Neoadjuvant Versus Adjuvant Chemotherapy in Advanced Ovarian Carcinoma
Article Information
Hanan Ramadan Nassar1, Ihab Samy Fayek2, Alfred Elias Namour1, Mostafa Maher El-Gammal3
1Medical Oncology department, National Cancer Institute, Cairo University, Egypt
2Surgical Oncology department, National Cancer Institute, Cairo University, Egypt
3Agouza police hospital, ministry of interior, Egypt
*Corresponding Author: Dr. Ihab Samy Fayek, Surgical Oncology Department, National Cancer Institute, Cairo University, Egypt
Received: 06 December 2019; Accepted: 02 January 2020; Published: 22 January 2020
Citation: Ihab Samy Fayek, Alfred Elias Namour, Nelly Alieldin, Iman Gouda Farahat. Impact of Different Prognostic Factors on the Survival of Patients with Malignant Granulosa Cell Tumors of the Ovary: An Institutional Based Study at the Egyptian National Cancer Institute. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 056-064.
View / Download Pdf Share at FacebookAbstract
Aim: Aim of this retrospective study was to assess whether there is an advantage of treating women with advanced ovarian cancer with chemotherapy before cytoreductive surgery (neoadjuvant chemotherapy (NACT)) compared with conventional treatment where chemotherapy follows maximal cytoreductive surgery.
Patients and Methods: Include patients with advanced epithelial ovarian cancer (stage III and IV) who underwent surgery from 2009–2015 with optimal cyto-reductive surgery (R0).
Results: The study included 60 patients the mean age for all patients was 53.7 years, a total of 60 patients diagnosed and treated in NCI and Agouza Police hospital in the specified period (2009-2015), with locally advanced epithelial ovarian carcinoma were divided into 2 groups according to the pattern of treatment where 30 patients seemed resectable and primary cytoreductive surgery was carried out (conventional group), and 30 patients seemed unresectable and neoadjuvant chemotherapy (NACT) was given to them followed by interval debulking surgery (IDS). On assessment of the survival we compared the whole number of both groups. The median overall survival time was 45.77 months in the conventional group and 49.06 months in the NACT group.
Conclusion: Primary chemotherapy followed by IDS in a selected group of patients doesn't appear to worsen the prognosis, but it permits a less aggressive surgery to be performed. NACT is not inferior to primary surgery.
Keywords
<p>Neoadjuvant; Chemotherapy; Ovarian carcinoma; Neoadjuvant chemotherapy</p>
Neoadjuvant articles, Chemotherapy articles, Ovarian carcinoma articles, Neoadjuvant chemotherapy articles
Neoadjuvant articles Neoadjuvant Research articles Neoadjuvant review articles Neoadjuvant PubMed articles Neoadjuvant PubMed Central articles Neoadjuvant 2023 articles Neoadjuvant 2024 articles Neoadjuvant Scopus articles Neoadjuvant impact factor journals Neoadjuvant Scopus journals Neoadjuvant PubMed journals Neoadjuvant medical journals Neoadjuvant free journals Neoadjuvant best journals Neoadjuvant top journals Neoadjuvant free medical journals Neoadjuvant famous journals Neoadjuvant Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals Ovarian carcinoma articles Ovarian carcinoma Research articles Ovarian carcinoma review articles Ovarian carcinoma PubMed articles Ovarian carcinoma PubMed Central articles Ovarian carcinoma 2023 articles Ovarian carcinoma 2024 articles Ovarian carcinoma Scopus articles Ovarian carcinoma impact factor journals Ovarian carcinoma Scopus journals Ovarian carcinoma PubMed journals Ovarian carcinoma medical journals Ovarian carcinoma free journals Ovarian carcinoma best journals Ovarian carcinoma top journals Ovarian carcinoma free medical journals Ovarian carcinoma famous journals Ovarian carcinoma Google Scholar indexed journals Neoadjuvant chemotherapy articles Neoadjuvant chemotherapy Research articles Neoadjuvant chemotherapy review articles Neoadjuvant chemotherapy PubMed articles Neoadjuvant chemotherapy PubMed Central articles Neoadjuvant chemotherapy 2023 articles Neoadjuvant chemotherapy 2024 articles Neoadjuvant chemotherapy Scopus articles Neoadjuvant chemotherapy impact factor journals Neoadjuvant chemotherapy Scopus journals Neoadjuvant chemotherapy PubMed journals Neoadjuvant chemotherapy medical journals Neoadjuvant chemotherapy free journals Neoadjuvant chemotherapy best journals Neoadjuvant chemotherapy top journals Neoadjuvant chemotherapy free medical journals Neoadjuvant chemotherapy famous journals Neoadjuvant chemotherapy Google Scholar indexed journals gynecologic cancers articles gynecologic cancers Research articles gynecologic cancers review articles gynecologic cancers PubMed articles gynecologic cancers PubMed