Nasal Glioma: Prenatal Imaging Diagnosis
Article Information
Clarós P1, Ribeiro I2, Clarós A1
1Clarós Clinic, Barcelona, Spain
2Clarós Clinic Scholarships, Barcelona, Spain
Pedro Clarós, Orcid ID: orcid.org/0000-0002-7567-0370
Andrés Clarós, Orcid ID: Orcid.Org/0000-0001-6084-3470
*Corresponding Author: Clarós P, Clarós Clinic, C/Los Vergós 31, Barcelona-08017, Spain
Received: 15 February 2019; Accepted: 25 February 2019; Published: 28 February 2019
Citation: Clarós P, Ribeiro I, Clarós A. Nasal Glioma: Prenatal Imaging Diagnosis. Journal of Radiology and Clinical Imaging 2 (2019): 033-041.
View / Download Pdf Share at FacebookAbstract
Nasal glioma is a rare congenital and benign tumor of the middle face. The prenatal diagnosis of this fetal tumor represents an important advance in care, as it is essential to guide the prenatal counseling of the couple and the orientation of prenatal and/or postnatal management. We report here our experience with nasal gliomas, reporting a case with prenatal diagnosis, evoked intrauterine with ultrasonographic examination and confirmed after birth. This allowed the psychological preparation of the parents by counseling on the interventions to come, that were performed at 26 months with good outcome.
Keywords
Diagnosis in uterus, Nasal glioma, Reconstructive surgery, Prenatal imaging
Diagnosis in uterus articles Diagnosis in uterus Research articles Diagnosis in uterus review articles Diagnosis in uterus PubMed articles Diagnosis in uterus PubMed Central articles Diagnosis in uterus 2023 articles Diagnosis in uterus 2024 articles Diagnosis in uterus Scopus articles Diagnosis in uterus impact factor journals Diagnosis in uterus Scopus journals Diagnosis in uterus PubMed journals Diagnosis in uterus medical journals Diagnosis in uterus free journals Diagnosis in uterus best journals Diagnosis in uterus top journals Diagnosis in uterus free medical journals Diagnosis in uterus famous journals Diagnosis in uterus Google Scholar indexed journals Nasal glioma articles Nasal glioma Research articles Nasal glioma review articles Nasal glioma PubMed articles Nasal glioma PubMed Central articles Nasal glioma 2023 articles Nasal glioma 2024 articles Nasal glioma Scopus articles Nasal glioma impact factor journals Nasal glioma Scopus journals Nasal glioma PubMed journals Nasal glioma medical journals Nasal glioma free journals Nasal glioma best journals Nasal glioma top journals Nasal glioma free medical journals Nasal glioma famous journals Nasal glioma Google Scholar indexed journals Reconstructive surgery articles Reconstructive surgery Research articles Reconstructive surgery review articles Reconstructive surgery PubMed articles Reconstructive surgery PubMed Central articles Reconstructive surgery 2023 articles Reconstructive surgery 2024 articles Reconstructive surgery Scopus articles Reconstructive surgery impact factor journals Reconstructive surgery Scopus journals Reconstructive surgery PubMed journals Reconstructive surgery medical journals Reconstructive surgery free journals Reconstructive surgery best journals Reconstructive surgery top journals Reconstructive surgery free medical journals Reconstructive surgery famous journals Reconstructive surgery Google Scholar indexed journals Prenatal imaging articles Prenatal imaging Research articles Prenatal imaging review articles Prenatal imaging PubMed articles Prenatal imaging PubMed Central articles Prenatal imaging 2023 articles Prenatal imaging 2024 articles Prenatal imaging Scopus articles Prenatal imaging impact factor journals Prenatal imaging Scopus journals Prenatal imaging PubMed journals Prenatal imaging medical journals Prenatal imaging free journals Prenatal imaging best journals Prenatal imaging top journals Prenatal imaging free medical journals Prenatal imaging famous journals Prenatal imaging Google Scholar indexed journals magnetic resonance imaging articles magnetic resonance imaging Research articles magnetic resonance imaging review articles magnetic resonance imaging PubMed articles magnetic resonance imaging PubMed Central articles magnetic resonance imaging 2023 articles magnetic resonance imaging 2024 articles magnetic resonance imaging Scopus articles magnetic resonance imaging impact factor journals magnetic resonance imaging Scopus journals magnetic resonance imaging PubMed journals