Not All Perineal Lesions are Extra-Intestinal Manifestations of Inflammatory Bowel Disease
Article Information
Mark Mahon*, Rachel Blasiak, Nichelle Simmons, Daniela Levanon
Jacobi Medical Center, NY, USA
*Corresponding Author: Mark Mahon, Jacobi Medical Center, 1400 Pelham Parkway S, Bronx, NY 10461, USA
Received: 30 June 2021; Accepted: 08 July 2021; Published: 01 October 2021
Citation:
Mark Mahon, Rachel Blasiak, Nichelle Simmons, Daniela Levanon. Not All Perineal Lesions are Extra-Intestinal Manifestations of Inflammatory Bowel Disease. Journal of Pediatrics, Perinatology and Child Health 5 (2021): 204-210.
View / Download Pdf Share at FacebookAbstract
This article is focused on the description of a clinical case that showcases a rare association between Inflammatory Bowel Disease and Hidradenitis Suppurativa. Our primary aim is to highlight this presentation at an atypical timing in the disease course, which necessitated escalation of treatment.
Keywords
Inflammatory Bowel Disease, Hidradenitis Suppurativa, Crohn’s Disease
Inflammatory Bowel Disease articles; Hidradenitis Suppurativa articles; Crohn?s Disease articles
Article Details
Abbreviations:
IBD: Inflammatory Bowel Disease; HS: Hidradenitis Suppurativa; CD: Crohn’s Disease
1. Introduction
Perineal lesions present a relatively broad differential across a number of pediatric subspecialties. Such lesions can present as a primary disease or a secondary manifestation of a systemic disease, an acute or chronic process. Concomitant symptomatology should always be considered to direct the necessary evaluation. In Inflammatory Bowel Disease (IBD), while perineal lesions are a common extra-intestinal manifestation, other etiologies should be entertained. This brief report focuses on an unusual dermatological association with IBD, presenting at an atypical time in the disease course. It showcases the need to increase awareness to this phenomenon in order to tailor treatment accordingly.
2. Case Presentation
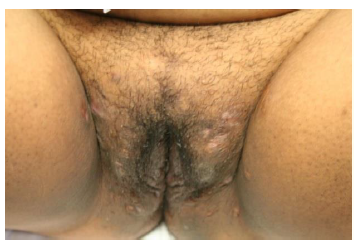
An 11-year-old female presented to the emergency department with the chief complaint of epistaxis. After acute care management, the patient reported the presence of painful lower extremity nodules and gluteal cleft lesions. A review of systems revealed fatigue, anorexia and diarrhea. The patient reported ongoing symptoms for two months prior to presentation. The growth chart demonstrated a 15lb (6.8 kg) weight loss over that time. The patient was up-to-date on vaccinations, denied recent travel, sexual activity or sick contacts. Vital signs recorded on presentation were a temperature of 101.100F, heart rate of 128 beats per minute, blood pressure of 103/65 mmHg, respiratory rate of 24 per minute and oxygen saturation of 100%. Her physical exam was remarkable for the following: mild periumbilical tenderness without evidence of peritonitis or organomegaly, papules and erosions to the labia majora and the intertriginous regions of the inguinal and gluteal folds [Figure 1], as well as a rash consistent with erythema nodosum on both lower extremities.
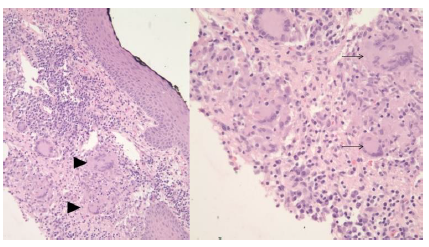
Baseline laboratory values were notable for a white cell count of 13.1/nL, an absolute lymphocyte count of 2.3/nL (a significant left shift), hypochromic microcytic anemia with a hemoglobin of 10.3 g/dL, mean corpuscular volume of 70.3 fL and thrombocytopenia of 55/nL. Inflammatory markers were elevated as well: C-reactive protein (99 mg/L) and erythrocyte sedimentation rate (120 mm/hr). Upon consultation with the Pediatric Gastroenterology service the patient was admitted for further evaluation. On admission, the patient underwent an investigation that included stool studies which were negative for blood and pathogens (including Clostridium Difficile). Fecal leukocytes were present. Due to the concern for IBD, a computed tomography (CT) of the abdomen and pelvis was obtained and revealed mural thickening of the ascending and transverse colon with fat stranding. Segmental colitis was then confirmed on colonoscopy, with additional evidence of cryptitis, reactive lymphoid hyperplasia and non-caseating granulomas in the sigmoid colon on histopathology. A punch biopsy performed on one of the gluteal lesions, at the same time, was documented as granulomatous dermatitis [Figure 2]. This was suspected, in the setting of IBD, to be cutaneous Crohn’s Disease (CD).
