Novel chitosan-based bone substitute. A summary of in vitro and in vivo evaluation
Article Information
Witold Bojar1*, Tomasz Ciach2 , Sylwia Flis3, Micha? Sza?wi?ski4, Maciej Jagielak5
1Department of Dental Medicine, Medical University of Warsaw, ul. Emilii Plater 21, 00-688 Warsaw, Poland
2Warsaw University of Technology, Biomedical Engineering Laboratory, Wary?skiego 1, 00-645 Warsaw, Poland
3National Medicines Institute, Chelmska 30/34, 00-725 Warsaw, Poland
4Department of Descriptive and Clinical Anatomy, Medical University of Warsaw, Cha?ubi?skiego 5, 02-004 Warsaw, Poland
5Klinika Stomatologiczna Ortognatyka, Al. Krakowska 54, 05-090 Raszyn, Poland
*Corresponding Author: Witold Bojar, Faculty of Dental Medicine, Medical University of Warsaw, ul. Emilii Plater 21, 00-688 Warsaw, Poland
Received: 26 April 2021; Accepted 03 May 2021; Published: 06 May 2021
Citation: Witold Bojar, Tomasz Ciach, Sylwia Flis, Michał Szałwiński, Maciej Jagielak. Novel chitosan-based bone substitute. A summary of in vitro and in vivo evaluation. Dental Research and Oral Health 4 (2021): 012-024.
View / Download Pdf Share at FacebookAbstract
Backgorund: Composite bone substitute materials have been raising more interest as an alternative for autologous transplants and pure xenogenic materials in oral surgery for last few years. These not immunogenic and completely resorbable biomaterials may become the basis for complete and predictable guided bone regeneration. In the majority of cases the deciding factor is ease of application of the material by a surgeon.
Objectives: The main objective of our project was to design and fabricate an osteoconductive, injectable and well tolerated by human tissues biomaterial for guided bone regeneration.
Materials and methods: For this purpose, a self-setting composite consisting of chitosan/tricalcium phosphate microparticles and sodium alginate was formulated. The obtained material was characterized as far as microsphere, agglomerates morphology and microstructure are concerned. Physical properties relating to setting time and mechanical properties were precisely investigated. Our material was evaluated according to EN ISO 10993 Biological evaluation of medical devices. Then, the implantation tests on small and big animal model were performed. Results: The tested material showed high degree of cytocompatibility, fulfilled the requirements of the International Standards, confirmed its osteoconductivity after implantation and seems to be a “user friendly” material for oral, orthopedics and neurosurgeons.
Conclusions: On the basis of positive in vitro and in vivo test results an attempt at introducing new biotechnology was made.
Keywords
Chitosan, Beta tricalcium phosphate, Alginate, Guided bone regeneration
Article Details
1. Introduction
Modern dentistry, orthopedics and neurosurgery are an area of medicine which, to a large extent, is based on new material technologies, and in the field of implantology special requirements have to be fulfilled for bone substitute materials. Although autologous bone has always been the gold standard, the most biologically viable material, its harvesting is a challenge [1,2]. Most likely for these reasons synthetic bone substitute materials attract more attention as an alternative for autologic transplants and xenogenic materials in oral surgery over the last few years. Those non-immunogenic and resorbable materials may provide the basis for complete, predictable and repetitive bone regeneration [3,4]. Materials of this type should be designed to allow blood vessel penetration and attachment of bone-forming cells. In such case there is no risk of a residual immunological and infection process, as it occurs with materials of biological origin. These can be completely biocompatible and be manufactured with precisely defined physical and crystalline properties with consistent batch quality. However, the materials currently available on the market hardly meet all the surgical and biological requirements. The aim of our project was the development and pre-clinical assessment of material which is biodegradable and well tolerated by tissues, which could be used for guided bone regeneration, with shape, size and setting time easily modifiable depending on planned application, also allowing for incorporation of active substances: signal particles of the healing process and stem or differentiated cells. The basis for this biocomposite is an organic substance- chitosan, and a non-organic one- tricalcium phosphate. The ability to combine them with other osteoconductive and osteoinductive substances allows the synthesis of biomaterial with required properties, whose high potential for bone reconstruction would go hand in hand with the security of application in humans.
