Novel Recognition of HER2 Amplification in Enteroblastic Colonic Adenocarcinoma. Diagnostic Clues and Future Perspectives
Article Information
Giuseppe Leoncini*, Giovanna Sabella, Massimo Milione
First Division of Pathology, Department of Pathology and Laboratory Medicine, IRCCS Foundation National Cancer Institute, Milan, Italy
*Corresponding Author: Giuseppe Leoncini, First Division of Pathology, Department of Pathology and Laboratory Medicine, IRCCS Foundation National Cancer Institute, Milan, Italy.
Received: 18 August 2022; Accepted: 25 August 2022; Published: 30 September 2022
Citation: Leoncini Giuseppe, Sabella Giovanna, Milione Massimo. Novel Recognition of HER2 Amplification in Enteroblastic Colonic Adenocarcinoma. Diagnostic Clues and Future Perspectives. Journal of Surgery and Research.5 (2022): 559-562.
View / Download Pdf Share at FacebookAbstract
Enteroblastic differentiation in sporadic colonic adenocarcinoma was a rare event that lacks to be fully elucidated. It was characterized by an aggressive biological behavior, with early metastatic spread and poor prognosis. Diagnostic clues and molecular features were not completely elucidated so far. Histological features including tubulo-papillary architecture, cleared neoplastic cells, fetal oncoproteins expression were diagnostic requirements. We describe a new case of enteroblastic colonic adenocarcinoma (CAED) in the sigmoid, exhibiting small intestinal phenotype, higher stage at diagnosis and early liver metastases. At molecular level, CAED shared both TP53 gene mutation and beta-catenin nuclear translocation with conventional colonic adenocarcinoma. Altered human epidermal growth factor receptor (HER) 2 signaling has been described in a small subset of conventional colonic adenocarcinoma. At best of our knowledge our report represents the first immunohistochemical recognition of HER2 gene amplification in CAED. Given its aggressive clinical behavior, clinicians should be aware of the possible HER2 amplification, that could point toward further treatment options.
Keywords
<p>Enteroblastic differentiation, HER2 amplification, Spalt-like transcriptor factor 4 (SALL-4), Glypican (GPC)3, Alfa-fetoprotein</p>
Enteroblastic differentiation articles; HER2 amplification articles; Spalt-like transcriptor factor 4 (SALL-4) articles; Glypican (GPC)3 articles; Alfa-fetoprotein articles
Enteroblastic differentiation articles Enteroblastic differentiation Research articles Enteroblastic differentiation review articles Enteroblastic differentiation PubMed articles Enteroblastic differentiation PubMed Central articles Enteroblastic differentiation 2023 articles Enteroblastic differentiation 2024 articles Enteroblastic differentiation Scopus articles Enteroblastic differentiation impact factor journals Enteroblastic differentiation Scopus journals Enteroblastic differentiation PubMed journals Enteroblastic differentiation medical journals Enteroblastic differentiation free journals Enteroblastic differentiation best journals Enteroblastic differentiation top journals Enteroblastic differentiation free medical journals Enteroblastic differentiation famous journals Enteroblastic differentiation Google Scholar indexed journals HER2 amplification articles HER2 amplification Research articles HER2 amplification review articles HER2 amplification PubMed articles HER2 amplification PubMed Central articles HER2 amplification 2023 articles HER2 amplification 2024 articles HER2 amplification Scopus articles HER2 amplification impact factor journals HER2 amplification Scopus journals HER2 amplification PubMed journals HER2 amplification medical journals HER2 amplification free journals HER2 amplification best journals HER2 amplification top journals HER2 amplification free medical journals HER2 amplification famous journals HER2 amplification Google Scholar indexed journals Spalt-like transcriptor factor 4 articles Spalt-like transcriptor factor 4 Research articles Spalt-like transcriptor factor 4 review articles Spalt-like transcriptor factor 4 PubMed articles Spalt-like transcriptor factor 4 PubMed Central articles Spalt-like