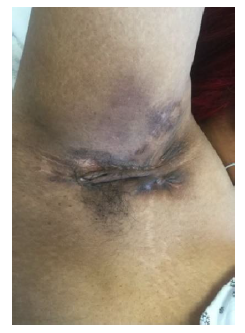
Empiric treatment was initiated with Metronidazole and Methylprednisolone for her gastrointestinal symptoms. In addition, serial Sitz Baths, Mupirocin and Hydrocortisone 1% were applied to the perineal lesions. However, the patient’s clinical course became complicated by the development of a rectovaginal fistula. This prompted treatment escalation with an Infliximab regimen. After two weeks, the patient was discharged with the diagnosis of CD with perineal involvement. The gastrointestinal course improved clinically on that treatment, mirroring the normalization of inflammatory markers and hematological indices. Within three years of diagnosis and treatment, while being followed by a multidisciplinary team, the patient reported progression of intertriginous lesions, this time in a new location; the axillae [Figure 3] with infra-mammary involvement. Another punch biopsy was performed on the former, non-perineal lesion, confirming the now clinical diagnosis of Hidradenitis Suppurativa (HS), on pathological examination.
3. Discussion
HS is a chronic inflammatory disease involving apocrine glands of the skin, characterized by recurrent and painful, deep-seated nodules, abscesses, sinus tracts and/or fistulas [1]. Apocrine glands are primarily found in inverse areas of the skin such as the groin and axillae. Generally developing after puberty, the majority of cases arise in the second to third decades of life. There is a prevalence of 1%, with women three times more likely to be affected than men [2]. Smoking and obesity have been identified as important parameters in disease progression and severity [3]. Disease severity has been defined using the Hurley’s Criteria. This classification system is used to grade the severity of intertriginous lesions according to Stages I-III (Table 1) [4]. Chronicity is a hallmark of the disease, so early lesions at a single site should undergo surveillance, to avoid misdiagnosis. The axillae and inguinal regions are the two primary sites of involvement. Topographic gender variation exists for non-axillary lesions with perineal, perianal and gluteal being male-predominant [5]. Initial presentations of non-axillary involvement call for a broad differential.
In considering the differential diagnosis for a perineal lesion, infectious, gastrointestinal and dermatological etiologies should be considered. Herpes Simplex Virus infection can result in genital ulcerations manifesting as a crop of vesicles that are often painful and associated with systemic symptoms such as fever and malaise (in primary infection). Although usually singular, a pilonidal abscess can occur when cysts within the gluteal cleft become occluded by hair follicles, acting as a nidus for bacterial growth, particularly Staphylococcal Aureus. Such cases tend to present with an erythe-matous, fluctuant and tender mass. Less severe manifestations include furuncles and carbuncles. In indolent, non-resolving lesions, an infective etiology should be sought to consider mycotic organisms or mycobacterium. Gastrointestinal associations with perineal lesions include extra-intestinal manifestation of CD in the form of anoperineal ulcerations that may be complicated by a fistulizing tract. Skin tags are also common. In addition, Bechet’s Disease (BD) may present with recurrent aphthous ulcers involving both the oral and genital mucosa. The latter is a chronic, autoimmune vasculitis with a multisystem involvement. Interestingly, BD and HS fall under the same category of neutrophilic dermatoses, a group of inflammatory skin disorders with neutrophilic infiltrates on histopathology [6].
There is a rare, but established association between CD and HS, strongest in severe phenotypes and pancolitis [7]. Up to 25% of patients with inflammatory bowel disease experience extra-intestinal manifestations, some as the primary presentation. Perineal pathology occurs in 50% of these cases [8]. This can complicate the diagnosis of HS when both disease states coexist. A timeline has been identified for HS, with the diagnosis made on average one decade after that of CD [9], making the association more likely to present with the adult gastroenterologist. Most CD associated perineal lesions involve the epithelium of the anal canal and contiguous mucosa. In the absence of another intertrig-inous apocrine gland involvement, the consideration of HS is difficult and can delay accurate diagnosis. To assist in the differentiation, inter-gluteal prevalence has been reported to be more common in IBD patients complicated by HS [10]. If perianal disease is present and fistulizing, then HS can be formally differentiated from CD, as such HS-tracts do not extend beyond the depth of the apocrine glands within the anal canal [11], one third distal to the dentate line.