2. Materials and Methods
The structure of the material was developed in cooperation with Biomedical Engineering Laboratory of the Faculty of Chemical and Process Engineering. This material is based on the biphasic system of chitosan (CH) and ß- tricalcium phosphate (TCP) with sodium alginate (Alg). This system comprises of a solid phase- the CH/TCP composite and a liquid, gel phase, which acts as a carrier and facilitates in vivo application. Chitosan (~95% degree of deacetylation) was purchased from Medical Heppe GmbH, β-tri-calcium phosphate from Sigma Aldrich and alginic acid sodium salt from brown algae was purchased from Fluka [5,6]. Chitosan/ β-tri-calcium phosphate beads were prepared by hydrodynamic formulation of CH/TCP slurry at a drop forming rate and were collected into a precipitation bath consisting of a NaOH solution. Chitosan/ β-TCP solution was prepared by suspending TCP powder in a 2% chitosan in 2% acetic acid solution [5,6]. The material is supposed to be injectable and after a couple of minutes following implantation, undergoes in situ networking. The functioning of the material would be based on combining chitosan granulation with βTCP which has been saturated with CaCl2 solution (which forms a stable gel with ions of calcium), mixing it with sodium alginate and placing the mixture on the cavity, so that the material undergoes networking on the site of implantation. Sodium alginate is supposed to be the carrier not only for the granules, but it also creates the possibility to introduce growth factors or morphogenetic cells. The concept also assumes quick sodium alginate degradation, as a result of which the porous structure of the material can be exposed enabling the growth of the new bone tissue. Sodium alginate gels have been proven to be carriers of cells and proteins that promote regeneration of mineralized tissues.
2.1 In vitro tests
The tested CH/TCP/Alg biomaterial was evaluated according to European / Polish Standard PN-EN ISO 10993 Biological evaluation of medical devices.The evaluation of the mutagenic action of tested biomaterials was carried out on the basis of the reference Ames test according to PN-EN ISO 10993-3:2009 Biological evaluation of medical devices. Results of genotoxicity, carcinogenicity and reproductive toxicity tests were published in 2012 [7]. The tests were performed within a research project No 3.83 at the National Medicines Institute [5]. After the normative in vitro tests, cytotoxicity assessment of the CH/TCP/Alg material on mouse fibroblasts, L929 was extended and additional tests with MTT method on human osteoblasts and osteosarcoma cells were performed. Commercially available, synthetic material was selected as the positive control (4Bone®, Biomatlante, France) [6]. Tests for in vitro cytotoxicity of evaluated biomaterial was also performed according to the European / Polish Standard PN-EN ISO 10993-5. Tests for in vitro cytotoxicity.
2.2 In vivo evaluation
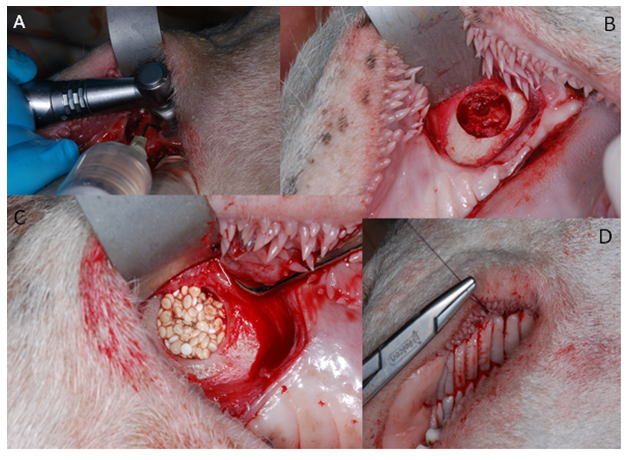
In tests on animals, the following features were assessed: skin irritation after epidermic application, intradermal reactivity and skin sensitization according to ISO 10993-10:2009 as well as systemic toxicity according to ISO 10993-11: 2009. Implantation tests on small animal model were to evaluate the dynamics of bone formation in rats’ skulls after implantation of the new chitosan/ tricalcium phosphate/ alginate biomaterial in comparison to the commercially available synthetic bone graft [7]. The Local Committee of the Animal Experimentation Ethics Commission, Poland approved the research protocol. 