magnetic resonance imaging medical journals magnetic resonance imaging free journals magnetic resonance imaging best journals magnetic resonance imaging top journals magnetic resonance imaging free medical journals magnetic resonance imaging famous journals magnetic resonance imaging Google Scholar indexed journals computed-tomography articles computed-tomography Research articles computed-tomography review articles computed-tomography PubMed articles computed-tomography PubMed Central articles computed-tomography 2023 articles computed-tomography 2024 articles computed-tomography Scopus articles computed-tomography impact factor journals computed-tomography Scopus journals computed-tomography PubMed journals computed-tomography medical journals computed-tomography free journals computed-tomography best journals computed-tomography top journals computed-tomography free medical journals computed-tomography famous journals computed-tomography Google Scholar indexed journals gadolinium injection articles gadolinium injection Research articles gadolinium injection review articles gadolinium injection PubMed articles gadolinium injection PubMed Central articles gadolinium injection 2023 articles gadolinium injection 2024 articles gadolinium injection Scopus articles gadolinium injection impact factor journals gadolinium injection Scopus journals gadolinium injection PubMed journals gadolinium injection medical journals gadolinium injection free journals gadolinium injection best journals gadolinium injection top journals gadolinium injection free medical journals gadolinium injection famous journals gadolinium injection Google Scholar indexed journals Radiation articles Radiation Research articles Radiation review articles Radiation PubMed articles Radiation PubMed Central articles Radiation 2023 articles Radiation 2024 articles Radiation Scopus articles Radiation impact factor journals Radiation Scopus journals Radiation PubMed journals Radiation medical journals Radiation free journals Radiation best journals Radiation top journals Radiation free medical journals Radiation famous journals Radiation Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals computed-tomography articles computed-tomography Research articles computed-tomography review articles computed-tomography PubMed articles computed-tomography PubMed Central articles computed-tomography 2023 articles computed-tomography 2024 articles computed-tomography Scopus articles computed-tomography impact factor journals computed-tomography Scopus journals computed-tomography PubMed journals computed-tomography medical journals computed-tomography free journals computed-tomography best journals computed-tomography top journals computed-tomography free medical journals computed-tomography famous journals computed-tomography Google Scholar indexed journals
Article Details
1. Introduction
Nasal gliomas are rare congenital masses that occur due to aberrant embryologic development of the fronto-nasal region. They are more often seen in newborns and children, with a male predominance of 3:1. Congenital nasal masses have an incidence of 1 in 20,000 to 40,000 live births, of which 5-6% are nasal gliomas [1, 2]. Although they are not neoplasms, the term nasal glioma, first applied by Reid in 1852, is still used. These ectopic neural tumors are also called, perhaps more accurately, as nasal cerebral heterotopia or sequestered encephaloceles. In addition, nasal gliomas are rare, even ENT specialists are not familiar with their management. They can be in an extra-nasal position in 60% of cases, intra-nasal in 30% and combined in 10% of cases [1-4]. The prenatal diagnosis of congenital malformations in general and nasal glioma in particular, has made great progress, allowing a better psychological preparation of the parents on the postnatal care. This previous preparation has improved the care and follow-up of children with congenital malformations [5].
1.1 Objective
We present our experience in the clinic over the last 24 years and report a case of nasal glioma diagnosed in-utero, with subsequent treatment in our institution.
2. Materials and Methods
We did a retrospective search of nasal gliomas treated in our institution. Our experience reports back to 1995 [6, 7]. We evaluated the mean age at treatment, sex, location and type of surgery performed.