Central articles gynecologic cancers 2023 articles gynecologic cancers 2024 articles gynecologic cancers Scopus articles gynecologic cancers impact factor journals gynecologic cancers Scopus journals gynecologic cancers PubMed journals gynecologic cancers medical journals gynecologic cancers free journals gynecologic cancers best journals gynecologic cancers top journals gynecologic cancers free medical journals gynecologic cancers famous journals gynecologic cancers Google Scholar indexed journals cytoreductive surgery articles cytoreductive surgery Research articles cytoreductive surgery review articles cytoreductive surgery PubMed articles cytoreductive surgery PubMed Central articles cytoreductive surgery 2023 articles cytoreductive surgery 2024 articles cytoreductive surgery Scopus articles cytoreductive surgery impact factor journals cytoreductive surgery Scopus journals cytoreductive surgery PubMed journals cytoreductive surgery medical journals cytoreductive surgery free journals cytoreductive surgery best journals cytoreductive surgery top journals cytoreductive surgery free medical journals cytoreductive surgery famous journals cytoreductive surgery Google Scholar indexed journals cytoreduction articles cytoreduction Research articles cytoreduction review articles cytoreduction PubMed articles cytoreduction PubMed Central articles cytoreduction 2023 articles cytoreduction 2024 articles cytoreduction Scopus articles cytoreduction impact factor journals cytoreduction Scopus journals cytoreduction PubMed journals cytoreduction medical journals cytoreduction free journals cytoreduction best journals cytoreduction top journals cytoreduction free medical journals cytoreduction famous journals cytoreduction Google Scholar indexed journals paclitaxel articles paclitaxel Research articles paclitaxel review articles paclitaxel PubMed articles paclitaxel PubMed Central articles paclitaxel 2023 articles paclitaxel 2024 articles paclitaxel Scopus articles paclitaxel impact factor journals paclitaxel Scopus journals paclitaxel PubMed journals paclitaxel medical journals paclitaxel free journals paclitaxel best journals paclitaxel top journals paclitaxel free medical journals paclitaxel famous journals paclitaxel Google Scholar indexed journals platinum compounds articles platinum compounds Research articles platinum compounds review articles platinum compounds PubMed articles platinum compounds PubMed Central articles platinum compounds 2023 articles platinum compounds 2024 articles platinum compounds Scopus articles platinum compounds impact factor journals platinum compounds Scopus journals platinum compounds PubMed journals platinum compounds medical journals platinum compounds free journals platinum compounds best journals platinum compounds top journals platinum compounds free medical journals platinum compounds famous journals platinum compounds Google Scholar indexed journals ovarian malignant tumors articles ovarian malignant tumors Research articles ovarian malignant tumors review articles ovarian malignant tumors PubMed articles ovarian malignant tumors PubMed Central articles ovarian malignant tumors 2023 articles ovarian malignant tumors 2024 articles ovarian malignant tumors Scopus articles ovarian malignant tumors impact factor journals ovarian malignant tumors Scopus journals ovarian malignant tumors PubMed journals ovarian malignant tumors medical journals ovarian malignant tumors free journals ovarian malignant tumors best journals ovarian malignant tumors top journals ovarian malignant tumors free medical journals ovarian malignant tumors famous journals ovarian malignant tumors Google Scholar indexed journals
Article Details
1. Introduction
Ovarian carcinoma (OC) represents about three percent of cancers, 10th ranking cancer and fifth leading cause of cancer-related deaths in women, with the most lethal prognosis among gynecologic cancers. Mortality rates are slightly higher for Caucasian women than for African-American women. In 2012, an estimated 22.280 new diagnoses and an estimated 15.500 deaths in the United States; less than 40% of women with OC are cured [1]. In NCI, Cairo University, tumors of the female genital system represented 4.7% of total female malignancies. A total of 135 cases of ovarian malignant tumors, accounting for 29.22% of gynecologic malignancies and 1.37% of total female malignancies are presented during the years 2003-2004 [2]. In the triennial report of 2000-2002 Gharbia population-based cancer registry (GPBCR), about 75 cases of OC were registered per year. Based on Aswan regional registry, in which over the year 2008, 35 cases of OC were registered, representing 5.6% of all female cancers cases [3].