45 adult male rats weighing 300-400g were used for the study. The 85mm-diameter defects in calvaria bone were prepared with a trephine bur, and then filled with the bone substitute materials. The material was evaluated in comparison with that available on the market, injectable alloplastic bone substitute (easy-graft®, Degradable Solutions, Switzerland), consisting of βTCP and a PLA biolinker, histopathologically and with the application of computer microtomography, after 1 and 3 months from the application to the prepared cavity in the calvaria. Although the results of performed in vitro and in vivo tests of the CH/TCP/Alg material proved to be favorable, guided by extreme care in future patients, analyzing literature and recommendations of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products concerning marketing medical devices it was decided to expand pre-clinical evaluation [8]. The last stage of the research consisted of the assessment of osteogenic properties on a big animal model- the Polish lowland sheep (breed BCP). The in vivo performance of CH/TCP/Alg material was evaluated in an implantation test, in 12 sheep. BioOss Collagen (Geistlich AG, Switzerland) was chosen as a reference material and a positive control. The experimental material was also combined with the stem cells isolated from the subcutaneous adipose tissue from the base of the tail. The biomaterials were implanted into mechanically created defects in a sheep's maxilla (Figure 1). The selection of three different control materials at the each stage of evaluation was determined by their disadvantageous results at the first steps of research. The tests were performed in four consecutive years.
2.3 Statistical analysis
The Medistat System (microcomputer statistical system for medicinal purposes, version 2.1; 1992) was used to check the statistical significance of the results of in vitro evaluation (P<0.05). Experimental data were expressed as mean ± standard deviation (SD) from at least 8 experiments. Data were analyzed using SigmaPlot version 11 software. Statistical analysis was performed using the one-way ANOVA. Values of P<0.05 were considered to be statistically significant. Just in the case of the Ames test mean ± standard deviation (SD) and t-Student test were used for analysis. The histopathological results of in vivo testing were presented in a descriptive and semi-quantative manner for each animal or group of animals.
3. Results
3.1 In vitro tests
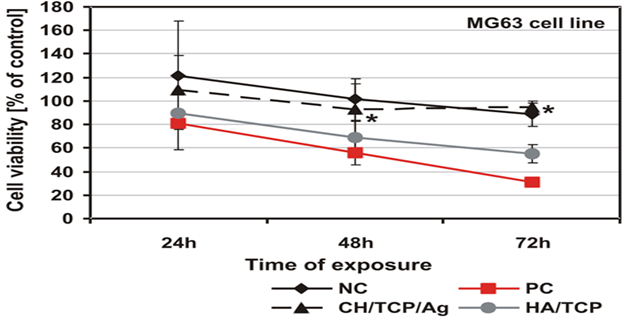
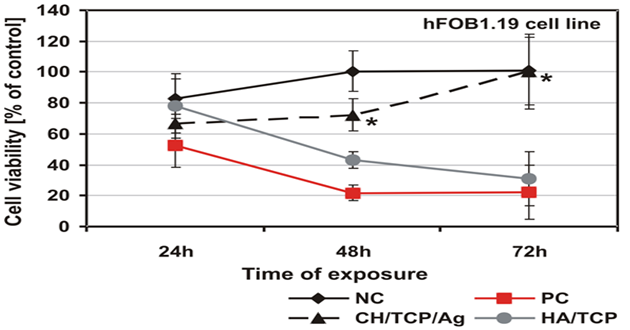
As was shown by the qualitative morphological evaluation of the cell cultures after 48-hr exposure to the extract of the tested material or after 24-hour direct contact with it, also confirmed in agar diffusion test, no malformed or degenerated cells were observed. The cells were free of intracytoplasmatic granules. Reduction of cell growth or lysis was not noted throughout all culture areas in each of the tests. The results of all the tests based on PN-EN ISO 10993-5:2009 showed a lack of cytotoxicity of the tested material [5]. Mutagenicity of the material was assessed in the Ames test, micronucleus test and comet assay. On the basis of the results obtained from the Ames test it could be stated that the material extract, in the tested range of concentrations, does not show any mutagenic properties. No relation between his+ revertants on the plate and the increase in concentration of the tested extract was found. In the tested samples no doubled numbers of his+ revertants in relation to the level in spontaneous version were found. On the basis of the results obtained from the micronucleus test no genotoxic activity was found in the case of the tested medical device. The results obtained from the comet assay showed that the CH/TCP/Alg material does not induce DNA damage in L929 cells and in CHO cells, for which the value of relative tail moment, in comparison to control cells, is statistically insignificant. The test results obtained from the comet assay were compliant with the results obtained from the Ames test and micronucleus test [5,6]. The results obtained in additional cytotoxicity tests [6] clearly proved lack of cytotoxicity of the experimental material and clear, negative influence on survival outcomes in the MG-63 and hFOB1.19 cells, thorough commercial material - positive control. According to PN-EN ISO 10993-5:2009 requirements the control material 4Bone® (Biomatlante, France) (consisted of HA and TCP) was cytotoxic after 72hrs of exposition of extract to the tested cells, and CH/TCP/Alg proved not to be cytotoxic in all the time points of the exposition (Figures 2 and 3) [6]. The results of the tests performed on the MG-63 and hFOB1.19 cell lines were quite differentiated for CH/TCP/Alg and control, commercial biomaterial. Both cell lines were not negatively affected by the extracts of CH/TCP/Alg. On the contrary, response of both cell lines unquestionably proved the cytotoxicity of MIS 4Bone® (Biomatlante, France). In addition, CH/TCP/Alg showed stimulative properties for proliferation of osteoblasts. Although it has been repeatedly reported that biocompatibility of tricalcium phosphate ceramic materials is high, this research showed important and significant diversification of two materials of similar chemical composition.