3. Results
We identified 22 cases of nasal glioma treated in our centre (Table 1). One case in particular, which was diagnosed prenatally, will be reported independently. Most children were reported to us during the first months (range 3 days-2 years), either referred by another clinician or through a direct consult. Children were usually asymptomatic; however, intranasal lesions could provide a degree of nasal obstruction. All children had an imaging screening with MRI (magnetic resonance imaging), that confirmed the lesion and, in specific cases, CT-scan (computed-tomography).
|
Mean age (months) |
6.45 ± 5.16 |
|
Sex |
|
|
Boys |
10 (45.5%) |
|
Girls |
12 (54.4%) |
|
Location |
|
|
Intra-nasal |
12 (54.5%) |
|
Extra-nasal |
10 (45.5%) |
|
Surgery |
|
|
Nasal endoscopy |
11 (50.0%) |
|
Lateral rhinotomy |
8 (36.4%) |
|
Paralateronasal |
2 (9.5%) |
|
External rhinoplasty |
1 (4.5%) |
Table 1: Characterization of population and treatment.
We decided the timing for surgery considering the impact of the lesion in the daily life, its growth pattern and surgical and anaesthetic risks. Whenever possible (accessible intranasal lesions), we opted for an endonasal approach with nasal endoscopy. However, in 50% of cases, we performed an external approach, either by lateral rhinotomy, para-latero-nasal or external rhinoplasty. The latter allows a good correction of deformity, if present, although giving a more limited view for excision. Whichever the technique, we reported no recurrences. Final diagnosis was provided by pathology, that showed in 86.4% neuro-glial cells and in 3 cases reactive astrocytes and fibrosis.
4. Case Report
4-month-old girl, received for evaluation of a right lateral mass, firm in consistency, deforming the nasal pyramid (Figure 1). Pre-natal history revealed the presence of a lateral-nasal mass during a routine 3D ultrasonographic examination at 28 weeks gestation (Figure 2). Pregnancy and childbirth went off without incidents. Clinically, the child presented with a right unilateral nasal obstruction due to compression of the nasal pyramid. There were no other malformations. Post-natal CT confirmed the presence of a mass of the right part of the root of the nose, which appeared to be continuous with the intranasal route of the persistent foramen caecum, suggesting a probable dermoid cyst of the dermal sinus. Post-natal MRI showed a well-defined bilobal tumor measuring 18 × 10 × 13 mm, isodense on T1-weighted, discretely hypo-dense in T2-weighted sequences, located in the right side of the nasal pyramid. The intranasal tumor was supported by the nasal septum. The mass had a very discrete homogeneous enhancement after gadolinium injection and showed no intra-cranial extension. There was no displacement of medial structures, or intra or extra-parenchymal bleeding lesions, supra nor infratentorial. Callous body was normal. There were no other intra or extra-cranial abnormalities (Figure 3).
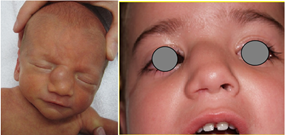
Figure 1: (a) at birth and (b) at 26 months old.
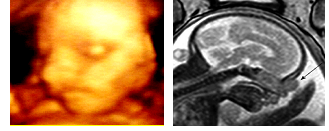
Figure 2: 3-D Ultrasonography at 28-weeks gestation showing the nasal mass.
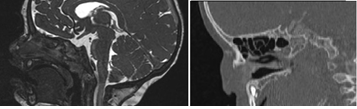
Figure 3: (a) MRI-sagital view (b) CT-axial view at birth.
With these elements, a strong presumption of the diagnosis of nasal glioma was posed, motivating surgical treatment, with care to preserve patient´s aesthetics. We performed a transcolumellar external rhinoplasty approach, with nasal pyramidal reconstruction step with auricular conchal composite graft. The operative sequences were simple, without nasal deformity. The patient spent a few days with nasal conformers to avoid secondary stenosis. Histology showed a nodular formation of pink color and firm consistency measuring 0.8 cm in diameter. The cut showed a whitish homogeneous coloring, of dense consistency. The nodule consisted of a number of interanastomotic formations, corresponding to glial tissue, which included reactive astrocytes. It was surrounded by collagenous fibrous tissue and accompanied by dilated capillaries, typical of nasal glioma. There were no obvious signs of atypia or malignancy.
