Neuroimaging Findings and Outcome in Pediatric Cephalocele with Chiari II-Like Stigmata
Article Information
Rida Salman1, Nilesh K. Desai1, Stephen F. Kralik1, Michael A. Belfort2, Livja Mertiri1, Gunes Orman1 , Thierry A.G.M. Huisman1
1Edward B. Singleton Department of Radiology
2Department of Obstetrics and Gynecology, Texas Children's Hospital and Baylor College of Medicine, Houston, TX 77030, United States
*Corresponding Author: Thierry A.G.M. Huisman, Edward B. Singleton Department of Radiology, Texas Children's Hospital and Baylor College of Medicine, 6701 Fannin St. Suite 470, Houston, TX, 77030, USA
Received: 10 July 2023; Accepted: 18 July 2023; Published: 22 August 2023
Citation: Rida Salman, Nilesh K. Desai, Stephen F. Kralik, Michael A. Belfort, Livja Mertiri, Gunes Orman , Thierry A.G.M. Huisman. Neuroimaging Findings and Outcome in Pediatric Cephalocele with Chiari II-Like Stigmata. Journal of Radiology and Clinical Imaging 6 (2023): 182-186.
View / Download Pdf Share at FacebookAbstract
Background and Objective: There is a known association between Chiari malformation and occipital encephalocele. We aim to identify the frequency of Chiari II-like neuroimaging findings and radiologic and clinical outcomes after surgical repair in pediatric patients with cephalocele.
Materials and Methods: Retrospective review of brain CT and MRI of children with cephalocele between 1/1/2011-11/30/2021 was performed in consensus. Demographic, clinical and surgical data were recorded. The location and size of defect, type of herniated tissue, posterior fossa size, hindbrain herniation (HH) into the upper spinal canal, tectal beaking (TB), cortical malformation (CM), corpus callosum abnormalities and hydrocephalus were collected. Clinical and radiologic outcome were noted.
Results: Twenty-six patients were included (19 females, 73%) with mean age at diagnosis 121+/-565 days. Neuroimaging showed occipital defect in 23 (89%), meningoencephalocele in 18 (69%) and small posterior fossa in 18 (69%). HH was present in 12 (46%), TB in 5 (19%) and CM in 11 (42%). No hydrocephalus in 15 (58%), normal corpus callosum in 11 (42%) patients. One patient did not have documented clinical follow-up note. Sixteen (64%) had good outcome. No statistically significant difference in clinical outcome regarding the radiologic findings at diagnosis. Small posterior fossa (p=0.04) and presence of HH (p=0.001) on initial imaging was associated with postsurgical HH. Defect location (p=0.03) and presence of hydrocephalus (p=0.01) on initial imaging was associated with postsurgical hydrocephalus.
Conclusions: Less than half of cephalocele patients demonstrate Chiari II-like findings on initial imaging. Variable changes in HH and hydrocephalus occur following surgery suggesting that it is an acquired deformity sequence.
Keywords
Cephalocele; Chiari-II malformation; Hindbrain herniation; Hydrocephalus
Cephalocele articles; Chiari-II malformation articles; Hindbrain herniation articles; Hydrocephalus articles
Cephalocele articles Cephalocele Research articles Cephalocele review articles Cephalocele PubMed articles Cephalocele PubMed Central articles Cephalocele 2023 articles Cephalocele 2024 articles Cephalocele Scopus articles Cephalocele impact factor journals Cephalocele Scopus journals Cephalocele PubMed journals Cephalocele medical journals Cephalocele free journals Cephalocele best journals Cephalocele top journals Cephalocele free medical journals Cephalocele famous journals Cephalocele Google Scholar indexed journals Chiari-II malformation articles Chiari-II malformation Research articles Chiari-II malformation review articles Chiari-II malformation PubMed articles Chiari-II malformation PubMed Central articles Chiari-II malformation 2023 articles Chiari-II malformation 2024 articles Chiari-II malformation Scopus articles Chiari-II malformation impact factor journals Chiari-II malformation Scopus journals Chiari-II malformation PubMed journals Chiari-II malformation medical journals Chiari-II malformation free journals Chiari-II malformation best journals Chiari-II malformation top journals Chiari-II malformation free medical journals Chiari-II malformation famous journals Chiari-II malformation Google Scholar indexed journals Hindbrain herniation articles Hindbrain herniation Research articles Hindbrain herniation review articles Hindbrain herniation PubMed articles Hindbrain herniation PubMed Central articles Hindbrain herniation 2023 articles Hindbrain herniation 2024 articles Hindbrain herniation Scopus articles Hindbrain herniation impact factor journals Hindbrain herniation Scopus journals Hindbrain herniation PubMed journals Hindbrain herniation medical journals Hindbrain herniation free journals Hindbrain herniation best journals Hindbrain herniation top journals Hindbrain herniation free medical journals Hindbrain herniation famous journals Hindbrain herniation Google