Neonatal Effects of Maternal Supplementation with Docosahexaenoic Acid and Lactoferrin on the Fetal Brain and Placenta in a Rabbit Model of Intrauterine Growth Restriction
Article Information
Miriam Illa1,2,*, Laura Pla1, Carla Loreiro1,3, Cristina Miranda1, Montse Mayol1, Britta Anna Kühne1,4, Marta Barenys4, Eduard Gratacós1,2,3,5, Elisenda Eixarch1,3,5
Affiliation:
1BCNatal | Fetal Medicine Research Center (Hospital Clínic and Hospital Sant Joan de Déu), Universitat de Barcelona, Barcelona, Spain
2Institut de Recerca Sant Joan de Déu, Esplugues de Llobregat, Spain
3Institut d’Investigacions Biomèdiques August Pi iSunyer (IDIBAPS), Barcelona, Spain
4GRET, INSA-UB and Toxicology Unit, Pharmacology, Toxicology and Therapeutical Chemistry Department, Faculty of Pharmacy, University of Barcelona, Barcelona, Spain
5Centre for Biomedical Research on Rare Diseases (CIBER-ER), Barcelona, Spain
*Corresponding author: Miriam Illa, BCNatal | Fetal Medicine Research Center (Hospital Clínic and Hospital Sant Joan de Déu), Universitat de Barcelona, Barcelona, Spain.
Received: December 03, 2022; Accepted: December 27, 2022; Published: January 10, 2023
Citation: Miriam Illa, Laura Pla, Carla Loreiro, Cristina Miranda, Montse Mayol, Britta Anna Kühne, Marta Barenys, Eduard Gratacós, Elisenda Eixarch. Neonatal Effects of Maternal Supplementation with Docosahexaenoic Acid and Lactoferrin on the Fetal Brain and Placenta in a Rabbit Model of Intrauterine Growth Restriction. Obstetrics and Gynecology Research 6 (2023): 01-14.
View / Download Pdf Share at FacebookAbstract
Objective:
The main objective is to evaluate the protective effects of maternal supplementation with docosahexaenoic acid (DHA) and lactoferrin (Lf) on the fetal brain and in the placenta structure in a rabbit model of Intrauterine growth restriction (IUGR).
Methods:
At 25 days of gestation, IUGR was surgically induced in pregnant rabbits. At that time, mothers were randomized to receive no treatment, oral DHA or oral Lf administration. Five days later, animals were delivered obtaining untreated IUGR, IUGR treated with DHA and IUGR treated with Lf. At postnatal day 1, a functional and structural evaluation was performed, including neuronal arborization in the frontal cortex, density of pre-oligodendrocytes in the corpus callosum and basic placental histology.
Results:
No differences in birth weight and survival were observed across study groups. On the contrary, functional and structural improvements were observed either in the brain and in the placenta.
Conclusions:
Our data suggest that maternal supplementation with DHA and Lactoferrin could be a beneficial nutritional intervention able to revert some of the IUGR-induced sequelae, including brain and placenta changes. These results support the implementation of clinical studies testing the potential role of DHA and Lf as protective strategies in human pregnancies affected by IUGR.
Keywords
Pregnancy, Nutrition, Supplement, Developmental origins of health and disease (DOHaD), Animal model, Intrauterine Growth Restriction, Neurodevelopment, Placental insufficiency, Docosahexaenoic acid, Lactoferrin
Pregnancy articles Pregnancy Research articles Pregnancy review articles Pregnancy PubMed articles Pregnancy PubMed Central articles Pregnancy 2023 articles Pregnancy 2024 articles Pregnancy Scopus articles Pregnancy impact factor journals Pregnancy Scopus journals Pregnancy PubMed journals Pregnancy medical journals Pregnancy free journals Pregnancy best journals Pregnancy top journals Pregnancy free medical journals Pregnancy famous journals Pregnancy Google Scholar indexed journals Nutrition articles Nutrition Research articles Nutrition review articles Nutrition PubMed articles Nutrition PubMed Central articles Nutrition 2023 articles Nutrition 2024 articles Nutrition Scopus articles Nutrition impact factor journals Nutrition Scopus journals Nutrition PubMed journals Nutrition medical journals Nutrition free journals Nutrition best journals Nutrition top journals Nutrition free medical journals Nutrition famous journals Nutrition Google Scholar indexed journals Supplement articles Supplement Research articles Supplement review articles Supplement PubMed articles Supplement PubMed Central articles Supplement 2023 articles Supplement 2024 articles Supplement Scopus articles Supplement impact factor journals Supplement Scopus journals Supplement PubMed journals Supplement medical journals Supplement free journals Supplement best journals Supplement top journals Supplement free medical journals Supplement famous journals Supplement Google Scholar indexed journals Developmental origins of health and disease articles Developmental origins of health and disease Research articles Developmental origins of health and disease review articles Developmental origins of health and disease PubMed articles Developmental origins of health and disease PubMed Central articles Developmental origins of health and disease 2023 articles Developmental origins of health and disease 2024 articles Developmental origins of health and disease Scopus articles Developmental origins of health and disease impact factor journals Developmental origins of health and disease Scopus journals Developmental origins of health and disease PubMed journals Developmental origins of health and disease medical journals Developmental origins of health and disease free journals Developmental origins of health and disease best journals Developmental origins of health and disease top journals Developmental origins of health and disease free medical journals Developmental origins of health and disease famous journals Developmental origins of health and disease Google Scholar indexed journals Animal model articles Animal model Research articles Animal model review articles Animal model PubMed articles Animal model PubMed Central articles Animal model 2023 articles Animal model 2024 articles Animal model Scopus articles Animal model impact factor journals Animal model Scopus journals Animal model PubMed journals Animal model medical journals Animal model free journals Animal model best journals Animal model top journals Animal model free medical journals Animal model famous journals Animal model Google Scholar indexed journals Intrauterine Growth Restriction articles Intrauterine Growth Restriction Research articles Intrauterine Growth Restriction review articles Intrauterine Growth Restriction PubMed articles Intrauterine Growth Restriction PubMed Central articles Intrauterine Growth Restriction 2023 articles Intrauterine Growth Restriction 2024 articles Intrauterine Growth Restriction Scopus articles Intrauterine Growth Restriction impact factor journals Intrauterine Growth Restriction Scopus journals Intrauterine Growth Restriction PubMed journals Intrauterine Growth Restriction medical journals Intrauterine Growth Restriction free journals Intrauterine Growth Restriction best journals Intrauterine Growth Restriction top journals Intrauterine Growth Restriction free medical journals Intrauterine Growth Restriction famous journals Intrauterine Growth Restriction Google Scholar indexed journals Neurodevelopment articles Neurodevelopment Research articles Neurodevelopment review articles Neurodevelopment PubMed articles Neurodevelopment PubMed Central articles Neurodevelopment 2023 articles Neurodevelopment 2024 articles Neurodevelopment Scopus articles Neurodevelopment impact factor journals Neurodevelopment Scopus journals Neurodevelopment PubMed journals Neurodevelopment medical journals Neurodevelopment free journals Neurodevelopment best journals Neurodevelopment top journals Neurodevelopment free medical journals Neurodevelopment famous journals Neurodevelopment Google Scholar indexed journals Placental insufficiency articles Placental insufficiency Research articles Placental insufficiency review articles Placental insufficiency PubMed articles Placental insufficiency PubMed Central articles Placental insufficiency 2023 articles Placental insufficiency 2024 articles Placental insufficiency Scopus articles Placental insufficiency impact factor journals Placental insufficiency Scopus journals Placental insufficiency PubMed journals Placental insufficiency medical journals Placental insufficiency free journals Placental insufficiency best journals Placental insufficiency top journals Placental insufficiency free medical journals Placental insufficiency famous journals Placental insufficiency Google Scholar indexed journals Docosahexaenoic acid articles Docosahexaenoic acid Research articles Docosahexaenoic acid review articles Docosahexaenoic acid PubMed articles Docosahexaenoic acid PubMed Central articles Docosahexaenoic acid 2023 articles Docosahexaenoic acid 2024 articles Docosahexaenoic acid Scopus articles Docosahexaenoic acid impact factor journals Docosahexaenoic acid Scopus journals Docosahexaenoic acid PubMed journals Docosahexaenoic acid medical journals Docosahexaenoic acid free journals Docosahexaenoic acid best journals Docosahexaenoic acid top journals Docosahexaenoic acid free medical journals Docosahexaenoic acid famous journals Docosahexaenoic acid Google Scholar indexed journals Lactoferrin articles Lactoferrin Research articles Lactoferrin review articles Lactoferrin PubMed articles Lactoferrin PubMed Central articles Lactoferrin 2023 articles Lactoferrin 2024 articles Lactoferrin Scopus articles Lactoferrin impact factor journals Lactoferrin Scopus journals Lactoferrin PubMed journals Lactoferrin medical journals Lactoferrin free journals Lactoferrin best journals Lactoferrin top journals Lactoferrin free medical journals Lactoferrin famous journals Lactoferrin Google Scholar indexed journals
Article Details
Introduction
Intrauterine growth restriction (IUGR) is defined as a significant reduction in the fetal growth rate affecting 7-10% of all pregnancies in developed countries[1]. One of the most prevalent causes of IUGR is placental insufficiency, which represents one of the major causes of perinatal morbidity and mortality[2]. IUGR has been related to poorer functional performance in the neonatal period[3,4], persisting during childhood and adolescence[5,6]. Although the exact structural substrate underlying neurodevelopmental impairments in IUGR is still under evaluation, different studies have suggested that abnormal oligodendrocyte maturation and neuronal arborization could be key processes[7-11]. Despite the clear impact of this condition, there are no effective therapies to either reverse IUGR or prevent its neurodevelopmental consequences.