transcriptor factor 4 2023 articles Spalt-like transcriptor factor 4 2024 articles Spalt-like transcriptor factor 4 Scopus articles Spalt-like transcriptor factor 4 impact factor journals Spalt-like transcriptor factor 4 Scopus journals Spalt-like transcriptor factor 4 PubMed journals Spalt-like transcriptor factor 4 medical journals Spalt-like transcriptor factor 4 free journals Spalt-like transcriptor factor 4 best journals Spalt-like transcriptor factor 4 top journals Spalt-like transcriptor factor 4 free medical journals Spalt-like transcriptor factor 4 famous journals Spalt-like transcriptor factor 4 Google Scholar indexed journals Glypican (GPC)3 articles Glypican (GPC)3 Research articles Glypican (GPC)3 review articles Glypican (GPC)3 PubMed articles Glypican (GPC)3 PubMed Central articles Glypican (GPC)3 2023 articles Glypican (GPC)3 2024 articles Glypican (GPC)3 Scopus articles Glypican (GPC)3 impact factor journals Glypican (GPC)3 Scopus journals Glypican (GPC)3 PubMed journals Glypican (GPC)3 medical journals Glypican (GPC)3 free journals Glypican (GPC)3 best journals Glypican (GPC)3 top journals Glypican (GPC)3 free medical journals Glypican (GPC)3 famous journals Glypican (GPC)3 Google Scholar indexed journals Alfa-fetoprotein articles Alfa-fetoprotein Research articles Alfa-fetoprotein review articles Alfa-fetoprotein PubMed articles Alfa-fetoprotein PubMed Central articles Alfa-fetoprotein 2023 articles Alfa-fetoprotein 2024 articles Alfa-fetoprotein Scopus articles Alfa-fetoprotein impact factor journals Alfa-fetoprotein Scopus journals Alfa-fetoprotein PubMed journals Alfa-fetoprotein medical journals Alfa-fetoprotein free journals Alfa-fetoprotein best journals Alfa-fetoprotein top journals Alfa-fetoprotein free medical journals Alfa-fetoprotein famous journals Alfa-fetoprotein Google Scholar indexed journals conventional colonic adenocarcinoma articles conventional colonic adenocarcinoma Research articles conventional colonic adenocarcinoma review articles conventional colonic adenocarcinoma PubMed articles conventional colonic adenocarcinoma PubMed Central articles conventional colonic adenocarcinoma 2023 articles conventional colonic adenocarcinoma 2024 articles conventional colonic adenocarcinoma Scopus articles conventional colonic adenocarcinoma impact factor journals conventional colonic adenocarcinoma Scopus journals conventional colonic adenocarcinoma PubMed journals conventional colonic adenocarcinoma medical journals conventional colonic adenocarcinoma free journals conventional colonic adenocarcinoma best journals conventional colonic adenocarcinoma top journals conventional colonic adenocarcinoma free medical journals conventional colonic adenocarcinoma famous journals conventional colonic adenocarcinoma Google Scholar indexed journals tubulo-papillary architecture, articles tubulo-papillary architecture, Research articles tubulo-papillary architecture, review articles tubulo-papillary architecture, PubMed articles tubulo-papillary architecture, PubMed Central articles tubulo-papillary architecture, 2023 articles tubulo-papillary architecture, 2024 articles tubulo-papillary architecture, Scopus articles tubulo-papillary architecture, impact factor journals tubulo-papillary architecture, Scopus journals tubulo-papillary architecture, PubMed journals tubulo-papillary architecture, medical journals tubulo-papillary architecture, free journals tubulo-papillary architecture, best journals tubulo-papillary architecture, top journals tubulo-papillary architecture, free medical journals tubulo-papillary architecture, famous journals tubulo-papillary architecture, Google Scholar indexed journals neoplastic cells articles neoplastic cells Research articles neoplastic cells review articles neoplastic cells PubMed articles neoplastic cells PubMed Central articles neoplastic cells 2023 articles neoplastic cells 2024 articles neoplastic cells Scopus articles neoplastic cells impact factor journals neoplastic cells Scopus journals neoplastic cells PubMed journals neoplastic cells medical journals neoplastic cells free journals neoplastic cells best journals