Scholar indexed journals Hydrocephalus articles Hydrocephalus Research articles Hydrocephalus review articles Hydrocephalus PubMed articles Hydrocephalus PubMed Central articles Hydrocephalus 2023 articles Hydrocephalus 2024 articles Hydrocephalus Scopus articles Hydrocephalus impact factor journals Hydrocephalus Scopus journals Hydrocephalus PubMed journals Hydrocephalus medical journals Hydrocephalus free journals Hydrocephalus best journals Hydrocephalus top journals Hydrocephalus free medical journals Hydrocephalus famous journals Hydrocephalus Google Scholar indexed journals computed tomography articles computed tomography Research articles computed tomography review articles computed tomography PubMed articles computed tomography PubMed Central articles computed tomography 2023 articles computed tomography 2024 articles computed tomography Scopus articles computed tomography impact factor journals computed tomography Scopus journals computed tomography PubMed journals computed tomography medical journals computed tomography free journals computed tomography best journals computed tomography top journals computed tomography free medical journals computed tomography famous journals computed tomography Google Scholar indexed journals magnetic resonance imaging articles magnetic resonance imaging Research articles magnetic resonance imaging review articles magnetic resonance imaging PubMed articles magnetic resonance imaging PubMed Central articles magnetic resonance imaging 2023 articles magnetic resonance imaging 2024 articles magnetic resonance imaging Scopus articles magnetic resonance imaging impact factor journals magnetic resonance imaging Scopus journals magnetic resonance imaging PubMed journals magnetic resonance imaging medical journals magnetic resonance imaging free journals magnetic resonance imaging best journals magnetic resonance imaging top journals magnetic resonance imaging free medical journals magnetic resonance imaging famous journals magnetic resonance imaging Google Scholar indexed journals tectal beaking articles tectal beaking Research articles tectal beaking review articles tectal beaking PubMed articles tectal beaking PubMed Central articles tectal beaking 2023 articles tectal beaking 2024 articles tectal beaking Scopus articles tectal beaking impact factor journals tectal beaking Scopus journals tectal beaking PubMed journals tectal beaking medical journals tectal beaking free journals tectal beaking best journals tectal beaking top journals tectal beaking free medical journals tectal beaking famous journals tectal beaking Google Scholar indexed journals cortical malformation articles cortical malformation Research articles cortical malformation review articles cortical malformation PubMed articles cortical malformation PubMed Central articles cortical malformation 2023 articles cortical malformation 2024 articles cortical malformation Scopus articles cortical malformation impact factor journals cortical malformation Scopus journals cortical malformation PubMed journals cortical malformation medical journals cortical malformation free journals cortical malformation best journals cortical malformation top journals cortical malformation free medical journals cortical malformation famous journals cortical malformation Google Scholar indexed journals hindbrain herniation articles hindbrain herniation Research articles hindbrain herniation review articles hindbrain herniation PubMed articles hindbrain herniation PubMed Central articles hindbrain herniation 2023 articles hindbrain herniation 2024 articles hindbrain herniation Scopus articles hindbrain herniation impact factor journals hindbrain herniation Scopus journals hindbrain herniation PubMed journals hindbrain herniation medical journals hindbrain herniation free journals hindbrain herniation best journals hindbrain herniation top journals hindbrain herniation free medical journals hindbrain herniation famous journals hindbrain herniation Google Scholar indexed journals surgical imaging articles surgical imaging Research articles surgical imaging review articles surgical imaging PubMed articles surgical imaging PubMed Central articles surgical imaging 2023 articles surgical imaging 2024 articles surgical imaging Scopus articles surgical imaging impact factor journals surgical imaging Scopus journals surgical imaging PubMed journals surgical imaging medical journals surgical imaging free journals surgical imaging best journals surgical imaging top journals surgical imaging free medical journals surgical imaging famous journals surgical imaging Google Scholar indexed journals
Article Details
1. Introduction