Clinical and experimental evidence suggests that early postnatal strategies such as breastfeeding[12], an individualized newborn developmental care and assessment program[13], and environmental enrichment[14] can partially ameliorate the neurodevelopmental impairment caused by IUGR. However, all these strategies have been applied after birth, when the adverse effects of IUGR on brain development have already been consolidated. It has been well described that a strategy applied during the prenatal period, a “critical window of opportunity”[15], could have a more pronounced effect. Adequate nutrition during pregnancy lay the foundations for neuroplasticity and ultimately the development of cognitive, motor, and socio-emotional skills throughout childhood and adulthood[16]. Proposing new nutritional strategies for the period of brain development, recognizing that fetal and early-life programming could exert a long-term influence on brain maturation, has the potential to be a cost- effective approach that might be encouraged by public health policies[17]. Among potential therapies that could be applied during the prenatal period, docosahexaenoic acid (DHA) and lactoferrin (Lf) supplements emerge as potential candidates. Previous evidences have demonstrated their neuroprotective role in several neurological disorders such as Parkinson’s disease and schizophrenia, and after perinatal insults such as acute hypoxic-ischemic events[18-21]. DHA, a long-chain polyunsaturated fatty acid, plays a fundamental role in central nervous system development and transfers across the placenta to reach the fetal circulation[22,23] and fetal brain[24]. Lf is a sialic acid-rich glycoprotein that also crosses the placenta and blood-brain barrier and has been involved in modulating cell- to-cell interactions and neuronal outgrowth[25]. In addition, preliminary evidence has linked both therapies with some positive effects on placental development. Reduced oxidative stress and placental apoptosis has been previously described in omega-3 supplemented pregnancies[26,27], whereas Lf has been involved in cytotrophoblast endothelial cell invasion and placental vasculature enhancement[28,29]. Furthermore, a key factor of DHA and lactoferrin supplements is that they are normal bioactive components of a great diversity of foods and maternal milk, and consequently can be safely used as supplements[30].
Despite all these evidences of the positive effect of DHA and Lf on brain and placental development, no previous studies have evaluated the potential neuroprotective role or placental effects of these two therapies in a well characterized model of placental insufficiency. We previously demonstrated the functional and structural brain sequels and placental histological alterations related to IUGR by using a surgical IUGR rabbit model[7]. In this study we aimed to evaluate the neuroprotective effects of DHA and Lf in the brain at the functional and structural level and in the placenta structure in this animal model. For this purpose, density of pre- oligodendrocytes (pre-OLs) in the corpus callosum (CC), neuronal arborization in the frontal cortex, and placenta structure in prenatally treated IUGR animals with DHA or Lf were evaluated and this data was compared with the already published data in untreated IUGR rabbit animals[7].
Materials and Methods
IUGR induction, experimental groups, and therapy administration
All procedures were performed following all the applicable regulations and guidelines of the Animal Experimental Ethics Committee (CEEA) of the University of Barcelona and were approved with the license number 03/17. The animals were provided by a certified commercial farm (Granja San Bernardo) and were acclimated at least for one week before surgery. Their clinical condition was assessed by veterinary staff before starting the study. The animals were housed following local standards. All surgery was performed under general anesthesia, and all efforts were made to minimize suffering. Also, animal work has been conducted fulfilling ARRIVE’s guidelines and reported accordingly[31].
At 25 days of gestation, a total of 19 pregnant rabbits (New Zeland) were subjected to the IUGR induction protocol. Sample size was calculated taking into account the mortality related to the IUGR model following previous experience in the group[7,14,32,33, 34] to include the required number of animals for each analysis (see each paragraph for detailed information). IUGR was induced by ligating 40-50% of the uteroplacental vessels of each gestational sac, whereas non- ligated gestational sacs provided normally-grown controls as previously described[32]. At the time of IUGR induction, the pregnant rabbits were randomly assigned to 3 groups: no treatment (n = 9), treatment with DHA (n = 5) and treatment with Lf (n = 5). At 30 days of gestation, cesarean section was performed obtaining living and stillborn animals and their placentas. Normally-grown animals (controls) and untreated IUGR pups were obtained from untreated mothers, whereas IUGR-DHA and IUGR-Lf pups were obtained from mothers treated with DHA or Lf, respectively. All experimental groups were contemporaneous, were evaluated following similar protocols and by the same examiners (MI, LP). Perinatal, functional and structural data from untreated IUGR and normally-grown controls had been discussed in a previous publication[7]. Perinatal data described in this study[7] was in agreement with previous data published in the surgical rabbit model [7,14,32,33,34], demonstrating the suitability of the model used in recreating perinatal complications related with IUGR including a significantly higher percentage of stillbirth (42.42% vs 11.11%, p = 0.01), and a reduced birth weight (38.65 ± 9.84 vs 48.48 ± 7.34, p < 0.01). The present work focuses in the evaluation of the potential protective effects of DHA and Lf prenatally in IUGR at the brain and placenta levels. To this end and to avoid the unnecessary repetition of animal experiments, based on ethical reasons, already published data from the untreated IUGR animals were used as positive control in this study. After cesarean section, mothers were sacrificed by an overdose of pentobarbital.