3.2 In vivo evaluation
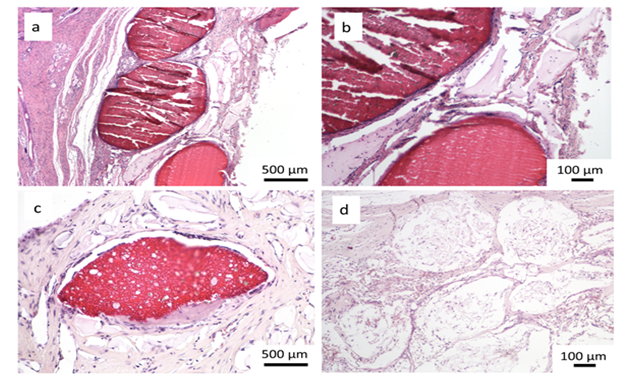
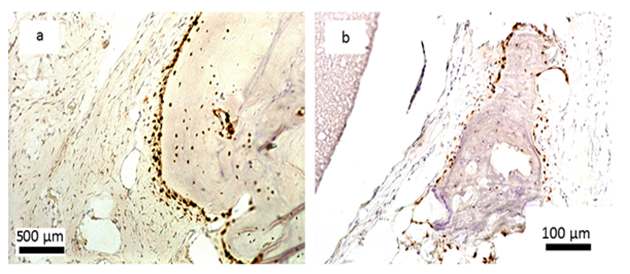
Encouraging results were also observed in vivo. The saline extract of the bone substitute material produced no irritant effects when injected intradermally to rabbits (no. of 3). The difference between the reaction to the tested sample and the reaction to the control sample was less than “1”. Likewise, no topical reaction was seen following direct application of the solid bone substitute to intact skin. Since rabbit skin is more sensitive than human skin to chemical irritants, the likelihood of local irritation from this material in humans is negligible [5,6]. The CH/TCP/Alg material did not show any sensitizing effects in 18 guinea pigs, so the risk for allergic reaction in humans should be considered unlikely. There were also no differences seen in mice (no. of 10) appearance and behaviour, as well as in animal weight gains between the tested and the control groups indicating that the bone substitute material does not produce systemic toxicity. The number of animals were according to EN ISO 10993 Standard and the research protocol was approved by the Local Ethical Commision for Animal Experimentation in Warsaw. Favorable in vitro and in vivo test results allowed the commencement of direct implantation to bone in a small animal model- Wistar rats. Diverse results were obtained, confirming high bone substitute potential of the new material and surprisingly undesirable inflammatory reactions around the control material (Figure 4). Around all the samples of the CH/TCP/Alg material, development of woven bone was observed after just a month. In 85% of cases there were no signs of purulent inflammation with neutrophils infiltration as well as granulomatosis with infiltration of giant and epithelioid cells. Observation under a light microscope, after three months, showed that around the biomaterial, trabecular bone developed without significant signs of inflammation. There was no granulomatosis, which could be noticed easily after 3 months in specimens with the control material assessed. The CH/TCP/Alg material proved its osteogenic properties also in immunohistochemical evaluation (Figure 5). Its advantage over the positive control material was particularly visible after a month [7]. The positive control material, commercially available easy-graft®, showed osteogenic potential after three months which is similar to the experimental one, however, presence of neutrophils - markers of an acute inflammation were revealed. Presence of macrophages and giant cells after 1 month proved, on the other hand, a foreign body inflammatory response. It was proven that development of new bone was inhibited at that time. A significant number of neutrophils and lymphoid cells were also found. The results of this experiment prove to be contrary to the producer's declarations on biocompatibility of this medical device [7]. The results of implantation on big animal model were also satisfying. Samples of sheep’s maxillas taken 4 months after implantation were histopathologically assessed. In vivo tests gave the results that confirmed favorable osteogenic properties of both evaluated biomaterials: BioOss Collagen (Geistlich AG, Switzerland) and CH/TCP/Alg. In the histopathological evaluation the following were observed: visible bone remodeling area with an active presence of osteoblasts and single osteoclasts on its verges. In the sites of mesenchymal remodeling there were ossification areas with active