Cephalocele refers to the extracranial herniation of intracranial structures through a defect in the cranium [1,2]. Cephalocele can be classified based on the defect location as anterior or posterior and on the type of protruding structures through this defect as meningoencephalocele (cerebrospinal fluid (CSF), brain tissue and meninges), meningocele (CSF and meninges), atretic (dura, fibrous and degenerated brain tissue) and gliocele (CSF-containing glial-lined cyst) [1,2]. Multiple congenital central nervous system anomalies are reported in association with cephalocele including, but not limited to, Chiari malformation, corpus callosum agenesis, microcephaly, schizencephaly, thalamic fusion, Dandy-Walker malformation and Walker-Warburg syndrome [2]. The prognosis of patients with cephalocele depends on multiple factors that can be determined by imaging studies including the location and size of the defect, herniated contents, the presence of hydrocephalus and associated malformations [3]. For instance, the presence of hydrocephalus was found to be associated with worse neurological outcome [2]. The classification of Chiari malformations has evolved over time since the original description by Chiari with multiple overlapping imaging features [4]. The herniation of posterior fossa contents along with low occipital and high cervical cephalocele is usually reported as Chiari III malformation [5,6]. However, many radiologists tend to be more descriptive of the Chiari II-like findings associated with occipital cephalocele rather than classifying these cases as a discrete entity of Chiari III malformation. In our institution, we noticed that some of these patients have “acquired” Chiari II-like findings (low positioning of the cerebellar tonsils) on their follow up scan. Hence, the aim of this study is to determine the frequency of Chiari II-like neuroimaging findings in pediatric patients with cephalocele and to evaluate the radiologic and clinical outcome after surgical repair of cephalocele as well as to assess the proposed suggestion that Chiari II-like findings are part of an acquired dynamic deformation sequence rather than a primary malformation.
2. Materials And Methods
The study was approved by the Institutional Review Board of our institution and patient consent was waived.
2.1 Study design and data collection
A retrospective study was performed in pediatric patients (age < 18 years) with a cephalocele between 1/1/2011 and 11/30/2021. Patients were identified through an electronic radiological database search using key words “cephalocele”, “meningoencephalocele”, “meningocele” and “encephalocele” on computed tomography (CT) and/or magnetic resonance imaging (MRI) reports. Only children with congenital cephalocele were included. Children who had an acquired cephalocele after trauma or surgical procedure were excluded. Demographic (age and sex) and surgical (intervention for cephalocele, surgical findings and ventricular shunt placement) data were recorded from the medical charts. Clinical findings obtained from the last clinical follow-up included patients’ developmental milestones which were summarized as good or poor outcome based on the pediatric neurosurgery and/or neurology notes. Death rate was also recorded. Pre- and postoperative radiologic findings at the initial diagnosis and at the last follow up were reviewed in consensus by two readers (GO with 8 years of pediatric neuroradiology experience and RS with 3 years of pediatric radiology experience). The pre-surgical imaging findings included location (occipital, parietal, frontonasal, and basal) and size of the defect (mild, moderate and severe), type of herniated tissue (meningocele and meningoencephalocele), posterior fossa size (normal, large, relatively small and significantly small), presence of hindbrain herniation (HH) into the upper spinal canal, tectal beaking (TB), cortical malformation (CM), corpus callosum abnormalities and hydrocephalus. The qualitative scoring classification of the defect size and posterior fossa size was based on subjective reader evaluation. Additional abnormalities were also recorded, when present, such as microcephaly, gray matter heterotopia and schizencephaly. The post-surgical imaging studies were evaluated for the hindbrain herniation and hydrocephalus outcome which were classified as: stable and absent, stable and present, improved, resolved, worse and new. The duration between surgical intervention for cephalocele and last follow-up imaging modality and duration between surgical intervention and last clinical visit were calculated.
2.2 Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, version 27. 0 (SPSS Corp., Armonk, NY, USA). Comparison of the pre-surgical radiologic findings were conducted between two groups of patients based on their clinical outcome (good versus poor). The comparison between these two groups regarding the new development or resolution of HH was conducted. Additional comparisons between the post-surgical hindbrain herniation and hydrocephalus outcome with pre-surgical radiologic findings were also conducted. Chi-square test was used. Statistical significance was defined as p ≤ 0.05.
