placenta. Sleep 42 (2019).
- Naeye R L. Functionally important disorders of the placenta, umbilical cord, and fetal membranes. Hum Pathol 18 (1987): 680-691.
- Kari R Risnes, Pål R Romundstad, Tom I L Nilsen, Anne Eskild, Lars J Vatten. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am J Epidemiol 170 (2009): 622-631.
- Judette M Louis, Mulubrhan F Mogos, Jason L Salemi, Susan Redline, Hamisu M Salihu. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998-2009. Sleep 37 (2014): 843-849.
- Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J 36 (2010): 849-855.
- Judette M Louis, Dennis Auckley, Robert J Sokol, Brian M Mercer. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol 202 (2010): 261 e261-e265.
- Sofia A Olivarez, Millie Ferres, Katherine Antony, Amarbir Mattewal, Bani Maheshwari, Haleh Sangi-Haghpeykar, et al. Obstructive sleep apnea screening in pregnancy, perinatal outcomes, and impact of maternal obesity. Am J Perinatol 28 (2011): 651-658.
- Sushmita Pamidi, Lancelot M Pinto, Isabelle Marc, Andrea Benedetti, Kevin Schwartzman, John Kimoff R. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol 210 (2014): 52 e51-52 e14.
- Francesca L Facco, Corette B Parker, Uma M Reddy, Robert M Silver, Matthew A Koch, Judette M Louis, et al. Association Between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obstet Gynecol 129 (2017): 31-41.
- Ding XX, Wu YL, Xu SJ, Zhang SF, Jia XM, Zhu RP, et al. A systematic review and quantitative assessment of sleep-disordered breathing during pregnancy and perinatal outcomes. Sleep Breath 18 (2014): 703-713.
- Yi-Hua Chen, Jiunn-Horng Kang, Ching-Chun Lin, I-Te Wang, Joseph J Keller, Herng-Ching Lin. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol 206 (2012): 136 e131-e135.
- Dennis L Spence, Rhonda C Allen, Monica A Lutgendorf, Virginia R Gary, John D Richard, Sara C Gonzalez. Association of obstructive sleep apnea with adverse pregnancy-related outcomes in military hospitals. Eur J Obstet Gynecol Reprod Biol 210 (2017): 166-172.
- Edwards N, Blyton D M, Kirjavainen T, Kesby G J, Sullivan C E. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med 162 (2000): 252-257.
- Bei Bei, Jeannette Milgrom, Jennifer Ericksen, John Trinder. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep 33 (2010): 531-538.
- Douglas N, Young A, Roebuck T, Ho S, Miller B R, Kee K, et al. Prevalence of depression in patients referred with snoring and obstructive sleep apnoea. Intern Med J 43 (2013): 630-634.
- Cass Edwards, Sutapa Mukherjee, Laila Simpson, Lyle J Palmer, Osvaldo P Almeida, David R Hillman. Depressive Symptoms before and after Treatment of Obstructive Sleep Apnea in Men and Women. J ClinSleep Med 11 (2015): 1029-1038.
- Norma I Gavin, Bradley N Gaynes, Kathleen N Lohr, Samantha Meltzer-Brody, Gerald Gartlehner, Tammeka Swinson. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106 (2005): 1071-1083.
- Jacques Dayan, Christian Creveuil, Maureen N Marks, Sue Conroy, Michel Herlicoviez, Michel Dreyfus, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med 68 (2006): 938-946.
- John Eastwood, Felix A Ogbo, Alexandra Hendry, Justine Noble, Andrew Page. The Impact of Antenatal Depression on Perinatal Outcomes in Australian Women. PLoS One 12 (2017): e0169907.
- Karen Redhead, Jennifer Walsh, Megan Galbally, John P Newnham, Stuart J Watson, Peter Eastwood, et al. Obstructive sleep apnea is associated with depressive symptoms in pregnancy. Sleep 43 (2020).
- Farnoosh Emamian, Habibolah Khazaie, Michele L Okun, Masoud Tahmasian, Amir A Sepehry. Link between insomnia and perinatal depressive symptoms: A meta-analysis. J Sleep Res 28 (2019): e12858.
- Meslier N, Gagnadoux F, Giraud P, Person C, Ouksel H, Urban T, et al. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J 22 (2003): 156-160.