DHA or Lf were orally administered to the pregnant rabbits once per day from day 25 until the day of the cesarean section. The therapy was administered using a syringe to cautiously release the solution into the mouth. The specific dose of DHA (37 mg/kg/day) and Lf (166 mg/kg/day) was calculated taking into account previous evidence[21,35]. Doses were adjusted with the formula for interspecies translation based on the body surface area (BSA)[36], obtained as follows:
BSA (m2) =9.9 x rabbit weight (g) / 10 000
and on the correction factor (Km) value, obtained by: km=body weight (kg) / BSA
Finally, the formula used for interspecies translation of
the HED (equivalent dose) was as follows[37]:
HED (mg/kg) = known dose (mg/kg) x km animal of known dose / rabbit Km
The DHA was obtained from Rendon Europe Laboratories and was presented in 3 g 100% pure powder from Microalga oil Schizochytrium sp. The Lf administrated was bovine Lf containing a low concentration of iron (9.2 mg of iron per 100 g of protein) and was obtained from Farmalabor.
Functional evaluation
Due to differences in the mortality rate obtained in the experimental groups, the final sample included in the functional evaluation differed between groups: 12 untreated IUGR, 18 IUGR-DHA, and 19 IUGR-Lf. At postnatal day 1, functional variables that previously demonstrated to be altered in IUGR[7] were evaluated in all offspring following the previous methodology described by Derrick et al.[38] and adapted by our group[32]. Briefly, at postnatal day 1, general motor skills, tone, reflexes, and olfactory sensitivity were evaluated in all offspring. For each animal, the testing was videotaped and variables were scored on a scale of 0 to 3 (0 = worst and 3 = best), except for tone, which was scored (0-4) according to the Ashworth scale[39] by two blinded observers (MI, LP). A detailed explanation of how each variable was assessed is given above.
The first part of evaluation lasted 1 minute and included the evaluation of: i. Animal posture; ii. Righting reflex (number of times the animal turned prone from the supine position in 10 tries); iii. Tone (assessed by an active flexion and extension of the fore limbs and hind limbs); ii. Circular motion (evaluation of the range of movements and jumping);
iii. Hind limb locomotion (assessed by grading the amount of spontaneous movement of the hind limbs); iv. Intensity of the movements; v. Duration of the movements; vi. Lineal movement (number of times the animal crossed a perpendicular line of 15 cm when walking straight); vii. The mean of the shortest fore-hind paw distance (evaluated when the animal walks in a straight line).
After this first minute of evaluation, suck and swallow, head turning, and olfaction were evaluated: i. Suck and swallow (assessed by the introduction of formula (Lactadiet with omega 3; Royal Animal) into the pup’s mouth with a plastic pipette); ii. Head turning (assessed by observing the head and body movements associated with the suction reflex);
iii. Olfaction (counting the time in seconds the animal moves the nose away from a cotton swab soaked with pure ethanol when this was placed close to the nose). The score grading used for each variable is detailed in Supplementarial Table 1 (S1 Table).
Sample collection
After the cesarean section, placentas were obtained, carefully washed in saline solution, weighed, fixed for 24 hours in 10% formalin and embedded in paraffin.
After functional evaluation, newborns were weighed and sacrificed by decapitation after the administration of ketamine (35 mg/kg) and xylazine (5mg/kg) intramuscularly. All brains were carefully dissected and fixed according to the analysis performed. Four brains from each group were randomly selected for the Golgi-Cox staining protocol and were processed according to it (detailed below). The other brains (11 untreated IUGR, 14 IUGR-DHA, 15 IUGR-DHA) were fixed for 24 hours in 10% formalin, dehydrated for 48 hours with sucrose 30% and finally frozen at −80 ºC.
Histological procedures
Placenta
Five placentas from each group were randomly selected for a basic histological evaluation based on findings from previous work in pregnant rabbits[7]. All the placentas were evaluated except one from the IUGR-DHA group, in which the analysis could not be performed owing to technical problems with the processing. Two consecutive slices (4 µm) of each placenta from paraffin blocks were stained following a hematoxylin and eosin standard protocol. The pathologist was blinded to the experimental groups. The analyses were performed on two different regions of the placenta: the decidua basalis (maternal part) and the labyrinth zone (fetal part). Ischemia, necrosis, fibrin, and calcifications were assessed in the decidua, while ischemia, vascular collapse, congestion, and calcifications were evaluated in the labyrinth zone. Ischemia and fibrin were expressed as a percentage, necrosis was assessed by the presence of karyorrhexis and karyolysis, and the remaining variables were evaluated with a semiquantitative system that graded each lesion from 0 to
The grading criteria followed were: 0) Unremarkable; 1) Minimal; 2) Mild; 3) Moderate; 4) Marked; 5) Severe.
O4-immunoreactive oligodendrocytes (O4+ cells)
Eight formalin-fixed brains per group were selected for the O4-immunoreactive oligodendrocyte evaluation (O4+ cells). The sample size for oligodendrocyte evaluation was calculated taking into consideration previous studies with the same animal model[7,33]. Three coronal sections of 40 μm per animal at the level of the genu CC and with reference to the bregma were obtained and processed as previously described[7,34]. Briefly, slices were incubated overnight with anti-oligodendrocyte marker O4 (1:50, Chemicon) followed by 1% Hoechst 33258 (1:1000, Thermofischer) incubation for cellular nucleus visualization. Finally, slices were incubated with the specific secondary antibody conjugated to 488 Alexa Fluor (1:400, MoBitec). Immunoreactive sites were observed under the confocal microscope and images were taken as previously described[7]. The number of the total cell nuclei (stained with Hoechst) and the number of cells with fluorescent staining around the nucleus (O4-immunoreactive oligodendrocytes) were counted using the software ImageJ. O4-immunoreactive oligodendrocyte density (immune- reactive cells/mm2) was then calculated. The evaluation for each experimental group was blinded for the evaluator (LP). The anti-O4 antibody was selected as a marker for the latter stages of oligodendrocyte maturation (O4-OL), which include pre-OLs, pre-myelinating OL, and myelinating OL[40]. Since pre-OL cells are the most predominant oligodendrocytes in rabbits at the day of evaluation, we assumed that the O4 marker used mainly stained the pre-OL cells[41].
Neuronal arborization (Golgi-Cox staining)
Four brains per group were included in the neuronal arborization analyses, similarly to previous studies[7,8]. Neuronal arborization evaluation was performed as previously described[7, 34]. Briefly, vibratome was used to obtain 100 µm serial and coronal sections that were processed for Golgi-Cox impregnation with the FD Rapid Golgi Stain kit (FD Neurotechnologies Inc.). Coronal slices with reference to the bregma were observed under ×40 objective magnification in an AF6000 epifluorescence microscope. Five pyramidal neurons that fulfilled the inclusion criteria from the frontal cortex (at the level of premotor cortex) per brain hemisphere were selected randomly and one image per neuron was obtained. In order to obtain the dendritic tree of each neuron, different Z-stacks per each image were needed. The inclusion criteria were: pyramidal neurons within layer II and III and complete filling of the dendritic tree with well- defined endings. The evaluation for each experimental group was blinded for the evaluators (LP, MM). The parameters evaluated were: i) area of the soma (obtained by manual delineation of the shape of the neuronal soma in a 2D image);
ii) total basal dendritic length (obtained after performing manual delineation of the length of each basal dendrite and then calculating the sum of all lengths from all basal dendritic branches); iii) basal dendritic complexity, including number of dendrites (obtained by manual counting) and number of intersections for each Sholl ring (following the sholl analyses technic previously published [42] and standarized in our model [7]). The study design is summarized in Figure 1 (Fig 1).