3. Results
Twenty-six patients were included (19 females, 73%) with mean age at diagnosis 121+/-565 days. Initial postnatal evaluation of cephalocele was made by MRI in 24 patients (92%) and by CT in 2 (8%). The defect was occipital in 23 patients (88%), parietal in 2 (8%) and frontonasal in 1 (4%). Eight patients had a meningocele (31%) and 18 had a meningoencephalocele (69%). Posterior fossa was normal in 7 patients (27%), large in 1 (4%) and small in 18 (69%). HH was present in 12 patients (46%), TB in 5 (19%) and CM in 11 (42%). There was no hydrocephalus in 15 patients (58%). The corpus callosum was normal in 11 patients (42%). The mean age at the time of surgical intervention was 131 +/- 573 days. Ventriculoperitoneal (VP) shunt placement and/or endoscopic third ventriculostomy (ETV) were performed in 15 patients (58%) with mean age 104 +/- 123 days. Mean clinical follow up duration was 42 (range 1-207) months after surgery. One patient did not have a clinical follow-up note documented in the chart. Sixteen children (64%) had good outcome and 9 (36%) had poor outcome. Three children (12%) died. Mean duration to last CT follow-up was 34 (range 1-166) months and to last MRI follow-up was 27 (range 1-147) months after surgery. Twenty-two patients had available imaging on follow up. On the follow-up imaging, HH was stable and absent in 6 patients (27%), stable and present in 3 (14%), improved in 2 (9%), resolved in 4 (18%), worse in 2 (9%) and new in 5 (23%). Hydrocephalus was absent in 7 patients (32%), improved in 2 (9%), resolved in 3 (14%), worse in 6 (27%) and new in 4 (18%). Tables 1 and 2 summarize the demographic, clinical, radiologic and surgical data. When comparing the radiologic findings at the initial scan between the two groups of good versus poor clinical outcome, there was no statistically significant difference in outcomes. Regarding post-surgical development and resolution of hindbrain herniation in these two groups, 19% (3/16) versus 22% (2/9) developed HH and 6% (1/16) versus 33% (3/9) had resolved HH, in patients with good and poor outcome, respectively. When comparing the initial and follow-up radiologic findings, we found that postsurgical HH was more common if it was present on preoperative imaging (p=0.001) and if there was small posterior fossa (p=0.04) on initial imaging. We also found that defect location (p=0.03) and presence of hydrocephalus (p=0.01) on initial imaging was associated with the presence of postsurgical hydrocephalus. Tables 3 and 4 summarize the comparisons between the different groups regarding radiologic findings and outcome, and clinical outcome. The imaging findings of two children with different clinical and radiologic outcome were shown in Figures 1 and 2.
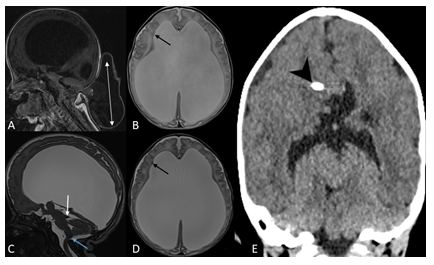
Figure 1: 1-day old female with occipital meningoencephalocele. Sagittal T1-weighted image (A) of brain MRI shows a large defect in the midline of the occipital bone through which a small amount of cerebellar tissue and meninges herniate (double arrow). There is small posterior fossa with posterior displacement of its content and brainstem towards the occipital defect and associated tectal beaking. Axial T2-weighted image (B) demonstrates severe lateral ventriculomegaly and periventricular nodular heterotopia (black arrow). The infant underwent surgical repair 7 days later and had a postoperative MRI in one week. Sagittal T2-SPACE (C) shows postoperative changes with resolution of hindbrain herniation, persistent dorsal angulation of the brainstem with kinking at cervicomedullary junction (blue arrow) and tectal beaking (white arrow). Axial T2-SPACE (D) demonstrates periventricular nodular heterotopia (black arrow) and worsening of supratentorial ventriculomegaly. This was followed by ventriculoperitoneal shunt placement (arrow head) with interval decompression of the lateral and third ventricles as demonstrated on coronal CT image of the brain (E). On further clinical follow-up within one year, patient was meeting her developmental milestones.