participation of osteoblasts. It should be stressed that high compatibility of both materials was observed in nearly all cases. In the case of negative control- defects left without biomaterial just with a blood clot, most often the features of passive hyperaemia and reactive fibrosis around small bone structures as well as fibrosis of perivascular parenchyma without traits of ossification were observed [8]. What is more, a combination of the experimental material with stem cells in the amount possible to obtain from a few grams of adipose tissue did not significantly improve the bone formation and requires further research. The research was approved by the Local Ethical Commision for Animal Experimentation in Lublin.
4. Discussion
Chitosan is a naturally occurring polysaccharide which is used in numerous fields of medicine and food industry. Its bioactivity is the result of a number of processes and phenomena such as biodegradation, membrane interactions, polycationic character or stimulation of organism immunity. It has been applied to the production of various medical devices, e.g. sponges for staunching blood flow, blood vessel prostheses, plasmapheresis membranes, foils for burns, bandages, developing porous structures in tissue engineering, implants for releasing medicines into blood [9,10].
In this project the most important and desired property of chitin and its derivates is their capability to form three-dimensional scaffoldings for guided bone and tissue regeneration, which has been described frequently in literature [11-17]. When searching the proper material for this purpose we should take into consideration its porous structure enabling tissue in-growth, capability to create reabsorbable matrix, biodegradation and lack of toxic substances released during lysis. Chitosan seems to have those desired properties both in biological and physicochemical areas. Its great ability to gel and to create various porous structures obtained during thermally induced separation method allows for the wide application in biomedical field. It should be mentioned that some authors [13,17], suggest that chitosan has osteoconductive and osteoinductive properties when it is used as a material for scaffoldings in tissue engineering. Pure phase beta-tricalcium phosphate - βTCP (tricalcium phosphate according to IUPAC) is also non-immunogenic and completely resorbable. When combined with chitosan they are materials that can be used for complete, predictable and repetitive bone regeneration. βTCP is supposed to take the function of osteoconductive factor, facilitating the development of bone tissue through chemical copying of natural bone [3,18,19]. The design of CH/TCP/Alg particle was in attempt to allow complete biodegradation after serving as a scaffold for the bone tissue. This may help to avoid any potential adverse reactions as opposed to xenogenic materials [3,20]. The priority was to obtain a material fully substituted by the own bone of a recipient. Therefore beta tricalcium phosphate, not hydroxyapatite was chosen as one of the components. After completing the engineering part of our research, further evaluation moved on to in vitro and in vivo testing. Cell cultures and bacterial strains provide a convenient, controllable and repetitive instrument for a preliminary evaluation of the biological response. Cytotoxicity and genotoxicity are important factors affecting the systemic compatibility of an implantable material. In general, cytotoxicity in vitro is a simulation of the biological response to the material through the exposition of the cell cultures to the extracts or direct contact to the material. Due to serious and life-threatening consequences these tests are gaining increasing public interest [19-23]. The reference research and the need for verifying normative recommendations led us to widen in vitro tests. More sensitive and susceptible for extracts of bone substitute materials cell lines were chosen for further evaluation [22,24]. In vitro testing is the most popular method for the characterization of bone grafting materials, especially as researchers embrace the doctrine of animal reduction.