Surgery with adjuvant chemotherapy is recommended for patients with epithelial ovarian cancer (EOC) stage IC and up. In locally advanced and metastatic EOC either primary surgery or neoadjuvant chemotherapy (NACT) followed by surgery is a reasonable modality [4]. Numerous combination chemotherapy regimens have been shown to produce responses in patients with OC. Since the mid-1990, a chemotherapy combination consisting of the combination of paclitaxel and a platinum compounds has been used [5].
Optimizing cytoreduction is by leaving minimal or no residual cancer. Interval debulking surgery (IDS) has gained popularity, mainly reducing the volume of residual disease after primary surgery plus chemotherapy or after chemotherapy alone as much as possible. Several trials have tried to validate IDS, the most important trial on NACT is certainly the EORTC55971 trial, constituting the first prospective multicentric trial to evaluate NACT followed by IDS versus primary surgery followed by adjuvant chemotherapy, concluding that neoadjuvant chemotherapy is not inferior to primary surgery [6]. Kehoe et al. 2013 presented the preliminary results of the CHORUS trial, with similar data as the EORTC55971 [7].
The aim of this study is to assess and evaluate the experience of the National Cancer institute (NCI), Cairo University and Agouza Police hospital (APH), Cairo- Egypt in treating Egyptian women with advanced EOC with NACT before cytoreductive surgery compared with the conventional treatment where chemotherapy follows optimal cytoreductive surgery, regarding the superiority of one treatment modality over the other.
2. Patients and Methods
This is a retrospective study include 60 Egyptian female patients with advanced EOC (stage III and IV) who underwent surgery from 2009–2015 with optimal cytoreductive surgery (R0), at the NCI, Cairo University, Egypt and APH, Cairo, Egypt. The following data were collected from the patients’ medical records: Age at diagnosis, presenting symptoms, Laboratory investigations (Liver function, kidney function, complete blood count and tumor markers), Tumor histological type, tumor FIGO stage& degree of differentiation, Type of surgery, Chemotherapy regimens (Adjuvant, Neoadjuvant or palliative), Chemotherapy responsiveness or resistance, Disease recurrence and disease-free period, and patients' survival. They were grouped into 2 groups (30 patients each) according to the pattern of treatment where 30 patients seemed resectable and primary cytoreductive surgery (with CC0 or CC1 intent) was carried out (conventional / adjuvant group), and 30 patients seemed unresectable and NACT was given to them followed by surgery (NACT group).
2.1 Exclusion criteria
Included all suboptimal (CC2, CC3) surgical cytoreductions, other pathological types of ovarian cancers, recurrent cases, patients with co-morbidities or low performance status which didn’t allow the completion of chemotherapy or the performance of surgery, and reluctant patients with irregular or interrupted follow-ups.
2.1.1 Outcome in both groups was studied regarding
- Overall survival (OS) calculated from date of presentation till date of death or last follow-up.
- Disease free survival (DFS) calculated from date of initial treatment till date of relapse or death or last follow-up.
2.2 Statistical analysis
Data was analyzed using IBM SPSS advanced statistics version 22 (SPSS Inc., Chicago, IL). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test was used to examine the relation between qualitative variables. For normally distributed quantitative data, comparison between two groups was done using Student t-test and Mann-Whitney test (non-parametric t-test) for not normally distributed quantitative data. Survival analysis was done using Kaplan-Meier method. Comparison between two survival curves was done using log-rank test. All tests were two-tailed. A p-value < 0.05 was considered significant.
2.2.1 Institutional review board (IRB) approval: The study only started after the approval of the IRB. No individual patient consent is needed as the study poses no risk of harm to any of the study subjects.
2.2.2 Protection of privacy and confidentiality of patients' information: Data collection and presentation was anonymous and both privacy and confidentiality were protected to the maximum possible standards.