neoplastic cells top journals neoplastic cells free medical journals neoplastic cells famous journals neoplastic cells Google Scholar indexed journals oncoproteins articles oncoproteins Research articles oncoproteins review articles oncoproteins PubMed articles oncoproteins PubMed Central articles oncoproteins 2023 articles oncoproteins 2024 articles oncoproteins Scopus articles oncoproteins impact factor journals oncoproteins Scopus journals oncoproteins PubMed journals oncoproteins medical journals oncoproteins free journals oncoproteins best journals oncoproteins top journals oncoproteins free medical journals oncoproteins famous journals oncoproteins Google Scholar indexed journals
Article Details
Introduction
Adenocarcinoma with enteroblastic differentiation was previously described in the stomach [1], whilst it was rare in the large bowel [2-5]. Esophagus [6] and small intestine [7] were other sites of involvement into the gastro-intestinal (GI) tract. It was also referred as clear cell adenocarcinoma, even though such terminology could be misleading, as clear cell morphology alone do not stands for enteroblastic differentiation. Moreover, the current WHO guidelines on digestive system [8] do not encompass the clear cell variant of colonic adenocarcinoma. CAED was clinically characterized by aggressive behavior, early liver metastases and poor prognosis [3]. It was also recently suggested that the small intestinal phenotype could represent a further predictor of aggressive behavior in CAED [4]. The prevalent molecular alteration is TP53 gene mutation, whereas microsatellite instability (MSI) was less frequently observed [9]. At best of our knowledge, HER2 immunohistochemical evaluation was never reported in CAED so far.
Case Report
A 71 years old male complained obstructive GI symptoms, pointing toward peritonism. CT scan revealed the presence of a huge mass in the sigmoid. At gross examination, surgical specimen revealed the presence of a circumferential stenotic mass, measuring 6 centimeters in greatest dimension, invading the sub-serosal fat. It showed a whitish cut surface with hemorrhagic foci and pushing margins. Microscopically, tumor cells showed a complex tubulo-papillary architecture. Tumor cells were characterized by oval atypical nuclei, cleared tall cytoplasm with occasional sub-nuclear vacuole (Figure 1A,B). The unconventional histology suggested further investigations and ancillary test. Histological prognostic factors included: 1) diffuse lymphatic invasion; 2) tumor metastases in 6 of 61 loco-regional lymph nodes. Tumor deposits, venous and perineural invasion were not found. Alcian blue with periodic-acid Schiff (PAS) stained negative for glycogen or mucins (Figure 1C). Intriguingly, strong and diffuse membranous HER2 staining was detected in almost all neoplastic cells (Figure 1D). Tumor cells expressed the caudal type homeobox (CDX)-2, fetal oncoproteins such as spalt-like transcriptor factor 4 (SALL-4) and glypican (GPC)-3, whereas alpha-fetoprotein (AFP) was expressed at low level. (Figure 2A,B,C,D). Diffuse CD10 membranous immunolabeling was detected, recapitulating the healthy small intestine, cytokeratin (CK) 20 was focally reactive. Special AT-rich binding protein (SATB)2 was negative. TP53 overexpression with mutational pattern was detected (Figure 3A,B,C,D). Polyclonal carcino-embryonic antigen (pCEA) and epithelial membrane antigen (EMA) were also expressed, beta-catenin nuclear translocation was also seen. Immunolabeling for both CK7 and Vimentin was negative. The mismatch repair proteins assay exhibited a preserved nuclear expression, consistent with microsatellite stability (MSS). Molecular analysis for KRAS, NRAS, BRAF genes mutations resulted negative. Given the peculiar histological features and immunohistochemical results, a conclusive diagnosis of colonic adenocarcinoma with enteroblastic differentiation was rendered. Tumor staging was performed according to the last AJCC release (8th edition) as pT3; pN2a. The clinical post-surgical course was uneventful. CT scan revealed multiple liver metastases three months after surgery.