Once diagnosed, the treatment options available for HS are dictated by disease severity using the Hurley Staging [12]. Topical Clindamycin (1-2%) or Resorcinol (15%), a topical antiseptic, are the first line option for localized Hurley Stage I, whereas oral antibiotics such as Tetracycline or Doxycycline are reserved for the more widespread Hurley Stage II lesions. Immunosuppressive agents and/or surgery are used for refractory cases. When associated with other pathology, treatment options should be discussed as part of a multi-disciplinary team to reduce side effects and enhance dual disease quiescence. Similar to our patient above, the majority of IBD-HS cases are diagnosed with the more severe phenotype, Hurley Stage III [9]. At this point, the use of an immunosuppressive agent, Tumor Necrosis Factor-alpha blocker (anti-TNF), is required to control symptoms. Dose escalation is often needed [13], with roughly 50% achieving clinical remission [14]. Deroofing and excision (local or wide) [12] are reserved for those who fail medical treatment.
|
Hurley Stage |
Dermatological Description |
Patient Description |
|
I |
Solitary, painful and deep-seated nodule that usually progresses to an abscess formation. |
“Lump, that is tender and painful to touch” |
|
II |
Recurrent nodules or abscess formation, that often coalesce forming larger secondary lesions with sinus tracts. |
“Much larger lump or swelling, that is draining fluid or oozing” |
|
III |
Multiple interconnected sinus tracts and abscesses, that have formed hypertrophic fibrous scar tissue. |
“Tight bands of tissue, that limits my arm moving” |
Table 1: Hurley Stages of Hidradenitis Suppurativa [4].
4. Conclusion
HS, when associated with CD, tend to present one decade after the initial IBD diagnosis. Most perineal lesions are presumed to be extra-intestinal manifesta-tions of IBD. However, as is demonstrated in this case, not all perineal lesions are so, even in a well established disease. The differential diagnosis should remain broad for perineal lesions, especially those arising early in the clinical course. This case presents a much shorter interval between IBD and HS diagnoses, and potentially a dual presentation, which has not been appreciated in the literature to date. Given that additional therapeutic modalities for HS exist, early diagnosis may change the treatment. This highlights a diagnostic challenge for the treating physician when an unrelenting perineal lesion persists, at times, despite early immunologic IBD treatment. In addition, it emphasizes the need to have increased surveillance during transition of care for this cohort of adolescent IBD patients in order to avoid a misdiagnosis in new or refractory perineal lesions.
Author Contributorship
MM and DL performed chart review and extraction of medical details. MM scripted the paper, with DL providing editorial guidance. RB provided detailed input regarding description of dermatological photographs, while NS provided histopathological description of slides. Both, RB and NS provided their relative subspeciality input. MM submitted the paper.
Funding
No funding was required for this submission.
Ethics Approval
Consent was obtained from the patient; no further ethical approval was required by the institution.
Informed Consent Statement
Informed consent was obtained from the patient for use of clinical photograph.
Acknowledgements
No further acknowledgements are indicated, outside of authorship.
Competing Interests
The authors declare no conflict of interest.
References
- Jemec GB. Hidradenitis suppurativa. New England Journal of Medicine 366 (2012): 158-164.
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 35 (1996): 191-194.
- Jemec GBE. Body weight in hidradenitis suppurativa. In: Marks R, Plewig G, editors. Acne and related disorders. Dunitz, London (1989): 375-376.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. Journal of the American Academy of Dermatology 81 (2019): 76-90.
- Jemec G, Revuz J, Leyden JJ. Hidradenitis suppurativa. Springer Science & Business Media (2006).
- Nelson CA, Stephen S, Ashchyan HJ, James WD, Micheletti RG, Rosenbach M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. Journal of the American Academy of Dermatology 79 (2018): 987-1006.
- Kamal N, Cohen BL, Buche S, et al. Features of patients with Crohn’s disease and hidradenitis suppurativa. Clinical Gastroenterology and Hepatology 14 (2016): 71-79.
- Seksik P, Contou JF, Cosnes A, et al. Hidradenitis suppurativa and Crohn’s disease. InHidradenitis suppurativa (2006): 50-57.
- Yadav S, Singh S, Varayil JE, et al. Hidradenitis suppurativa in patients with inflammatory bowel disease: a population-based cohort study in Olmsted County, Minnesota. Clinical Gastroenterology and Hepatology 14 (2016): 65-70.
- Van der Zee HH, de Winter K, Van Der Woude CJ, et al. The prevalence of hidradenitis suppurativa in 1093 patients with inflammatory bowel disease. British Journal of Dermatology 171 (2014): 673-675.
- Culp CE. Chronic hidradenitis suppurativa of the anal canal. Diseases of the colon & rectum 26 (1983): 669-676.
- Saunte DM, Jemec GB. Hidradenitis suppurative: advances in diagnosis and treatment. Jama (2017).
- Machet L, Samimi M, Delage M, et al. Systematic review of the efficacy and adverse events associated with infliximab treatment of hidradenitis suppurativa in patients with coexistent inflammatory diseases. Journal of the American Academy of Dermatology 69 (2013): 649-650.
- Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. New England Journal of Medicine 369 (2013): 754-762.