- Lisa M Blair, Kyle Porter, Binnaz Leblebicioglu, Lisa M Christianl. Poor Sleep Quality and Associated Inflammation Predict Preterm Birth: Heightened Risk among African Americans. Sleep 38 (2015): 1259-1267.
- Izolde Bouloukaki, Charalampos Mermigkis, Nikolaos Tzanakis, Eleftherios Kallergis, Violeta Moniaki, Eleni Mauroudi, et al. Evaluation of Inflammatory Markers in a Large Sample of Obstructive Sleep Apnea Patients without Comorbidities. Mediators Inflamm (2017): 4573756.
- Bilgay Izci Balserak, Grace W Pien, Bharati Prasad, Dimitrios Mastrogiannis, Chang Park, Laurie T Quinn, et al. Obstructive Sleep Apnea Is Associated with Newly Diagnosed Gestational Diabetes Mellitus. Ann Am Thorac Soc 17 (2020): 754-761.
- Chunfang Qiu, Daniel Enquobahrie, Ihunnaya O Frederick, Dejene Abetew, Michelle A Williams. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health (2017): 17.
- Sirimon Reutrakul, Nausheen Zaidi, Kristen Wroblewski, Helen H Kay, Mahmoud Ismail, David A Ehrmann, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care 34 (2011): 2454-2457.
- Ekasitt Wanitcharoenkul, Naricha Chirakalwasan, Somvang Amnakkittikul, Suranut Charoensri, Sunee Saetung, Suwannee Chanprasertyothin, et al. Obstructive sleep apnea and diet-controlled gestational diabetes. Sleep Med 39 (2017): 101- 107.
- Lévy P, Bonsignore M R, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J 34 (2009): 243-260.
- Paschalis Steiropoulos, Nikolaos Papanas, Evangelia Nena, Efstratios Maltezos, Demosthenes Bouros. Continuous positive airway pressure treatment in patients with sleep apnoea: does it really improve glucose metabolism?. Curr Diabetes Rev 6 (2010): 156-166.
- Ghada Bourjeily, Valery A Danilack, Margaret H Bublitz, Janet Muri, Karen Rosene-Montella, Heather Lipkind. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med 66 (2020): 233-240.
- Hamisu M Salihu, Lindsey King, Priyanshi Patel, Arnut Paothong, Anupam Pradhan, Judette Louis, et al. Association between maternal symptoms of sleep disordered breathing and fetal telomere length. Sleep 38 (2015): 559-566.
- Yu Sun Bin, Peter A Cistulli, Jane B Ford. Population-Based Study of Sleep Apnea in Pregnancy and Maternal and Infant Outcomes. J Clin Sleep Med 12 (2016): 871-877.
- Schoenfeld A, Ovadia Y, Neri A, Freedman S. Obstructive sleep apnea (OSA)-implications in maternal-fetal medicine. A hypothesis. Med Hypotheses 30 (1989): 51-54.
- Charbonneau M, Falcone T, Cosio M G, Levy R D. Obstructive sleep apnea during pregnancy. Therapy and implications for fetal health. Am Rev Respir Dis 144 (1991): 461-463.
- Anna W KNEITEL, Marjorie C TREADWELL, Louise M O’BRIEN. Effects of maternal obstructive sleep apnea on fetal growth: a case-control study. J Perinatol 38 (2018): 982-988.
- Sushmita Pamidi, Isabelle Marc, Gabrielle Simoneau, Lorraine Lavigne, Allen Olha, Andrea Benedetti, et al. Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax 71 (2016): 719-725.
- Nicole T Brown, Jessica M Turner, Sailesh Kumar. The intrapartum and perinatal risks of sleep-disordered breathing in pregnancy: a systematic review and meta-analysis." Am J Obstet Gynecol 219 (2018): 147-161 e141.
- Ayana Telerant, Galit Levi Dunietz, Ariel Many, Riva Tauman. Mild Maternal Obstructive Sleep Apnea in Non-obese Pregnant Women and Accelerated Fetal Growth. Sci Rep 8 (2018): 10768.