Discussion
The recognition of CAED was considered a rare event in colorectal surgical specimens, as it was described in few reports [2-6,10] (Table 1).
|
Author |
Year |
Cases n° |
Site |
Histology |
Immunohistochemistry |
Molecular assays |
|
Shiratori Y. [2] |
2020 |
1 |
Transverse colon |
Clear cell adenocarcinoma |
Positive staining: GPC3, CK20, CDX2 |
none |
|
Murakami T. [3] |
2020 |
5 |
Sigmoid colon, rectum |
Clear cell adenocarcinoma (4/5) |
Positive staining: GPC3, SALL4 |
none |
|
Hepatoid adenocarcinoma (1/5) |
AFP, in hepatoid variant |
|||||
|
Ogiwara S [5] |
2019 |
1 |
Sigmoid colon |
Hepatoid adenocarcinoma |
Positive staining: GPC3, SALL4 |
RAS, not muted |
|
Furuya Y [6] |
2019 |
1 |
Ascending colon |
Tubular adenocarcinoma |
Positive staining: CEA, AFP, EMA, AE1AE3, CK20, ckit, beta-catenin, |
none |
|
Kurosawa T [4] |
2022 |
42* |
Left sided in 62,5% |
Small intestinal, Large intestinal, Gastric, Mixed types. |
GPC3, SALL-4, AFP |
none |
|
Right sided 34,8% |
CD10, MUC2, MUC5A, MUC6 |
|||||
|
Leoncini Gpc |
2022 |
1 |
Sigmoid colon |
Colonic adenocarcinoma with enteroblastic differentiation |
Positive staining: GPC3, SALL-4, AFP, EMA,CDX2, CD10, CK20, |
KRAS. NRAS, BRAF. |
|
Nuclear beta-catenin, p53 mt |
MSS |
|||||
|
HER2: positive (score +++) |
Table 1: The table summarizes the reported case series (51 cases) of colonic adenocarcinoma with enteroblastic differentiation (AED), including the present case. Note that cases marked with (*) were evaluated on tissue microarrays (TMA). Abbreviation: pc: present case; mt: mutational pattern (over-expressed in more than 90% of neoplastic cells)
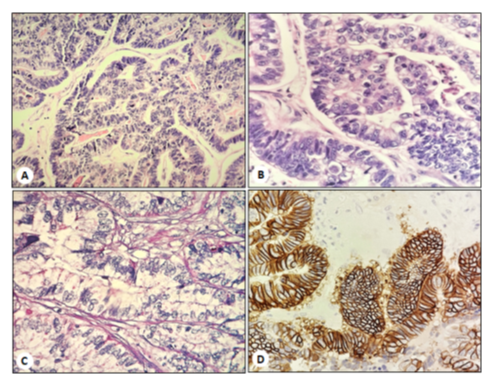
Figure 1: Neoplastic tubules and papillary structures, covered by tall cells with clear cytoplasm (Hematoxylin and eosin, magnification x10); B. Note the cleared cytoplasms with occasional subnuclear vacuole (Hematoxylin and eosin, magnification x40); C. Alcian blue - periodic-acid Schiff (PAS) reaction did not show any glycogen or mucin cytoplasmic storage (A-PAS, magnification x40); D. Strong and diffuse membranous immunostaining for HER2 in tumor cells (magnification x40).
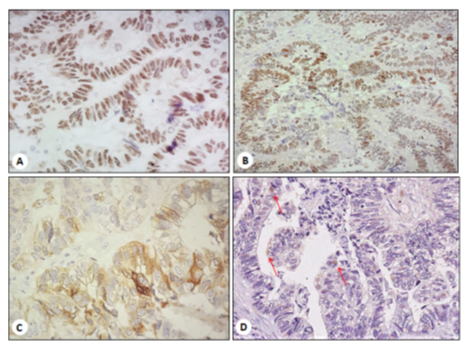
Figure 2: A. CDX2, strong nuclear immunostaining (magnification x40); B. SALL4, strong nuclear immunostaining (magnification x20); C. GPC-3, diffuse cytoplasmic expression (magnification x40); D. AFP, focal immunolabeling in single cell cytoplasms (red arrows) (magnification x20).