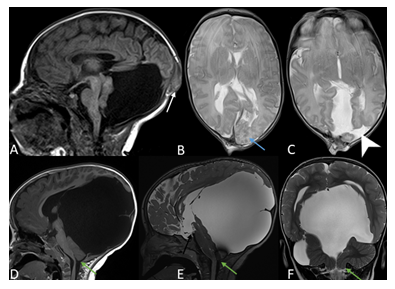
Figure 2: 1-day old male with occipital meningoencephalocele. Sagittal T1-weighted image (A) of the brain MRI shows a small posterior calvarial defect through which dysplastic occipital brain parenchyma and meninges herniate (white arrow). The posterior fossa is large and encysted with superior displacement of the tentorium and torcular herophili, dysplastic cerebellar hemisphere and vermis with no hindbrain herniation. The corpus callosum is fully formed. Axial T2-weighted images (B, C) demonstrate left occipital polymicrogyria (blue arrow) and left occipital open lip schizencephaly (arrow head). Two days later, the infant underwent surgical repair. On follow-up scans, there has been interval increased size of the encysted large posterior fossa with secondary increased mass effect on the posterior fossa content and supratentorial structures, as demonstrated on the sagittal T1-weighted (D), sagittal and coronal T2-SPACE (E, F). There is also secondary hindbrain herniation with low-lying cerebellar tonsils to the level of C1 (green arrow). A defect is noted in the third ventricular floor related to interval third ventriculostomy (black arrow). The patient showed delayed developmental milestones.
4. Discussion
The majority of our patients (89%) had an occipital encephalocele which is known to be more common in the Western countries compared to sincipital encephalocele which is more commonly found in Asian population [3]. Posterior fossa malformations are commonly described with occipital encephaloceles including Chiari malformation and Dandy-Walker malformation with associated corpus callosum abnormalities [1,3,7]. In our cohort, several Chiari II-like findings were found on preoperative scans including small posterior fossa (69%) with hindbrain herniation into the upper spinal canal (46%) and tectal beaking (19%). Corpus callosum abnormalities were also found in 58% of our patients. In addition, hydrocephalus was present in 11 patients (42%) on preoperative scans with 9/11 (82%) being present with occipital cephalocele, in concordance with reported incidence up to 60-90% in patients with posterior cephalocele and 10-15% with anterior cephalocele [2]. On the postoperative scans, we noticed that 5 patients (23%) developed HH while the rest had either stable, improved, resolved or worse herniation suggesting a dynamic and fluctuating pattern of this key Chiari II feature. Therefore, Chiari II-like findings might be considered as part of a dynamic acquired deformation sequence rather than being part of the classic classification of Chiari III malformation when cephalocele is present [5,6,8]. Furthermore, variable cortical malformations were found in our cohort, including polymicrogyria, dysgyria, stenogyria and gyral interdigitation which are commonly described in Chiari II malformation [9,10]. It is postulated that the presence of polymicrogyria and other Chiari II features including HH might be explained by the continuous CSF drainage through the defect leading to lack of ventricular distention which is essential to induce calvarial and neural development [11]. This follows the unified theory in which an open, non-skin covered neural tube defect is believed causative of the classical Chiari 2 malformation. A variable pattern of hydrocephalus was also noted on the follow-up scans depending on the defect location with worse or new hydrocephalus in 10 patients with occipital cephalocele while patients with parietal or frontonasal defects had either improved or resolved hydrocephalus. Among the 20 patients with occipital cephalocele and follow-up scans, only 4 children (20%) developed new hydrocephalus after surgical repair of cephalocele, as opposed to a much higher incidence in a recent case series of 17 patients with occipital encephalocele, 7 of which (41%) developed hydrocephalus after surgery [12]. Suggested mechanisms leading to postoperative hydrocephalus include CSF dynamics changes and torsion or stenosis of the aqueduct of Sylvius [1,12]. In our study, 64% of children had a good neurological outcome which is comparable to a range of 52-73% reported in historical series [13-15]. However, this is much higher than recent reports which only found 18-40% of children were neurologically normal [2,12]. This might be due to lower percentage of hydrocephalus in our study (42%) compared to 65% in other recent case series [12], since the presence of hydrocephalus was found to be statistically significantly associated with worse clinical outcome [2]. Although there was no statistically significant difference between the groups of good and poor outcome in our cohort regarding the presence or absence of hydrocephalus, we did notice a better outcome when hydrocephalus is absent as detailed in Table 3 with 11 patients with good outcome versus 3 with bad outcome. The limitations of this study are due to its relatively small sample size and retrospective nature; no standardized uniform clinical functional assessment was adopted to objectively evaluate the clinical outcome of children and longer clinical follow-up might identify developing learning or developmental disabilities in children who were labeled to have good clinical outcome after surgery. Otherwise, most queried information was available for the majority of patients. Prospective studies focusing on the risk factors for poor neurological outcome following surgical repair of cephalocele, such as neural tissue volume within the cephalocele, hydrocephalus and associated anomalies, would help clinicians and surgeons in dictating management and counseling the parents about the predicted outcome and long term consequences.