These tests should be used principally as a first stage test for acute toxicity and cytocompatibility to avoid unnecessary use of animals in the evaluation of cytologically inappropriate materials [23]. However, in vitro evaluation is not able to demonstrate the tissue response to biomaterials, being confined to the response of individual cell lines. Sometimes in vitro tests may also overestimate the level of material toxicity [22]. One major limitation to bone culture is also the lack of controlled physiological loading [23]. When considering the small animal model as one of the three steps of a novel biomaterial’s in vivo evaluation, rat seems to be the proper species, as many researchers confirmed [25-27], despite its dissimilarity with human bone and size of the bones making rats unsuitable for testing multiple implants simultaneously [23]. The results of implantation tests were undoubtedly encouraging. The histological evaluation revealed the presence of newly formed bone tissue around the experimental biomaterial without significant acute and chronic inflammatory responses. There were clear signs of bone formation at 4 and 12 weeks what was confirmed in immunohistochemical evaluation. It was indicated that in specimens at both 4 and 12 weeks following implantation, the graft particles of CH/TCP/Alg were in direct proximity to the sites exhibiting signs of de novo bone formation. The osteoclasts and osteoblasts are suggested to be attracted by the surface of the graft particles [7]. Unfortunately the chosen positive control (EG) acted like a negative control, but this was not our intention and should be undoubtedly investigated in future. The presence of multinucleated giant cells and a foreign body-type inflammatory reaction are known to markedly inhibit new bone formation [28]. Some of the graft material particles were disintegrated and replaced by mesenchymal and inflammatory cells at 4 weeks. At 12 weeks bone formation was observed only in some of them. In fact these evident results were surprising for us and are not consistent with data published by the manufacturer. In case of control material a cell based resorption of the material occurred inside the particles followed by new bone formation, which is consistent with results published by Chazono et al and Araujo et al [29,30]. On the basis of implementation character of the research we decided to widen preclinical in vivo evaluation [5]. According to many authors a dog is the most suitable model for human bone from a biological point of view [20,23,30]. However, an adult sheep is more similar in terms of weight and bone dimensions, which allows for implantation of e.g. human endoprostheses [31]. We also have in mind the negative ethical implications of using companion animals for medical research. Another indication for using sheep is the fact that we may choose animals which are approaching the end of their breeding cycle The animals are not sacrificed just for the research but are utilized for goods commonly obtained from sheep breeding. In order to design and carry out this experiment we managed to start with the cooperation of the department of Small Ruminants Breeding and Agricultural Advisory at the University of Life Sciences in Lublin. Here we must acknowledge that the results of implantation tests on big animal model were not as variable and evident as on rats. The essential outcome of the study was the lack of significant difference between the experimental biomaterial and the positive control- BioOss Collagen (Geistlich AG, Switzerland). BioOss® is unquestionable a material with proven clinical efficacy [32-38].
It is unarguably difficult to extrapolate the long-rage osteogenic potential of our experimental allogenic material on the basis of 4 month study [8]. The fact of initial disintegration of the particles is somehow promising. Our intention was to design a completely resorbable material which stimulates the bone formation and is replaced by autogenous bone without remnants. At this point it is also impossible to legitimate the beneficial influence of the adipose derived stem cells in obtained amount composed with graft material for the bone formation. It seems like the number of cells isolated per gram of the sheep adipose tissue (1,1-2,32x105 cells) and the amount of 2g of the collected tissue does not enhance the bone formation and that requires the further testing. The evaluation of using stem cells in oral surgery is carried out by numerous research centers. Our results may confirm the need for culturing preosteoblasts in vitro before colonizing the grafts [39]. Unfortunately still only autogenous grafts have capacity to activate all basic mechanisms of bone regeneration: osteogenic activity, osteoinduction and osteoconduction [39,40]. However significant progress in tissue engineering may provide to the medics composite biomaterials with desirable properties in near future [39]. This research project seems to be a noticeable part of search for highly biocompatible bone substitutes which may be the carriers for medicinal products. Our biomaterial was carefully evaluated in the broad spectrum of tests. Its biocompatibility and bone regeneration potential was affirmed, its safety and the method of manufacturing was detailed [5-8]. On the basis of positive results an attempt at introducing new biotechnology was made [19]. The patent application no. P-395212 pertaining to the described biomaterial was accepted by the Polish Patent Office. In the near future we plan to file an application to the Bioethical Commission for clinical testing.