Statistical analyses
The software packages STATA 14.0 and GraphPad 5 were used for statistical analyses and graphical representation. For quantitative variables, normality was assessed by the Shapiro- Wilk test and homoscedasticity was determined by Levene’s Test, except for variables with more than 30 observations (Golgi-Cox variables) for which normal distribution was already assumed. For ordinal variables, non-normal distribution was assumed. The descriptives of the variables were expressed as mean and standard deviation (SD) for normal distributions, whereas median and interquartile range (IQR) were used for non-normal distributions and ordinal variables. Appropriate statistical tests were used according to the variable: ANOVA with Dunnett’s multiple comparison test was used for continuous variables (birth weight, placental ischemia and fibrin deposition, some functional variables, pre-OL and arborization parameters), Kruskal-Wallis with Dunn’s multiple comparison test for ordinal variables (placental calcifications, collapse and congestion and some functional variables) and chi-squared for binary variables (survival). Statistical significance was declared at p < 0.05 in all variables evaluated.
Results
Perinatal data
In comparison with untreated IUGR animals, treated IUGR did not present any statistical improvement in stillbirth and birth weight, independently of the treatment group (stillbirth: untreated IUGR 42%; IUGR-DHA 57%, p=0.03; IUGR-Lf 53%, p= 0.11; birth weight: untreated IUGR 38.65
g (9.84); IUGR-DHA 40.4 g (6.81), p=0.39; IUGR-Lf 37.7 g
(7.93), p=1.00).
Functional results
Animals prenatally treated with DHA (IUGR-DHA) presented a significant improvement in righting reflex, intensity, sucking and swallowing and head turning, with no improvement in the circular motion variable. Regarding IUGR-Lf group, a significant improvement in sucking and swallowing was observed with no significant improvement in the rest of variables (Table 1).
Placental findings
Results are mean and standard deviation (mean (SD)) for continuous variables (expressed as a percentage) and median and interquartile range (median(IQR)) for ordinal variables (expressed as a semiquantitative grading system, score = 0-5).
The nomenclature of Y (yes) or N (no) was used for the variable of necrosis. *p<0.05
No differences were observed in the placental weight across groups (untreated IUGR: 5.23 g; IUGR-DHA:
4.96 g; IUGR-Lf: 5.05 g). Overall, DHA and Lf groups presented a significant reduction of the percentage of ischemia in the decidua basalis in comparison to untreated IUGR. In addition, IUGR-Lf group showed a significant decrease in ischemia and, a reduction of vascular collapse accompanied by an increase in vascular channel dilation in the labyrinth zone in comparison with untreated IUGR (Table 2 and Fig 2).
Representative images of calcification (arrow) in decidua basalis among the study groups.
Representative images of vascular channel congestion (arrow) in the labyrinth zone among the study groups. Abbreviations: IUGR = intrauterine growth restriction, DHA = docosahexaenoic acid, Lf = Lactoferrin.
O4-immunoreactive oligodendrocyte results
A significant increase in the percentage of O4- immunoreactive oligodendrocytes density (immune- reactive cells/mm2) was observed in the IUGR-DHA group in comparison with the untreated IUGR group (DHA vs untreated IUGR: 1.13 cells/mm2 (0.18) vs 0.86 cells/mm2 (0.21), p = 0.04). No statistical differences were observed in the IUGR-Lf groups when comparing to untreated IUGR (shown in Fig 3).
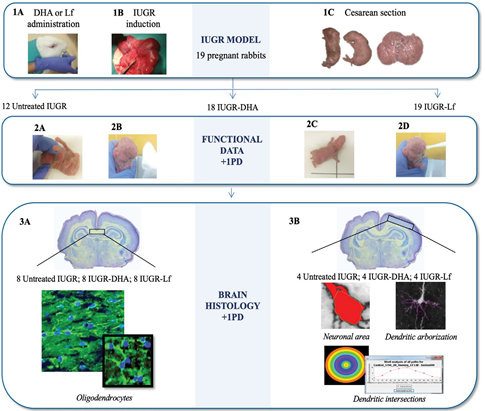
Fig 1: Study design.
Table 1: Functional results in the study groups
|
Variables |
Untreated IUGR (n=12) |
IUGR-DHA (n = 18) |
IUGR-Lf (n= 19) |
|
Righting reflex, num |
8 (5) |
10 (0)* |
6 (4) |
|
Circular motion, sc |
1 (2) |
2 (1) |
2 (1) |
|
Intensity, sc |
2 (1) |
3 (0)* |
2 (1) |
|
Sucking and swallowing, sc |
1 (2) |
3 (1)* |
3 (0)* |
|
Head turning, sc |
2 (2) |
3 (0) * |
3 (1) |
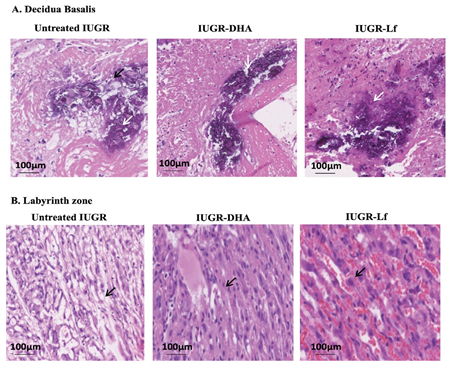
Fig 2: Placenta histological findings in the study groups.
- Representative images of calcification (arrow) in decidua basalis among the study
- Representative images of vascular channel congestion (arrow) in the labyrinth zone among the study Abbreviations: IUGR = intrauterine growth restriction, DHA = docosahexaenoic acid, Lf = Lactoferrin.
Table 2: Placental histopathological findings in the study groups
|
DECIDUA BASALIS |
|||
|
Variables |
Untreated IUGR (n= 5) |
IUGR-DHA (n = 5) |
IUGR-Lf (n= 5) |
|
Ischemia (%) |
99.8 (0.45) |
88.3 (5.69)* |
89.0 (9.62)* |
|
Necrosis (Y/N) |
Y |
Y |
Y |
|
Fibrin (%) |
4.40 (0.55) |
4.25 (0.50) |
4.40 (0.55) |
|
Calcification (0-5) |
3 (3.5) |
3 (1.5) |
2 (1.5) |
|
LABYRINTH ZONE |
|||
|
Variables |
Untreated IUGR (n= 5) |
IUGR-DHA (n= 5) |
IUGR-Lf (n= 5) |
|
Ischemia (%) |
96.0 (1.73) |
97.0 (2.45) |
89.0 (4.18)* |
|
Collapse (0-5) |
3 (0) |
2 (1.5) |
0 (1.5)* |
|
Congestion (0-5) |
0 (0) |
0 (1.5) |
4 (1.5)* |
|
Calcification (0-5) |
4 (4) |
3 (1.5) |
4 (1.5) |
Neuronal arborization
IUGR-DHA group showed higher values in the total dendritic length, area of soma and number of intersections per Sholl ring (shown in Fig 4 and Table 3) and a significant increase in the number of primary and secondary dendrites, with no significant changes in the more distal dendrites when compared with the pups of untreated animals (shown in Fig 4 and Table 3). The IUGR-Lf animals presented a decrease in the majority of the neuronal arborization parameters when compared with the pups of untreated IUGR (shown in Figs 4 and 5 and Table 3).significance was declared at p < 0.05 between untreated IUGR and IUGR-DHA or IUGR-Lf (*). Abbreviations: untreated IUGR = intrauterine growth restriction with no treatment; IUGR-DHA = intrauterine growth restriction treated with DHA; IUGR-Lf = intrauterine growth restriction treated with Lf.
Discussion
The present study demonstrates that maternal supplementation with DHA and Lf in an experimental model of IUGR has positive effects on placental structure and brain structure and function of IUGR fetuses.