3. Results
The mean age of all the patients was 53.7 years (Table 1). The Patterns of treatment used in both groups regarding the number of cycles and chemotherapeutics used were summarized in Table 2. Abdominal distension and pain were the most common complaints at presentation; Laterality is almost the same in both groups. Table 3 summarizes the patients’ complaints, Laterality, and complications related to treatment in both groups.
|
Valid N |
Mean |
Standard Deviation |
Minimum |
Maximum |
||
|
Group |
Adjuvant |
30 |
54.0 |
11.3 |
27.0 |
74.0 |
|
Neoadjuvant |
30 |
53.4 |
13.1 |
21.0 |
76.0 |
|
Table 1: Age distribution in years in both groups.
|
Group |
Pattern of treatment |
Frequency |
Percentage |
|
Adjuvant |
cytoreductive surgery then 6 cycles Taxol/carboplatin |
30 |
100 |
|
Neoadjuvant |
3 cycles Taxol/carboplatin then cytoreductive surgery then 3 cycles Taxol/carboplatin |
19 |
63.3 |
|
6 cycles Taxol/carboplatin then cytoreductive surgery |
8 |
26.7 |
|
|
6 cycles Taxol/carboplatin then cytoreductive surgery then 2 cycles Taxol/carboplatin |
1 |
3.3 |
|
|
6 cycles Taxol/carboplatin then cytoreductive surgery then 6 cycles Taxol/carboplatin |
2 |
6.7 |
Table 2: Chemotherapeutic patterns and medications used in the study.
|
Variable |
Group |
Total |
||
|
Complaint |
|
Adjuvant |
Neoadjuvant |
|
|
Abdominal distension |
Count |
13 |
12 |
25 |
|
% within Group |
43.3% |
40.0% |
41.7% |
|
|
Abdominal pain |
Count |
9 |
14 |
23 |
|
% within Group |
30.0% |
46.7% |
38.3% |
|
|
Vaginal bleeding |
Count |
7 |
2 |
9 |
|
% within Group |
23.3% |
6.7% |
15.0% |
|
|
Accidentally discovered |
Count |
1 |
2 |
3 |
|
% within Group |
3.3% |
6.7% |
5.0% |
|
|
Laterality |
Adjuvant |
Neoadjuvant |
||
|
Right |
Count |
6 |
6 |
12 |
|
% within Group |
20.0% |
20.0% |
20.0% |
|
|
Left |
Count |
10 |
9 |
19 |
|
% within Group |
33.3% |
30.0% |
31.7% |
|
|
Bilateral |
Count |
14 |
15 |
29 |
|
% within Group |
46.7% |
50.0% |
48.3% |
|
|
Complication |
Adjuvant |
Neoadjuvant |
||
|
G.I.T upset |
Count |
2 |
0 |
2 |
|
% within Group |
6.7% |
0.0% |
3.3% |
|
|
Hypersensitivity to Taxol |
Count |
2 |
0 |
2 |
|
% within Group |
6.6% |
0.0% |
3.3% |
|
|
Injury to renal vein |
Count |
1 |
0 |
1 |
|
% within Group |
3.3% |
0.0% |
1.6% |
|
|
Injury to transverse colon |
Count |
1 |
0 |
1 |
|
% within Group |
3.3% |
0.0% |
1.6% |
|
|
Myelo-suppression |
Count |
15 |
13 |
28 |
|
% within Group |
50% |
43.3% |
46.6% |
|
|
Peripheral neuritis |
Count |
2 |
0 |
2 |
|
% within Group |
6.6% |
0.0% |
3.3% |
|
Table 3: Patients’ presentations, Laterality, and complications related to treatment in both groups.
Myelo-suppression was the most common complication during treatment in both groups, in the adjuvant group surgical complications were more common than in neoadjuvant group, in form of injury to the renal vein and injury to the transverse colon. Stage distributions of the tumors among both arms in our study were summarized in (Table 4). Figures 1 and 2 illustrate the overall survival (OS) and Disease free survival (DFS) per month by stage, respectively. The median follow-up period was 48.7 months (range 14.2-73.6 months). The median DFS was 41 months approximately (Table 5).
|
Stage |
Adjuvant |
Neoadjuvant |
Total |
|
|
IIIA,B |
Count |
15 |
13 |
28 |
|
% within Group |
50.0% |
43.3% |
46.7% |
|
|
IIIC |
Count |
7 |
7 |
14 |
|
% within Group |
23.3% |
23.3% |
23.3% |
|
|
IV |
Count |
8 |
10 |
18 |
|
% within Group |
26.7% |
33.3% |
30.0% |
Table 4: Stage distributions of the tumors among the study population.
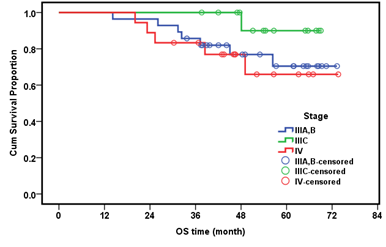
Figure 1: Overall Survival per month by stage.