Figure 4: Histological evaluation after 4 and 12 weeks after implantation; a, b, c- CH/TCP/Alg biomaterial, d- control; a,b- woven bone tissue around the graft observed after 4 weeks, c- new bone formation and degradation of the graft after 12 weeks, d- slight bone formation and inflammatory response around the commercially available bone substitute material [7].
Acknowledgments
The tests were performed within a research project No 3.83 at the National Medicines Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaminski A, Zasacka M, Wanyura H. Demineralized bone matrix- preparation and application in dental treatment. Czas Stomat 50 (2007): 601-610.
- Krzyma?ski G, J?drzejczak W. Autologous bone marrow-derived stroma fibroblastoid cells grown in vitro for the treatment of defects of mandibular bones. Transplant Proc 28 (1996): 3528-3530.
- Horovitz RA, Mazor Z, Foitzik C, et al. Β-Tricalcium Phosphate as Bone Substitute Material: Properties and Clinical Applications. Titanium 1 (2009): 1-11.
- Artzi Z, Nemcovsky H, Tal H. Efficacy of Porous Bovine Bone Mineral in Various Types of Osseous Deficiencies: Clinical Observations and Literature Review. Int J Periodontics Restorative Dent 21 (2001): 395-405.
- Bojar W. Novel, chitosan-based, bone substitute composite material. Synthesis, in vitro and in vivo evaluation. Narodowy Instytut Leków, Warszawa (2015).
- Bojar W, Ciach T, Kucharska M, et al. Cytotoxicity evaluation and crystallochemical analysis of a novel and commercially available bone substitute material. Adv Clin Exp Med 24 (2015): 511-516.
- Bojar W, Kucharska M, Ciach T, et al. Bone regeneration potential of the new chitosan based alloplastic biomaterial. J Biomater Appl 28 (2014): 1060-1068.
- Bojar W, Kucharska M, Ciach T, et al. In vivo performance of the experimental chitosan based bone substitute- advanced therapy medicinal product. A study in sheep. Acta Poloniae Pharmaceutica- Drug Research 73 (2016): 209-217.
- Kucharska M, Walenko K, Butruk B, et al. Fabrication and characterization of chitosan microspheres agglomerated scaffolds for bone tissue engineering. Materials Letters 64 (2010): 1059-1062.
- Kucharska M, Butruk B, Walenko K, et al. Fabrication of in-situ foamed chitosan/b-TCP scaffolds for bone tissue engineering application. Materials Letters 85 (2012): 124-127.
- Przekora A, Pa?ka K, Macherzy?ska B, et al. Structural properties, Young’s modulus and cytotoxicity assessment of chitosan-based composites . Engineering of Biomaterials 114 (2012): 52-58.
- Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials 24 (2003): 2339-2349.
- Li Z, Ramay HR, Hauch KD, et al. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 26 (2005): 3919-3928.
- Kim IY, Seo SJ, Moon HS, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26 (2008): 1-21.
- Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials 24 (2003): 2339-2349.
- Shyh MK, Shwu JC, Cheng-CG, et al. Guided tissue regeneration with use of β-TCP / Chitosan composite membrane. J Appl Polymer Science 112 (2009): 3127-3134.
- Hsu S, Whu SW, Tsai CL, et al. Chitosan as Scaffold Materials: Effects of Molecular Weight and Degree of Deacetylation. J Polym Res 11 (2004): 141-147.
- Kozakiewicz M, Marciniak-Hoffman A, Denkowski M. Long term comparison of application of two beta-tricalcium phosphates in oral surgery. Dent Med Probl 46 (2009): 384-388.
- Bojar W, Kucharska M, Bubak G, et al. Formulation and preclinical evaluation of the new alloplastic injectable bone substitute material. Acta of Bioengineering and Biomechanics 14 (2012): 39-44.