Perinatal data and placental evaluation
Our data showed that prenatal administration of DHA and Lf in an IUGR rabbit model was not related to improvements in survival and birth weight. These results are in line with previous studies showing no improvements in birth weight, nor in fetal survival after Omega-3 supplementation in human IUGR pregnancies[43] and after Lf supplementation in an IUGR rat model[44].
Regarding the histological findings in the placenta, DHA was related with a reduction in the proportion of ischemia in the decidua basalis. This is in line with previous experimental data that showed a reduction in the placental oxidative stress in the placenta of normal pregnancies of rats supplemented with an omega-3 diet[26]. In the clinical setting, reduced placental apoptosis has been described in normal pregnancies after DHA administration[27]. In the same line, Lf group showed a reduction in the proportion of ischemia in the decidua basalis and in the labyrinth zone. Furthermore, significant vascular channel dilation in comparison with the placentas of untreated IUGR animals was observed. This finding could be explained by the fact that Lf, especially apoLf, enhances matrix metalloproteinase-2 expression in the cytotrophoblast, which promotes endothelial cell invasion[28] and increases placental vasculature[29]. However, this improvement did not lead to an improvement in perinatal outcomes, perhaps due to the fact that Lf only improved the vasculature with
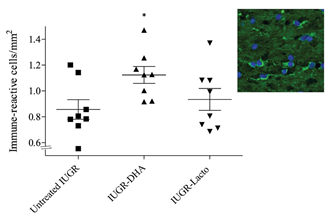
Fig 3: O4-immunoreactive oligodendrocytes in the CC in the study groups.
*p<0.05 between the untreated IUGR and IUGR-DHA. Abbreviations: untreated IUGR = intrauterine growth restriction with no treatment; IUGR-DHA = intrauterine growth restriction treated with DHA; IUGR-Lf = intrauterine growth restriction treated with Lf.
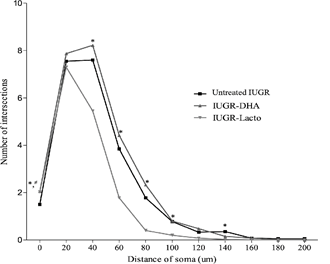
Fig 4: Number of intersections per Sholl ring in the study groups.
* p<0.05 between the untreated IUGR and IUGR-DHA. # p<0.05 between the untreated IUGR and IUGR-Lf. Abbreviations: untreated IUGR = intrauterine growth restriction with no treatment; IUGR- DHA = intrauterine growth restriction treated with DHA; IUGR-Lf
= intrauterine growth restriction treated with Lf.
no significant effects on fibrin deposition and necrosis, which could be determinants of perinatal outcomes. We acknowledge that further studies including fetal and maternal Doppler evaluations and specific histological evaluation including vascular immunohistochemistry, matrix metalloproteinase-2 and gene expression would be required in order to elucidate the exact mechanism underlying DHA and Lf in the placenta. However, such studies are beyond the scope of this paper.
Neurodevelopmental effects of the perinatal administration of DHA in IUGR
Overall, our data demonstrated the positive effect of DHA in ameliorating the functional and structural brain changes induced by IUGR in the neonatal period. Maternal administration of DHA was associated with functional improvements in most of the functional variables that have been demonstrated to be impaired in the untreated IUGR animals, similar to those previously reported in a neonatal acute hypoxic-ischemic[45] and in transient focal cerebral ischemia[46] mouse models.
Regarding structural brain results, we observed a significant improvement of the pre-OLs (O4+ cells) in the DHA group. These results are in line with previous findings described by our group in an in vitro IUGR rabbit neurosphere model, where the impairment of O4+ cells differentiation was reversed after exposure of IUGR neurospheres to DHA[47]. This O4+ cells increase was described either after in the in vitro exposure of IUGR neurospheres to DHA, or after in vivo administration of DHA and subsequent evaluation
Table 3: Dendritic arborization parameters in the study groups.
|
Variables |
Untreated IUGR (n = 40) |
IUGR-DHA (n = 40) |
IUGR-Lf (n = 40) |
|
Area of soma, µm2 |
366.97 (76.7) |
446.1 (165.0)* |
313.8 (95.2) |
|
Total intersections, no. |
47.03 (13.4) |
52.3 (16.3) |
34.2 (7.74)* |
|
Total dendritic length, µm |
623.01 (173.3) |
723.8 (230.8)* |
472.1 (121.3)* |
|
Primary dendrites, no. |
6.83 (1.71) |
8.95 (2.50)* |
7.60 (2.07) |
|
Secondary dendrites, no. |
7.65 (2.17) |
9.70 (3.38)* |
7.48 (2.49) |
|
Tertiary dendrites, no. |
4.75 (2.30) |
3.93 (2.83) |
2.85 (2.43)* |
|
Quaternary dendrites, no. |
2.10 (2.35) |
1.43 (2.10) |
0.83 (1.28)* |
|
Quinary dendrites, no. |
0.75 (1.26) |
0.43 (0.98) |
0.10 (0.44)* |
|
Senary dendrites, no. |
0.30 (0.97) |
0.00 (0.00)* |
0.00 (0.00)* |
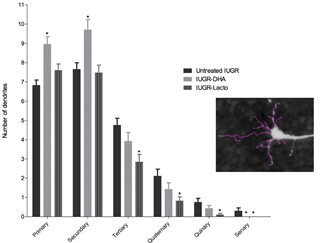
Fig 5: Number of each basal dendritic branch in the frontal cortex in the study groups in vitro neurospheres. Also, in that study we evaluated the potential effects of DH ribed to be essential for normal motor circuit function[52].
Regarding neuronal arborization, our results also suggest a protective role of DHA on several parameters. In our previous paper, untreated IUGR animals showed a trend to present decreased primary and secondary dendrites with a significant increase in the number of the more distal dendrites in comparison to normally growth animals (controls)[7]. In this study, DHA presented a significant increase of the primary and secondary dendrites, which goes in the same direction with previous data showing positive effects of Omega 3 fatty acids on dendritic arborization and new spine formation in the hippocampus during normal ageing[53,54] and on neurite length and branching in hippocampal embryonic neuronal cell cultures[55,56]. Interestingly, previous literature has demonstrated the link between enhanced neuronal dendritic growth in the motor cortex with motor functional improvements[57]. In this study, neuronal arborization has been evaluated in the frontal cortex, which has been involved in associative learning in rabbits[58]. Therefore, the functional correspondence of these neuronal arborization results in the frontal cortex might be assessed by evaluating other functional domains, rather than motor and reflex variables, such as social interaction, anxiety traits, or cognition.
Neurodevelopmental effects of the perinatal administration of Lf in IUGR
Maternal administration of Lf was associated with significant improvements in sucking and swallowing functional variable of the pups compared with untreated IUGR pups. This could not be contrasted with previous evidence as there is a lack of functional data reported in hypoxic-ischemia and IUGR studies evaluating the neuroprotective role of Lf in this setting.
At a structural level, in our study Lf seemed to have no effect in the pre-OL cell population (O4+ cells). This finding is aligned with the previous study described by our group in the IUGR rabbit neurosphere model, where no increase in the percentage of O4+ cells was observed after the administration of Lf, either in vitro or in vivo. Similar to DHA findings, no apparent effect was detected in O4+ cells in control neutrospheres after Lf administration[47]. On the contrary, these findings go against previous evidence in which a positive effect of Lf on the oligodendrocyte lineage was described, although the effect was in earlier stages of oligodendrocyte development: oligodendrocyte precursor NG2+[59]. It should be noted that we only included the evaluation of O4+ immunoreactivity oligodendrocytes (O4+ cells). The evaluation of earlier oligodendrocyte stages (such as NG2+) would be required in future studies to further evaluate the real role of Lf in the WM in our animal model.