- Xing Ge, Fangbiao Tao, Kun Huang, Leijing Mao, Sanhuan Huang, Ying Niu, et al. Maternal Snoring May Predict Adverse Pregnancy Outcomes: A Cohort Study in China. PLoS One 11 (2016): e0148732
- Harris J L, Krueger T R, Parer J T. Mechanisms of late decelerations of the fetal heart rate during hypoxia. Am J Obstet Gynecol 144 (1982): 491-496.
- Murata Y, Martin C B, Ikenoue T, Hashimoto T, Taira S, Sagawa T, et al. Fetal heart rate accelerations and late decelerations during the course of intrauterine death in chronically catheterized rhesus monkeys. Am J Obstet Gynecol 144 (1982): 218-223.
- Patterson A J, Zhang L. Hypoxia and fetal heart development. Curr Mol Med 10 (2010): 653-666.
- David Gozal, Stephen R Reeves, Barry W Row, Jennifer J Neville, Shang Z Guo, Andrew J Lipton, et al. Respiratory effects of gestational intermittent hypoxia in the developing rat." Am J Respir Crit Care Med 167 (2003): 1540-1547.
- Roush S F, Bell L. Obstructive sleep apnea in pregnancy. J Am Board Fam Pract 17 (2004): 292-294.
- Figen Kir Sahin, Gulengul Koken, Emine Cosar, Filiz Saylan, Fatma Fidan, Mehmet Yilmazer, et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynaecol Obstet 100 (2008): 141-146.
- Sofia A Olivarez, Bani Maheshwari, Meghan McCarthy, Nikolaos Zacharias, Ignatia van den Veyver, Lata Casturi, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol 202 (2010): 552 e551-557.
- Kotecha S. Lung growth: implications for the newborn infant. Arch Dis Child Fetal Neonatal Ed 82 (2000): F69-F74.
- Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of human fetal breathing during the last 10 weeks of pregnancy. Obstet Gynecol 56 (1980): 24-30.
- Stéphanie Fournier, Shelby Steele, Cécile Julien, Sébastien Fournier, Roumiana Gulemetova, Céline Caravagna, et al. Gestational stress promotes pathological apneas and sex-specific disruption of respiratory control development in newborn rat. J Neurosci 33 (2013): 563-573.
- Fiona B McDonald, Eugene M Dempsey, Ken D O'Halloran, et al. Effects of Gestational and Postnatal Exposure to Chronic Intermittent Hypoxia on Diaphragm Muscle Contractile Function in the Rat. Front Physiol 7 (2016): 276.
- Stephen M Johnson, Karanbir S Randhawa, Jenna J Epstein, Ellen Gustafson, Austin D Hocker, Adrianne G Huxtable, et al. Gestational intermittent hypoxia increases susceptibility to neuroinflammation and alters respiratory motor control in neonatal rats. Respir Physiol Neurobiol 256 (2018): 128-142.
- Lahti-Pulkkinen M, Mina T H, Riha R L, Räikkönen K. Maternal antenatal daytime sleepiness and child neuropsychiatric and neurocognitive development. Psychol Med 49 (2019): 2081-2090.
- Kira Nahum Sacks, Michael Friger, Ilana Shoham-Vardi, Ruslan Sergienko, Efrat Spiegel, Daniella Landau, et al. Long-term neuropsychiatric morbidity in children exposed prenatally to preeclampsia." Early Hum Dev 130 (2019): 96-100.
- Riva Tauman, Luba Zuk, Shimrit Uliel-Sibony, Jessica Ascher-Landsberg, Shlomit Katsav, Mira Farber, et al. The effect of maternal sleep-disordered breathing on the infant's neurodevelopment. Am J Obstet Gynecol 212 (2015): 656 e651-657.
- Yu Sun Bin, Peter A Cistulli, FRACP, FCCP, Christine L Roberts, FAFPHM, et al. Childhood Health and Educational Outcomes Associated With Maternal Sleep Apnea: A Population Record-Linkage Study. Sleep 40 (2017).
- Ren S Y, Xu X. Role of autophagy in metabolic syndrome-associated heart disease. Biochim Biophys Acta 1852 (2015): 225-231.
- Jui-Chih Chang, Wei-Fen Hu, Wen-Sen Lee, Jian-Hong Lin, Pei-Ching Ting, Huai-Ren Chang, et al. Intermittent Hypoxia Induces Autophagy to Protect Cardiomyocytes From Endoplasmic Reticulum Stress and Apoptosis. Front Physiol 10 (2019): 995.