- Santos FA, Pochapski MT, Martins MC, et al. Comparison of biomaterial implants in the dental socket: histological analysis in dogs. Clin Implant Dent Relat Res 12 (2010): 18-25.
- Heddle JA et al. The bone marrow micronucleus test. W Handbook of mutagenicity test procedures. Amsterdam: Elsevier 10 (1984): 441-457.
- Pizzoferrato A, Ciapetti G, Stea S, et al. Cell culture methods for testing biocompatibility. Clin Mater 15 (1994): 173-190.
- Pearce AI, Richards RG, Milz S, et al. Animal models for implant biomaterial research in bone: a review. European Cells and Materials 13 (2007): 1-10.
- Nahid M, Bottenberg P. Importance of cell cultures in biocompatible dental materials research. Rev Belge Med Dent 58 (2003): 189-196.
- Marins LV, Cestari TM, Sottovia AD, et al. Radiographic and histological study of perennial bone defect repair in rat calvaria after treatment with blocks of porous bovine organic graft material. J Appl Oral Sci 12 (2004): 62-69.
- Moreschi E, Hernandes L, Dantas JA, et al. Effect of dolomite in the repair of bone defects in rats: histological study. Histol Histopathol 25 (2010): 1547-1556.
- Inoda H, Yamamoto G, Hattori T. Histological investigation of osteoinductive properties of rh-BMP2 in a rat calvarial bone defect model. Journal of Cranio_Maxillo-Facial Surgery 32 (2004): 365-369.
- Lassus J, Salo J, Jiranek WA, et al. Macrophage activation results in bone resorption. Clin Orthop 352 (1998): 7-15.
- Chazono M, Tanaka T, Komaki H, et al. Bone formation and bioresorption after implantation injectable beta tricalcium phosphate granules-hyaluronate complex in rabbit bone defects. Journal of Biomedical Materials Research 70 (2004): 542-549.
- Araujo MG, Liljenberg B, Lindhe J. β-tricalcium phosphate in the early phase of socket healing: an experimental study in dog. Clin Oral Impl Res 21 (2010): 445-454.
- Bruder SP, Kurth AA, Shea M, et al. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. Journal of Orthopaedic Research 6 (1998): 155-162.
- Dominiak M, ?ysiak-Drwal K, Gerber H. Use of selected xenografts in the treatment of multiple intraosseus defects after apicectomy- case report. Dent Med Probl 45 (2008): 77-81.
- Markowska J, Radwan-Oczko M, Zi?tek M. Clinical and radiographic evaluation of Bio-Oss for the treatment of periodontal intra-bony defects. Polim Med 35 (2005): 1-12.
- Ciechowicz K, Plakwicz P, Thun-Szretter K, et al. The use of bone substitutes in regeneration of jaw bone defects. Data from Oral Surgery Department in Warsaw, during the 1999-2001 years. Prot Stom 52 (2002): 273-278.
- Mendonca TA, Conz MB, Barros TC, et al. Physiochemical characterization of two deproteinized bovine xenografts. Braz Oral Res 22 (2008): 5-10.
- Maiorana C, Redemagni M, Rabagliati M. Treatment of maxillary ridge resorption by sinus augmentation with iliac cancellous bone, anorganic bovine bone and endosseous implants: A clinical and histologic report. Int J Oral Maxillofac Implants 15 (2000): 873-878.
- Browaeys H, Bouvry P, Bruyn H. A literature review on biomaterials in sinus augmentation procedures. Clin Implant Dent Res 9 (2007): 166-177.
- Wojtowicz A, Perek J, Urbanowska E, et al. The treatment of maxillary bone defects used autologic pre-osteoblasts on allogenic bone scaffolds. Dent Med Probl 50 (2013): 20-29.
- Pi?tka T, Krzyma?ski G, Doma?ski W, et al. Allogenic frozen bone and autologenic bone marrow grafts in the treatment of extensive jawbone defects. Czas Stomatol 5 (2007): 312-320.
- Pagni G, Pellegrini G, Giannobile WV, et al. Postextraction alveolar ridge preservation: biological basis and treatments. Int J Dent 1 (2012): 151030.