Regarding the neuronal arborization results, the prenatal administration of Lf was related with significant reduction in the area of soma, total number of intersections and total dendritic length. Also, we observed that Lf presented a significant decrease in the dendrites that had been found to be increased in untreated IUGR in comparison to normally growth animals: tertiary, quaternary, and quinary dendrites[7]. Going in line with these results, preliminary data in our IUGR neurosphere model (in vitro evaluation) also replicated these findings: sialid acid, the major metabolite of Lf, was able to reduce the total neurite length to control level in IUGR neurospheres[60]. Although the interpretation of these findings remains elusive, recent evidences observed by our group may provide a plausible explanation for these results. The molecular mechanism involved in the dendrites increase could be secondary to an increase in integrin-β1 molecule, which is a molecule that promotes neuronal migration, neurite length and dendrite arborization. IUGR neurospheres presented a significant higher expression of this integ-rin- β1molecule[61]. Regarding the effects of Lf on the neuronal arborization results, it has been suggested to be secondary to an interference in this intregrin-β1 molecule. Lf plays a role in the synthesis of sialyted glycoproteins including the polysialic acid (Poly-SA). Burgess et al. (2007) discovered that Poly- SA molecule limited the neurite elongation by preventing the interaction between integrin- β1and laminin[62]. Therefore, Lf may prevent the increase in neuronal arborization (in vivo experiments) and neurite length (in vitro experiments) through the increase in the sialyted glycoproteins in the brain. Going in the same direction, other studies performed at different neurodevelopmental stages and also using other animal species were aligned with these findings: a positive effects of Lf on neuroplasticity, cell migration, and the differentiation of neuronal progenitor cells during the postnatal period in piglets were observed[25,63]. Similarly, Lf demonstrated a tendency to improve synaptogenesis in a hypocaloric IUGR rat model supplemented with Lf during gestation and lactation at postnatal day 7[59]. Future evaluation of more mature stages of neuronal connectivity in our animal model such as dendrite spine formation and synaptogenesis and cultured neurospheres studies with decreasing laminin concentration would provide additional insight regarding the mechanisms by which Lf exerts its neuroprotective role as suggested in previous studies.
Strengths and limitations
Among the strengths of the study was the use of a well described experimental model[32,64,65] that has consistently shown to reproduce the perinatal (birth weight and survival rate) and neurodevelopmental effects of IUGR in humans. In addition, among all the animal species used in investigations of IUGR, the rabbit has been described as presenting higher similarities to humans in terms of brain maturation in comparison with other species, making this a reliable model for obtaining data that is translational to humans[66]. Regarding the reliability of the reported results, it is noteworthy that we obtained a good agreement between the vivo evaluation (present study) with our previous in vitro evaluation by using the IUGR neurosphere model[47]. Besides, in this study the DHA and Lf effect has been evaluated at different levels: through histology of the placenta, in functional evaluation, and through WM and grey matter (GM) assessments, giving us robustness to the conclusions of the effects of these therapies on our model.
We acknowledge that there are limitations in the study. Lack of a true control or sham group is a major limitation as no baseline comparison could be done. Although DHA and Lf did not present any effect in control neurospheres from our IUGR in vitro model[45], future in vivo studies including normally grown animals prenatally treated with DHA or Lf should also be considered. Another limitation of the study was that histological analyses were restricted to the frontal cortex and CC. Other brain regions such as the hippocampus or ventricular zone have been documented to be key structures altered in IUGR[67,68] and would therefore be interesting to evaluate. Although the molecular mechanisms and pathways involved in the effects of DHA and Lf are beyond the scope of this study, the description of the molecular mechanisms by which both therapies exert their neuroprotective role would be useful in understanding the differences in the structural effects of both therapies. Also, sex is an important factor in brain development, although gender could not be assessed in the study groups, as this could not be clearly identified at neonatal stage. Finally, we acknowledge that it would have been interesting to carry out a pharmacokinetic study to determine the concentrations achieved after the administered doses. The study was not designed to carry out such analysis, mainly due to a lack of a specific laboratory that would give us this service. Further studies designed to evaluate this issue would be of value.
Conclusions
In summary, our study provides novel evidence highlighting the potential use of supplements in pregnancies affected by IUGR to improve fetal outcomes. Our data suggest that maternal DHA and Lf supplementation is a beneficial intervention able to revert some of the IUGR-induced sequelae, including brain and placenta changes. Derived evidence from this study supports the implementation of clinical studies testing the potential role of DHA and Lf as protective strategies in human pregnancies affected by IUGR. One promising opportunity in the clinical study might be to develop a combined supplementation including DHA agent and Lf to prevent the IUGR-induced adverse effects on the oligodendrocyte and on neuronal development, respectively and to corroborate their positive effects into human placenta insufficiency.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/Table/S1, Table S1: Functional evaluation
— score grading in the study groups.
Author Contributions
Conceptualization, Miriam Illa; Data curation, Miriam Illa and Laura Pla; Formal analysis, Miriam Illa and Laura Pla; Investigation, Miriam Illa, Laura Pla, Carla Loreiro, Cristina Miranda and Montserrat Mayol; Methodology, Miriam Illa, Laura Pla, Carla Loreiro, Cristina Miranda and Montserrat Mayol; Resources, Miriam Illa, Eduard Gratacós and Elisenda Eixarch; Software, Laura Pla and Carla Loreiro; Supervision, Eduard Gratacós and Elisenda Eixarch; Validation, Miriam Illa, Laura Pla and Britta Anna Kühne; Writing - original draft, Miriam Illa and Laura Pla; Writing - review & editing, Miriam Illa, Britta Anna Kühne, Marta Barenys, Eduard Gratacós and Elisenda Eixarch. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “La Caixa” Foundation [LCF/PR/GN14/10270005 and LCF/PR/GN18/10310003];
the Instituto de Salud Carlos III [PIE15/00027, PI15/00130, PI18/01763] within the Plan Nacional de I+D+I and cofinanced by ISCIII-Subdirección General de Evaluación together with the Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa”, AGAUR under grant 2017 SGR nº 1531 and Cellex Foundation. E. Eixarch and C. Loreiro have received funding from the Departament de Salut [SLT008/18/00156 and SLT006/17/00325]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by Animal Experimental Ethics Committee (CEEA) of the University of Barcelona and were ap-proved with the license number 03/17, data of ap-proval 2017.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the animal facility of Hospital Sant Joan de Déu for their help in maintaining the animal welfare. We also thank Ms. Maria Calvo, Ms. Anna Bosch, and Ms. Elisenda Coll from Centres Científics i Tecnològics - Universitat de Barcelona for their technical support in using the confocal microscope.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kady S M, Gardosi Perinatal mortality and fetal growth restriction. Best Pract Res Clin Obstet Gynaecol 2004; 18: 397-410.
- Malhotra A, Allison B J, Castillo-Melendez M, Jenkin G, Polglase G R, Miller S L. Neonatal morbidities of fetal growth restriction: Pathophysiology and impact. Front Endocrinol (Lausanne) 2019; 10: 1-18.
- Figueras F, Oros D, Cruz-Martinez R, Padilla N, Hernandez-Andrade E, Botet F, et Neurobehavior in term, small-for-gestational age infants with normal placental function. Pediatrics 2009; 124: e934-941.
- Feldman R, Eidelman A I. Neonatal state organization, neuromaturation, mother-infant interaction, and cognitive development in small-for-gestational-age premature Pediatrics 2006;118:e869-878.