- Motoo Yamauchi, Hiroshi Nakano, Junko Maekawa, Yukinori Okamoto, Yoshinobu Ohnishi, Takahiro Suzuki, et al. Oxidative stress in obstructive sleep apnea. Chest 127 (2005): 1674-1679.
- Tinnakorn Chaiworapongsa, Piya Chaemsaithong, Lami Yeo, Roberto Romero. Preeclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 10 (2014): 466-480.
- Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 147 (2015): 266-274.
- Peter Celec, Ingrid Jurkovicová, Roman Buchta, Ivan Bartík, Roman Gardlík, Roland Pálffy, et al. Antioxidant vitamins prevent oxidative and carbonyl stress in an animal model of obstructive sleep apnea. Sleep Breath 17 (2013): 867-871.
- Nazia Khan, Geralyn Lambert-Messerlian, Joao Filipe Monteiro, Julius Hodosy, L’ubomíra Tóthová, Peter Celec, et al. Oxidative and carbonyl stress in pregnant women with obstructive sleep apnea. Sleep Breath 22 (2018): 233-240.
- Carlson J T, Rångemark C, Hedner J A. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens 14 (1996): 577-584.
- Alonso-Fernandez A, Garcia-Rio F, Arias M A, Hernanz A, de la Pena M, Pierola J, et al. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax 64 (2009): 581-586.
- Rodgers G M, Taylor R N, Roberts J M. Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells. Am J Obstet Gynecol 159 (1988): 908-914.
- Roberts J M, Taylor R N, Musci T J, Rodgers G M, Hubel C A, McLaughlin M K, et al. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 161 (1989): 1200-1204.
- Anushua Sinha, Deborah Yokoe, Richard Platt. Intrapartum antibiotics and neonatal invasive infections caused by organisms other than group B streptococcus. J Pediatr 142 (2003): 492-497.
- Paschalis Steiropoulos, Ioannis Kotsianidis, Evangelia Nena, Venetia Tsara, Evdoxia Gounari, Olga Hatzizisi, et al. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep 32 (2009): 537-543.
- Mohsenin V, Urbano F. Circulating antiangiogenic proteins in obstructive sleep apnea and hypertension. Respir Med 105 (2011): 801-807.
- Sharon E Maynard, Jiang-Yong Min, Jaime Merchan, Kee-Hak Lim, Jianyi Li, Susanta Mondal, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111 (2003): 649-658.
- Richard J Levine, Sharon E Maynard, Cong Qian, Kee-Hak Lim, Lucinda J England, Kai F Yu, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350 (2004): 672-683.
- Ghada Bourjeily, Patrizia Curran, Kristen Butterfield, Hasina Maredia, Marshall Carpenter, Geralyn Lambert-Messerlian. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med 43 (2015): 81-87.
- Fenglian Zhao, Junling Yang, Ranji Cui. Effect of Hypoxic Injury in\ Mood Disorder. Neural Plast 2017 (2017): 6986983.
- Jeanine Kamphuis, Peter Meerlo, Jaap M Koolhaas, Marike Lancel. Poor sleep as a potential causal factor in aggression and violence. Sleep Med 13 (2012): 327-334.
- Dilys J Freeman, Frances McManus, Elizabeth Ann Brown, Lynne Cherry, John Norrie, Jane E Ramsay, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 44 (2004): 708-714.
- Musacchia X J. Fever and hyperthermia: introduction. Fed Proc 38 (1979): 27-29.
- Vgontzas A N, Pejovic S, Zoumakis E, Lin H-M, Bentley C M, Bixler E O, et al. Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J Clin Endocrinol Metab 92 (2007): 4199-4207.
- Gláucia Carneiro, Sônia Maria Togeiro, Lílian F Hayashi, Fernando Flexa Ribeiro-Filho, Artur Beltrame Ribeiro, Sérgio Tufik, et al. Effect of continuous positive airway pressure therapy on hypothalamic-pituitary-adrenal axis function and 24-h blood pressure profile in obese men with obstructive sleep apnea syndrome. Am J Physiol Endocrinol Metab 295 (2008): E380-E384.
- Grunstein R R, Stewart D A, Lloyd H, Akinci M, Cheng N, Sullivan C E. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep 19 (1996): 774-782.