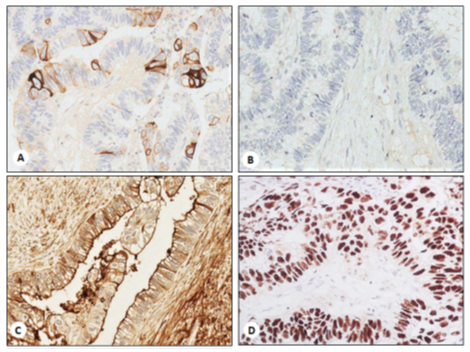
Figure 3: CK20, focal cytoplasmic and membranous expression (magnification x40); B. SATB2 negative stain (magnification x40); C. CD10, strong and diffuse membranous expression (magnification x40); D. TP53 mutational pattern, namely strong nuclear expression in more than 90% of neoplastic cells (magnification x40).
It can be found in both the right and the left colon, even though more frequently left-sided [4]. The first challenge in CAED is represented by its histological features, as tubulo-papillary structures with clear cells lining were not common in colonic adenocarcinomas. In the past decades, several clear cells adenomas and adenocarcinomas were described in the colorectum. The clear cell change has been reported to account for about 1% of colonic adenomas and associated adenocarcinomas [9,10], but the clear cell feature alone did not stand for enteroblastic differentiation, unless the oncoprotein expression was evaluated. Diagnostic inclusion criteria were far to be established for CAED and only the gastric subset has been included in the WHO classification of digestive tumors [11]. The clear cell morphology was never emphasized in GI tract, so that a clear cell variant was not provided for colonic adenocarcinoma. Thus, clear cells features alone seemed to be not enough to qualify the enteroblastic differentiation in an otherwise conventional adenocarcinoma. Growing evidences suggested the use of ancillary tests to provide a further definition about the enteroblastic differentiation in GI adenocarcinomas [12,13]. The immunohistochemical expression of GPC3, SALL4 and AFP should be evaluated in clear cell adenocarcinomas and at least two of three markers must be at least focally expressed in the tumor, even though a single marker was claimed by some Authors to be enough for qualifying the enteroblastic signature [4]. Nonetheless, non-enteroblastic tumors could express oncoproteins, as seen for the hepatoid variant of adenocarcinoma [12], but a differential should be focused on histology in such rare instances, as hepatoid adenocarcinomas usually exhibited a solid growth pattern. Another intriguing feature was represented by the divergent small intestinal differentiation showed by the present case, as highlighted by the CDX2 and CD10 positive expression. Conversely, SATB2 that is known to mark both normal and neoplastic colonic mucosa was negative. The small intestinal differentiation was recently described as a frequent finding in CAED, and it was associated to early metastatic spread and poor prognosis [4]. On the other hand, CD10 can be found to mark a small subset of conventional colonic adenocarcinomas [14,15]. The fetal midgut resemblance seemed to establish a distance between CAED and the conventional colonic adenocarcinoma, even though a common molecular trait was represented by both TP53 gene mutation and beta-catenin nuclear translocation. Interestingly, the immunohistochemical evidence of HER2 amplification in CAED tumor cells resulted in line with the molecular finding of higher HER2 gene amplification rate in CAED, compared to conventional colonic adenocarcinoma, along with a MSS dominant phenotype [16]. Altered HER2 signaling was involved as oncogenic driver in many cancer types, such as breast and gastric cancers. Note worthy, HER2 targeting represented a crucial therapeutic tool, since the biomarker can positively predict the patient benefit, and it is emerging as further treatment option in colorectal cancer as well [17]. In conclusion, both histological and immunohistochemical requirements should be met for qualifying colonic adenocarcinomas as enteroblastic. The recognition of HER2 amplification in CAED should be emphasized, paving the way for novel treatment options and target therapies.