- Leitner Y, Fattal-Valevski A, Geva R, Eshel R, Toledano- Alhadef H, Rotstein M, et Neurodevelopmental outcome of children with intrauterine growth retardation: A longitudinal, 10-Year prospective study. J Child Neurol 2007; 22: 580-587.
- Tideman E, Mar K S, Ley Cognitive function in young adults following intrauterine growth restriction with ab- normal fetal aortic blood flow. Ultrasound Obs Gynecol 2007; 29: 614-618.
- Pla L, Illa M, Loreiro C, Lopez M C, Vázquez-Aristizabal P, Anna B, et al. Structural brain changes at the neonatal period in a rabbit model of Intrauterine Growth Dev Neurosci 2021; 42: 217-229.
- Dean J M, McClendon E, Hansen K, Azimi-Zonooz A, Chen K, Riddle A, et al. Prenatal Cerebral Ischemia Disrupts MRI-Defined Cortical Microstructure Through Disturbances in Neuronal Arborization. Sci Transl Med 2013; 5: 168ra7-168ra7.
- Dieni S, Rees Dendritic morphology is altered in hippocampal neurons following prenatal compromise. J Neurobiol 2003; 55: 41-52.
- Back S A, Han B H, Luo N L, Chricton C A, Xanthoudakis S, Tam J, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia- ischemia. J Neurosci 2002; 22: 455-463.
- Tolcos M, Bateman E, O’Dowd R, Markwick R, Vrijsen K, Rehn A, et al. Intrauterine growth restriction affects the maturation of myelin. Exp Neurol 2011; 232: 53-65.
- Rao M R, Hediger M L, Levine R J, Naficy A B, Vik Effect of breastfeeding on cognitive development of infants born small for gestational age. Acta Paediatr 2002; 91: 267-274.
- Als H, Duffy F H, McAnulty G, Butler S C, Lightbody L, Kosta S, et NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J Perinatol 2012; 32: 797-803.
- Illa M, Brito V, Pla L, Eixarch E, Arbat-Plana A, Batallé D, et Early Environmental Enrichment Enhances Abnormal Brain Connectivity in a Rabbit Model of Intrauterine Growth Restriction. Fetal Diagn Ther 2018; 44: 184-193.
- Andersen S L. Trajectories of brain development : point of vulnerability or window of opportunity? Neurosci Biobehav Rev 2003; 27: 3-18.
- Cusick S E, Georgieff M K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J 2016: 175: 16-21.
- Lewis, A J, Galbally, M, Gannon T. et al. Early life programming as a target for prevention of child and adolescent mental disorders. BMC Med 12, 33 (2014).
- Rousseau E, Michel P P, Hirsch E C. The Iron-Binding Protein Lactoferrin Protects Vulnerable Dopamine Neurons from Degeneration by Preserving Mitochondrial Calcium Mol Pharmacol 2013; 84: 888-898.
- Zugno A I, Chipindo H L, Volpato A M, Budni J, Steckert AV, Oliveira MB De, et al. Omega-3 prevents behavior re-sponse and brain oxidative damage in the ketamine model of Neuroscience 2014; 259: 223-231.
- Arteaga O, Revuelta M, Urigüen L, Martínez-Millán L, Hilario E, Álvarez A. Docosahexaenoic Acid Reduces Cerebral Damage and Ameliorates Long-Term Cognitive Impairments Caused by Neonatal Hypoxia-Ischemia in Mol Neurobiol 2016; 54: 7137-7155.
- Looij Y Van De, Ginet V, Chatagner A, Toulotte A, Somm E, Hüppi PS, et al. Lactoferrin during lactation protects the immature hypoxic- ischemic rat brain. Ann Clin Transl Neurol 2014; 1: 955-967.
- Innis S Essential Fatty Acid Transfer and Fetal Development. Placenta 2005; 26: 70-75.
- Gil-Sánchez A, Larqué E, Demmelmair H, Acien M I, Faber F L, Parrilla J J, et Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am J Clin Nutr 2010; 92: 115-122.
- Ikeno M, Okumura A, Hayakawa M, Kitamura Y, Suganuma H, Yamashiro Y, et al. Fatty acid composition of the brain of intrauterine growth retardation rats and the effect of maternal docosahexaenoic acid enriched diet. Early Hum Dev 2009; 85: 733-735.
- Wang B. Molecular Determinants of Milk Lactoferrin as a Bioactive Compound in Early Neurodevelopment and J Pediatr 2016; 173: 29-36.
- Jones M L, Mark P J, Mori T A, Keelan J A, Waddell B Maternal Dietary Omega-3 Fatty Acid Supplementation Reduces Placental Oxidative Stress and Increases Fetal and Placental Growth in the Rat. Biol Reprod 2013;88(37): 1-8.
- Wietrak E, Kaminski K, Leszczynska-Gorzelak B, Oleszczuk Effect of Docosahexaenoic Acid on Apoptosis and Proliferation in the Placenta: Preliminary Report. Biomed Res Int 2015.
- Lopez V, Kelleher S L, Lönnerdal Lactoferrin receptor mediates apo- but not holo-lactoferrin internalization via clathrin-mediated endocytosis in trophoblasts. Biochem J 2008; 411: 271-278.
- Chen J, Khalil R Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. Prog Mol Biol Transl Sci 2017; 148: 87-165.
- Boquien C Human milk: An ideal food for nutrition of preterm newborn. Front Pediatr 2018; 6: 1-9.
- du Sert N P, Hurst V, Ahluwalia A, Alam S, Avey M T, Baker M, et al. The arrive guidelines 2.0: Updated guidelines for reporting animal PLoS Biol 2020; 18: e300041.
- Eixarch E, Figueras F, Hernández-Andrade E, Crispi F, Nadal A, Torre I, et An experimental model of fetal growth restriction based on selective ligature of uteroplacental vessels in the pregnant rabbit. Fetal Diagn Ther 2009; 26: 203-211.
- Barenys M, Illa M, Hofrichter M, Loreiro C, Pla L, Klose J, et Rabbit neurospheres as a novel in vitro tool for studying neurodevelopmental effects induced by intrauterine growth restriction (IUGR). Stem Cells Transl Med 2020; 10: 1-13.
- Pla L, Kühne BA, Guardia-Escote L, Vázquez-Aristizabal P, Loreiro C, Flick B, Gratacós E, Barenys M, Illa M. Protocols for the Evaluation of Neurodevelopmental Alterations in Rabbit Models In Vitro and In Vivo. Front 2022 Jul 22; 4: 918520.
- Greenberg J A, Bell S J, Ausdal W Van. Omega-3 Fatty Acid Supplementation During Rev Obstet Gynecol 2008; 1: 162-169. PMCID: PMC2621042.
- Zehnder A M, Hawkins M G, Trestrail E A, Holt R W, Kent M Calculation of body surface area via computed tomography-guided modeling in domestic rabbits (Oryctolagus cuniculus). Am J Vet Res 2012; 73: 1859-1863.
- Anroop B. Nair S J. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27-31.
- Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, et Preterm Fetal Hypoxia-Ischemia Causes Hypertonia and Motor Deficits in the Neonatal Rabbit: A Model for Human Cerebral Palsy? J Neurosci 2004; 24: 24-34.
- Damiano D L, Quinlivan J M, Owen B F, Payne P, Nelson K C, Abel M F. What does the Ashworth scale really measure and are instrumented measures more valid and precise? Dev Med Child Neurol 2002; 44: 112-118.
- Kuhn S, Gritti L, Crooks D, Dombrowski Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019: 8: 1424.