5. Conclusion
Neuroimaging findings in children with cephalocele might help predicting outcome, particularly Chiari II-like findings which might be related to a progressive acquired deformity sequence rather than a primary posterior fossa malformation.
Author contributions:
Rida Salman: Conceptualization; Data curation; Formal analysis; Methodology; Roles/Writing - original draft. Nilesh K. Desai: Conceptualization; Writing - review & editing. Stephen F. Kralik: Writing - review & editing. Michael A. Belfort: Writing - review & editing. Livja Mirtiri: Writing - review & editing; Gunes Orman: Conceptualization; Methodology; Supervision; Validation; Writing - review & editing; Thierry A.G.M. Huisman: Conceptualization; Writing - review & editing..
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest:
None
References
- Markovic I, Bosnjakovic P, Milenkovic Z. Occipital Encephalocele: Cause, Incidence, Neuroimaging and Surgical Management. Curr Pediatr Rev. 16 (2020): 200-205.
- Da Silva SL, Jeelani Y, Dang H, et al. Risk factors for hydrocephalus and neurological deficit in children born with an encephalocele. J Neurosurg Pediatr.15 (2015): 392-398.
- Pal NL, Juwarkar AS, Viswamitra S. Encephalocele: know it to deal with it. Egypt J Radiol Nucl Med 52 (2021):105.
- Azahraa Haddad F, Qaisi I, Joudeh N, et al. The newer classifications of the chiari malformations with clarifications: An anatomical review. Clin Anat. 31 (2018):314-322.
- Kanesen D, Rosman AK, Kandasamy R. Giant Occipital Encephalocele with Chiari Malformation Type 3. J Neurosci Rural Pract. 9 (2018):619-621.
- Mekouar Y, Laoudiyi D, Haboussi MR, et al. Chiari type III malformation: Case report and review of literature. Radiol Case Rep. 17 (2021): 628-630.
- Pejic M, Luecke K, Meoded A, et al. Pediatric Cephaloceles: A Multimodality Review. Appl Radiol. 49 (2020): 26-32.
- Andica C, Soetikno RD. Chiari malformation type III: Case report and review of the literature. Radiol Case Rep. 8 (2015):831.
- Geerdink N, van der Vliet T, Rotteveel JJ, et al. Essential features of Chiari II malformation in MR imaging: an interobserver reliability study--part 1. Childs Nerv Syst. 28 (2012): 977-985.
- Paschereit F, Schindelmann KH, Hummel M, et al. Cerebral Abnormalities in Spina Bifida: A Neuropathological Study. Pediatr Dev Pathol.25 (2022): 107-123.
- Stevenson KL. Chiari Type II malformation: past, present, and future. Neurosurg Focus. 16 (2004): E5.
- Nagy MR, Saleh AE. Hydrocephalus associated with occipital encephalocele: surgical management and clinical outcome. Egypt J Neurosurg 36 (2021): 6.
- Docherty JG, Daly JC, Carachi R. Encephaloceles: a review 1971-1990. Eur J Pediatr Surg. 1 (1991) :11-13.
- Date I, Yagyu Y, Asari S, et al. Long-term outcome in surgically treated encephalocele. Surg Neurol. 40 (1993):125-130.
- Martínez-Lage JF, Poza M, Sola J, et al. The child with a cephalocele: etiology, neuroimaging, and outcome. Childs Nerv Syst.12 (1996): 540-550.