Acknowledgements
Funding not provided
Authors’ contribution statement
Giuseppe Leoncini: paper’s conception, histological and immunohistochemical assessment, design and drafting.
Giovanna Sabella: data setting, histological and immunohistochemical assessment, molecular analysis.
Milione Massimo: paper’s critical revision and final approval.
References
- Yamada R, Horiguchi SI, Onishi T, et al. Early Gastric Cancer with Purely Enteroblastic Differentiation and No Conventional Adenocarcinoma Component. Case Rep Pathol 28 (2018): 362-393.
- Shiratori Y, Suzuki K, Ikeya T. Colonic clear cell adenocarcinoma with enteroblastic differentiation. Clin J Gastroenterol 13 (2020): 1196-1199.
- Murakami T, Yao T, Yatagai N, et al. Colorectal adenocarcinoma with enteroblastic differentiation: a clinicopathological study of five cases. Histopathology 6 (2020): 325-332.
- Kurosawa T, Murakami T, Yamashiro Y, et al. Mucin phenotypes and clinicopathological features of colorectal adenocarcinomas: Correlation with colorectal adenocarcinoma with enteroblastic differentiation. Pathol Res Pract 3 (2022): 153-184.
- Ogiwara S, Furihata M, Fukami K, et al. Hepatoid Adenocarcinoma with Enteroblastic differentiation in the Sigmoid Colon: Lessons From a Rare Case. Am J Gastroenterol 4 (2019): 684-685.
- Furuya Y, Wakahara T, Akimoto H, et al. Clear cell adenocarcinoma with enteroblastic differentiation of the ascending colon. J Clin Oncol 9 (2011): 647-649.
- Gushima R, Narita R, Shono T, et al. Esophageal adenocarcinoma with enteroblastic differentiation arising in ectopic gastric mucosa in the cervical esophagus: a case report and literature review. J Gastrointestin Liver Dis 6 (2017): 193-197.
- Mitsuma K, Taniguchi H, Kishi Y, et al. A case of adenocarcinoma with enteroblastic differentiation of the ampulla of Vater. Pathol Int 6 (2016): 230-235.
- Oyama Y, Nishida H, Kusaba T, et al. Colon adenoma and adenocarcinoma with clear cell components - two case reports. Diagn Pathol 14 (2019): 37-39.
- Shi C, Scudiere JR, Cornish TC, et al. Clear cell change in colonic tubular adenoma and corresponding colonic clear cell adenocarcinoma is associated with an altered mucin core protein profile. Am J Surg Pathol 4 (2010): 1344-1350.
- Nagtegaal ID, Odze RD, Klimstra D, et al. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 6 (2020): 182-188.
- Kwon M J, Byeon S, Kang S Yet al. Gastric adenocarcinoma with enteroblastic differentiation should be differentiated from hepatoid adenocarcinoma: A study with emphasis on clear cells and clinicopathologic spectrum. Pathol Res Pract 5 (2019): 152-155.
- Murakami T, Yao T, Mitomi H, et al. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer 9 (2016): 498-507.
- Ohji Y, Yao T, Eguchi T, et al. Evaluation of risk of liver metastasis in colorectal adenocarcinoma based on the combination of risk factors including CD10 expression: multivariate analysis of clinicopathological and immunohistochemical factors. Oncol Rep 7 (2007): 525-530.
- Fujimoto Y, Nakanishi Y, Sekine S, et al. CD10 expression in colorectal carcinoma correlates with liver metastasis. Dis. ColonRectum 4 (2005): 1883-1889.
- Yamashiro Y, Saito T, Hayashi T et al. Molecular and clinico-pathological features of colorectal adenocarcinoma with enteroblastic differentiation. Histopathology 77 (2020): 492-502.
- Guarini C, Grassi T, Pezzicoli G, et al. Beyond RAS and BRAF: HER2, a New Actionable Oncotarget in Advanced Colorectal Cancer. Int J Mol Sci 22 (2021): 68-73.