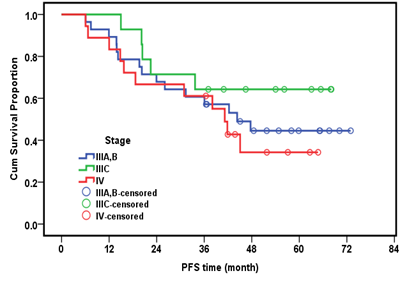
Figure 2: Disease free survival per month by stage.
|
Valid N |
Mean |
Standard Deviation |
Median |
Minimum |
Maximum |
|
|
Follow-up time (month) |
60 |
49.90 |
14.75 |
48.70 |
14.18 |
73.62 |
|
DFS time (month) |
60 |
38.86 |
19.76 |
41.05 |
6.05 |
72.96 |
Table 5: Follow-up and disease free survival (months) of all the patients in the study.
On assessment of the survival we compared the whole number of both groups. The median OS time was 45.77 months in the conventional group and 49.06 month in the NACT group with an insignificant P value (P=0.329). Table 6, Figure 3. The median OS was not reached since more than half of the patients remain alive till the end of the study. The cumulative OS for the whole studied group (n=60) was 73.7%.
|
|
No. |
Cumulative survival at 36 months (%) |
Cumulative survival at 60 months (%) |
p-value |
|
Whole group |
60 |
88.3% |
73.7% |
|
|
Adjuvant group |
30 |
83.2% |
69.3% |
0.320 |
|
Neo-adjuvant group |
30 |
93.3% |
79.0% |
Table 6: Cumulative survival in both groups and correlation.
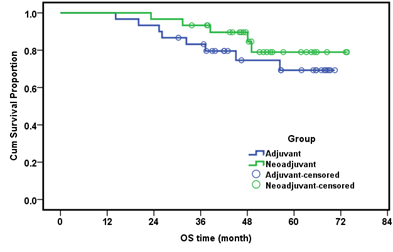
Figure 3: Overall survival (month) in both groups.
The median DFS was 37.35 months in the adjuvant group and 43.45 months in the neoadjuvant group with an insignificant P value (P=0.609) Figure 4. In a multivariate analysis of both the conventional group and NACT group, the OS was not significantly affected by any of the study parameters (pathologic type, grade, stage, laterality). As both groups of our study are subjected to optimal cytoreductive surgery we concluded that the value of NACT is to obtain optimum cytoreduction by means of less aggressive surgery. In our study, debulking surgery in NACT group was less aggressive than in the conventional group and bears less postoperative mortality.
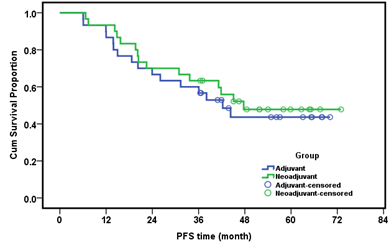
Figure 4: Disease free survival (month) in both groups.
4. Discussion
Epithelial ovarian cancer (EOC) causes more deaths than any other malignancy affecting the female reproductive system, with advanced stages (III – IV) in up to 75% of cases [8]. The EORTC 55971 trial and the CHORUS trial, both indicated that primary chemotherapy optimize cytoreduction, with less postoperative mortality [6, 7]. Subsequent clinical studies have confirmed these outcome measures and have also concluded that the rate of stoma formation is significantly reduced in those patients having neoadjuvant chemotherapy (NACT) [9, 10]. Hegazy et al, 2005 years earlier stated that NACT followed by IDS in a select group of patients doesn't worsen the prognosis, permitting a less aggressive surgery [11].
The Japan Clinical Oncology Group protocol 0602 in a phase III trial findings suggested that patients in the NACT arm had significantly less bowel/organ resection, blood loss, albumin transfusion and grade 3-4 adverse events after surgery [12]. Jacob et al., 1991 concluded that patients who underwent optimal IDS had minimal residual disease and appeared to have a longer median OS than those who did not (18.1 versus 7.5 months; P=0.02) [13]. In another study using historical controls, addressing the long term survival of 59 patients undergoing NACT, There was no statistical difference in the OS or progression free survival (PFS) between the study (five cycles of platinum-based NACT) and control group (treated with primary debulking surgery (PDS) followed by a minimum of 6 cycles of platinum-based chemotherapy), Over 50% > 1 cm of residual disease, after PDS. Interestingly, patients who underwent NACT and IDS had a longer OS comparing them to those who only received NACT (1.47 years versus 0.64 year; P < 0.0001) [14].