- Saadani-makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, et al. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral Am J Obstet Gynecol 2008; 199: 651. e1-651. e7.
- Sholl D a. Dendritic organization in the neurons of the visual and motor cortices of the J Anat 1953; 87: 387-PMCID: PMC1244622.
- Saccone G, Berghella V, Maruotti G M, Sarno L, Martinelli Omega-3 supplementation during pregnancy to prevent recurrent intrauterine growth restriction: Systematic review and meta-analysis of randomized controlled trials. Ul-trasound Obstet Gynecol 2015; 46: 659-664.
- Somm E, Larvaron P, van de Looij Y, Toulotte A, Chatagner A, Faure M, et al. Protective effects of maternal nutritional supplementation with lactoferrin on growth and brain metabolism. Pediatr Res 2014; 75: 51-61.
- Mayurasakorn K, Niatsetskaya Z V, Sosunov S A, Williams J J, Zirpoli H, Vlasakov I, et al. DHA but Not EPA Emul-sions Preserve Neurological and Mitochondrial Function after Brain Hypoxia-Ischemia in Neonatal PLoS One 2016;11:e0160870.
- Jian X post-stroke therapeutic regimen with omega-3 polyunsaturated fatty acids that promotes white matter integrity and beneficial microglial responses after cerebral ischemia, Pu H, Hu X, Wei Z, Hong D, Zhang W, et A post-stroke therapeutic regimen with omega-3 polyunsaturated fatty acids that promotes white matter integrity and beneficial microglial responses after cerebral ischemia. Transl Stroke Res 2016; 7: 548-561.
- Kühne B A, Vázquez.Aristizabal P, Fuentes-Amella M, Pla L, Loreiro C, Gómez-Catalán J et Docosahexaenoic acid and Melatonin prevent impaired oligodendrogenesis induced by Intrauterine growth restriction. Biomedicines 2022; 10: 1205.
- Pu H, Guo Y, Zhang W, Huang L, Wang G, Liou A K, et Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. J Cereb Blood Flow Metab 2013; 33: 1474-1484.
- Tuzun F, Kumral A, Dilek M, Ozbal S, Ergur B, Yesilirmak D C, et al. Maternal omega-3 fatty acid supplementation protects against lipopolysaccharide-induced white matter injury in the neonatal rat brain. J Matern Neonatal Med 2012; 25: 849-854.
- Caputo M P, Williams J N, Drnevich J, Radlowski E C, Larsen R J, Sutton B P, et al. Hydrolyzed Fat Formula Increases Brain White Matter in Small for Gestational Age and Appropriate for Gestational Age Neonatal Front Pediatr 2020; 8: 32.
- Strømmen K, Blakstad E W, Moltu S J, Almaas A N, Westerberg A C, Amlien I K, et al. Enhanced nutrient supply to very low birth weight infants is associated with improved white matter maturation and head growth. Neonatology 2015; 107: 68-75.
- Pepper R E, Pitman K A, Cullen C L, Young K M. How do cells of the oligodendrocyte lineage affect neuronal circuits to influence motor function, memory and mood? Front Cell Neurosci 2018; 12: 1-14.
- Cutuli D. Functional and Structural Benefits Induced by Omega-3 Polyunsaturated Fatty Acids During Curr Neuropharmacol 2017; 15: 534-542.
- Crupi R, Marino A, Cuzzocrea S. n-3 Fatty Acids : Role in Neurogenesis and Neuroplasticity. Curr Med Chem 2013; 20: 2953-2963.
- Calderon F, Kim H Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 2004; 90: 979-988.
- Carbone B E, Abouleish M, Watters K E, Vogel S, Ribic A, Schroeder O H U, et al. Synaptic Connectivity and Cortical Maturation Are Promoted by the ω-3 Fatty Acid Docosahexaenoic Cereb Cortex 2020; 30: 226-240.
- Biernaskie J, Corbett Enriched Rehabilitative Training Promotes Improved Forelimb Motor Function and Enhanced Dendritic Growth after Focal Ischemic Injury. J Neurosci 2001; 21: 5272-5280.
- Woodruff-Pak DS, Agelan A, Valle L Del. A rabbit model of Alzheimer’s disease: Valid at neuropathological, cogni- tive, and therapeutic levels. J Alzheimer’s Dis 2011; 11: 371-383.
- Looij Y Van De, Larpin C, Cabungcal J H, Sanches E F, Toulotte A, Do KQ, et Nutritional intervention for developmental brain damage: Effects of lactoferrin supplementation in hypocaloric induced intrauterine growth restriction rat pups. Front Endocrinol (Lausanne) 2019; 10: 1-14.
- Kühne B A, Gutiérrez L, Sánchez E, Guardia-Escote L, Pla L, Loreiro C, et , Lactoferrin prevents adverse effects of intrauterine growth restriction (IUGR) on neurite length: investigations in an in vitro rabbit neurosphere model. Front Cellular Neurosc 2022 submitted.
- Kühne B A, E Teixidó, Ettcheth M, Puig T, Planas M, Feliu L, et , Application of the adverse outcome pathway to identify molecular changes in prenatal brain programming induced by IUGR: Discoveries after EGCG exposure. Food Che Toxicol. 2022: 9: 113506.
- Burgess, A. et al. (2007) ‘Polysialic acid limits septal neurite outgrowth on laminin’, Brain Research, 1144(1): 52-58.
- Chen Y, Zheng Z, Zhu X, Shi Y, Ii FAT, Wang B. Lactoferrin Promotes Early Neurodevelopment and Cognition in Postnatal Piglets by Upregulating the BDNF Signaling Pathway and Polysialylation. Mol Neurobiol 2015; 52: 256-269.
- Eixarch E, Batalle D, Illa M, Muñoz-Moreno E, Arbat- Plana A, Amat-Roldan I, et al. Neonatal neurobehavior and diffusion MRI changes in brain reorganization due to intrauterine growth restriction in a rabbit model. PLoS One 2012; 7: e31497.
- Illa M, Eixarch E, Muñoz-Moreno E, Batalle D, Leal- Campanario R, Gruart A, et Neurodevelopmental Effects of Undernutrition and Placental Underperfusion in Fetal Growth Restriction Rabbit Models. Fetal Diagn Ther 2017; 42:189-197.
- Workman A D, Charvet C J, Clancy B, Darlington RB, Finlay Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 2013; 33: 7368-7383.
- Lodygensky G A, Seghier M L, Warfield S K, Tolsa C B, Sizonenko S, Lazeyras F, et al. Intrauterine growth restriction affects the preterm infant’s Pediatr Res 2008; 63: 438-443.
- Ruff C A, Faulkner S D, Rumajogee P, Beldick S, Foltz W, Corrigan J, et al. The extent of intrauterine growth restriction determines the severity of cerebral injury and neurobehavioural deficits in PLoS One 2017; 12: e0184653.
Supplementary Materials:
|
Variables |
Score |
|
Righting reflex |
Number of times the animal turns |
|
Circular motion |
0: No movement 1: Slight movement, slight jump 2: Good range of motion, maintains for 1 or 2 steps, occasional jump 3: Entire range of motion, at least 3 steps, rapid jumps |
|
Intensity |
0: No movement 1: Slight activity 2: Distinct forceful movements 3: Rapid forceful movements |
|
Sucking and swallowing |
0: No movement of jaw, all milk dribbles out 1: Some movement of jaw, most milk dribbles out 2: Definite suck and swallow, some milk in nose 3: Good suck and swallow, no milk in nose |
|
Head turning |
0: No movement 1: Slight occasional movement of the head 2: Distinct movement of the head 3: Rapid forceful movement of the head and body |