- Andre Schmoller, Frank Eberhardt, Kamila Jauch-Chara, Ulrich Schweiger, Peter Zabel, Achim Peters, et al. Continuous positive airway pressure therapy decreases evening cortisol concentrations in patients with severe obstructive sleep apnea. Metabolism 58 (2009): 848-853.
- David E Henley, Georgina M Russell, Jennie A Douthwaite, Susan A Wood, Fiona Buchanan, Rosemary Gibson, et al. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J Clin Endocrinol Metab 94 (2009): 4234-4242.
- Baumgartner A, Dietzel M, Saletu B, Wolf R, Campos-Barros A, Gräf K J, et al. Influence of partial sleep deprivation on the secretion of thyrotropin, thyroid hormones, growth hormone, prolactin, luteinizing hormone, follicle stimulating hormone, and estradiol in healthy young women. Psychiatry Res 48 (1993): 153-178.
- Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest 104 (1993): 781-787.
- Christian Guilleminault, Riccardo Stoohs, Young-do Kim, Ronald Chervin, Jed Black, Alex Clerk. Upper airway sleep-disordered breathing in women. Ann Intern Med 122 (1995): 493-501.
- Jennifer Lee, Elizabeth E Eklund, Geralyn Lambert-Messerlian, Glenn E Palomaki, Kristen Butterfield, Patrizia Curran, et al. Serum Progesterone Levels in Pregnant Women with Obstructive Sleep Apnea: A Case Control Study. J Womens Health (Larchmt) 26 (2017): 259-265.
- Jamie C M Lam, Aimin Xu, Sidney Tam, Pek-Ian Khong, Tzy-Jyun Yao, David C L Lam, et al. Hypoadiponectinemia is related to sympathetic activation and severity of obstructive sleep apnea. Sleep 31 (2008): 1721-1727.
- Yasuhiko Nakagawa, Ken Kishida, Shinji Kihara, Mina Sonoda, Ayumu Hirata, Atsutaka Yasui, et al. Nocturnal reduction in circulating adiponectin concentrations related to hypoxic stress in severe obstructive sleep apnea-hypopnea syndrome. Am J Physiol Endocrinol Metab 294 (2008): E778-784.
- Balcan B, Thunström E, Yucel-Lindberg T, Lindberg K, Ay P, Yüksel Peker. Impact of CPAP treatment on leptin and adiponectin in adults with coronary artery disease and nonsleepy obstructive sleep apnoea in the RICCADSA trial. Sleep Med 67 (2020): 7-14.
- Gracy X Rosario, Toshihiro Konno, Michael J Soares. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol 314 (2008): 362-375.
- Genbacev O, Joslin R, Damsky C H, Polliotti B M, Fisher S J. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 97 (1996): 540-550.
- Adelman D M, Gertsenstein M, Nagy A, Simon M C, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev 14 (2000): 3191-3203.
- Rajakumar A, Brandon H M, Daftary A, Ness R, Conrad K P. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25 (2004): 763-769.
- Stacy Zamudio, Yuanhong Wu, Francesca Ietta, Alessandro Rolfo, Ashley Cross, Timothy Wheeler, et al. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol 170 (2007): 2171-2179.
- Kevin P Robb, Tiziana Cotechini, Camille Allaire, Arissa Sperou, Charles H Graham. Inflammation-induced fetal growth restriction in rats is associated with increased placental HIF- 1alpha accumulation." PLoS One 12 (2017): e0175805.
- Jayasri Nanduri, Ying-Jie Peng, Guoxiang Yuan, Ganesh K Kumar, Nanduri R Prabhakar. Hypoxia-inducible factors and hypertension: lessons from sleep apnea syndrome. J Mol Med (Berl) 93 (2015): 473-480.
- Redline R W. Classification of placental lesions. Am J Obstet Gynecol 213 (2015): S21-28.
- Yee Khong T, Eoghan E Mooney, Ilana Ariel, Nathalie C M Balmus, Theonia K Boyd, Marie-Anne Brundler, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement." Arch Pathol Lab Med 140 (2016): 698-713.
- Akbulut M, Sorkun HC, Bir F, Eralp A, Duzcan E. Chorangiosis: the potential role of smoking and air pollution. Pathol Res Pract 205 (2009): 75-81.