Despite those findings, the impact of NACT remained controversial. To further explore the clinical ramifications, Bristow and Chi performed a meta-analysis of platinum-based NACT followed by IDS, analyzing 21 studies meeting their inclusion criteria. The mean weighted overall survival was 24.5 months. Of interest, the median survival increased 1.9 months with each 10% increase in the rate of maximal cytoreduction, and median survival was also increased with usage of Taxanes chemotherapy. However, with each increase in the number of NACT cycles, the median survival was decreased by 4.1 months. They concluded that NACT in lieu of PDS was associated with inferior OS [15].
In a similar study to ours, a total of 172 patients with stage III or IV EOC were evaluated between 1998 and 2005 were retrospectively reviewed, demonstrated the superiority of platinum and taxane NACT [16]. In our study a total of 60 patients with stage III or IV EOC were evaluated between 2009 and 2015. All patients were submitted to debulking surgery with optimal cytoreduction (CC0, CC1). NACT was given in 30 patients (Neoadjuvant group) and PDS was performed in 30 patients (conventional group). Surgical aggressiveness was significantly lower in the neoadjuvant group than the conventional group. The Cumulative survival at 36 months was 83.2 % in the conventional group and 93.3% in NACT group. The Cumulative survival at 60 months was 69.3% in the conventional group and 79% in NACT group (P value=0.32).
5. Conclusion
Primary chemotherapy followed by interval debulking surgery in select group of patients doesn't appear to worsen the prognosis, but it permits a less aggressive surgery to be performed. Until there is further confirmatory data, NACT should be restricted to those most unlikely to undergo optimal primary surgery or those too medically compromised. Further studies are needed with larger sample size and longer follow-up to assess the surgical morbidity and mortality in both groups and to confirm the advantages and drawbacks of NACT.
References
- Cancer facts and figures. Atlanta, GA: American Cancer Society (2015).
- Mokhtar N, Gouda I, Adel I. Cancer Pathology Registry: 2003-2004, and Time Trend Analysis (2004).
- Ibrahim AS, Khaled HM, Mikhail NNH, et al. Cancer Incidence in Egypt: Results of the National Population-Based Cancer Registry Program. Journal of Cancer Epidemiology (2014): ID: 437971.
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363 (2010): 943-953.
- McGuire WP, Markman M. Primary ovarian cancer chemotherapy: current standards of care. British Journal of Cancer 89 (2003): S3-S8.
- Vergote I, du Bois A, Amant F, et al. Neoadjuvant chemotherapy in advanced ovarian cancer: on what do we agree and disagree?. Gynecol Oncol 128 (2013): 6-11.
- Kehoe S, Hook J, Nankivell M, et al. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: Results from the MRC CHORUS trial. J Clin Oncol 31 (2013).
- Sun JH, Ji ZH, Yu Y, e al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat advanced/recurrent epithelial ovarian cancer: results from a retrospective study on prospectively established database. Translational Oncol 9 (2016): 130-138.
- Hoskins PJ. Which is the better surgical strategy for newly diagnosed epithelial ovarian cancer: primary or interval debulking? Curr Opin Oncol 23 (2011): 501-506.
- Thrall MM, Gray HJ, Symons RG, et al. Neoadjuvant chemotherapy in the Medicare cohort with advanced ovarian cancer. Gynecol Oncol 123 (2011): 461-466.
- Hegazy MA, Hegazi RA, Elshafei MA, et al. Neoadjuvant chemotherapy versus primary surgery in advanced ovarian carcinoma. World journal of surgical oncology 3 (2005): 57.
- Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 64 (2016): 22-31.
- Jacob JH, Gershenson DM, Morris M, et al. Neoadjuvant chemotherapy and interval debulking for advanced epithelial ovarian cancer. Gynecol Oncol 42 (1991): 146-150.
- Schwartz PE, Rutherford TJ, Chambers JT, et al. Neoadjuvant chemotherapy for advanced ovarian cancer: long-term survival. Gynecol Oncol 72 (1999): 93-99.
- Bristow RE, Chi D. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol 103 (2006): 1070-1076.
- Hou JY, Kelly MG, Yu H, et al. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol Oncol 105 (2007): 211-217.