5. Discussion
Nasal glioma is a rare entity, 22 cases in almost 40 years of experience in our clinic despite our expertise in the paediatric field [6, 7]; about 1 per 20,000 to 40,000 live births for several authors [1-4]. Only about 250 cases have been reported in the literature [8]. In our study, the location was 54.5%% intranasal, which isn´t in agreement to the literature, that describes 60% extra nasal, 30% intranasal and an intra and extra-nasal association in 10% of cases. They have also been found in the nasopharynx, paranasal sinuses, tonsillar lodges, scalp and orbit [1-4]. The origin of these malformations of the nasofrontal region in the second month of pregnancy may be nasal gliomas, dermoid cysts and encephaloceles. Encephaloceles is the general term, which includes meningoceles, meningoencephaloceles and meningoencephalo-cystoceles, depending on the neural tissue herniating through the cranial opening. In the naso-frontal zone, closure of the fonticulus frontalis and the foramen caecum may be defective, resulting in nasofrontal and nasoethmoidal encephaloceles [8, 9]. Dermoid cysts are attributable to the occurrence of herniation through the foramen cecum to the pre-nasal skin which, in the regression of the central nervous system, leaves meningeal tissue or skin in its tube. The latter may spontaneously fist in the skin of the nose or around it and mesodermal elements such as hair, sweat and sebaceous glands may be present [4, 8].
Various hypotheses have been proposed to explain the development of nasal glioma [4, 9-11]. It is generally accepted that brain tissue has migrated through the foramen cecum and lost contact with the central nervous system, although 15-20% of them maintain a fibrous stem connection with it [4, 9-11]. Thus, nasal glioma and nasal encephalocele have a common origin. But while nasal gliomas are sequestered masses, encephaloceles are extensions of brain tissue through a bone defect. In some borderline cases it is difficult to distinguish a glioma from an encephalocele [11]. However, there is no variation in nasal glioma size with the increase in intracranial pressure (compression of the internal jugular vein), in contrast to encephalocele, which constitutes the sign of Furstenberg. Given modern advances in the quality and resolution of prenatal ultrasound and MRI, the diagnosis and therapeutic planning of nasal gliomas, encephaloceles, and other craniofacial masses have recently transitioned from postnatal to prenatal [8] as was the case with our patient. Routine ultrasound and three-dimensional reconstructions of fetal images provided obstetricians and radiologists with the ability to detect these lesions. In the present case, the accuracy of the ultrasonographic examination has allowed to evoke the diagnosis during fetal life, as so well demonstrated De Biaso [12]. This had a psychological impact and allowed to discuss the rest of the paraclinic to confirm the diagnosis as well as the time of care. Additional diagnostic images could have been obtained prenatally, including fetal MRI, which has the additional advantage of not using ionizing radiation [11]. In our case, the MRI was performed in the postnatal period, which could explain the delay between diagnostic suspicion and management. Fetal MRI provides information on soft cerebral tissues (bulbs, frontal lobes, screened plaque, crista galli) and searches for other abnormalities or connections with intracranial contents [13]. It also helps to eliminate some differential diagnosis of facial malformations such as encephalocele, teratoma, retinoblastoma, hemangioma, and others. Nasal gliomas often appear iso-intense to gray matter on T1-weighted imaging, heterogeneous on T2-weighted imaging and with none to little enhancement with contrast. [8].
Postnatal imaging evaluation paradigm of nasal masses has evolved because of concerns regarding cancer risk in pediatric patients exposed to ionizing radiation, since there is a significantly increased lifetime radiation risk over adult CT due to increased patient dose per milliampere-second (mAs) and increased lifetime risk per unit dose [14]. High resolution multiplanar MRI is the preferred modality to evaluate these lesions, as it allows an optimal visualization of any intracranial extension, which will help guide the surgical approach [15]. Its limitations distinguishing true communication from the intracranial box from the fibrous stem that is sometimes seen with nasal gliomas don´t seem to be an issue, according to Adil et al. [16]. In their study of 21 patients evaluated with MRI alone, none had a subtotal resection or unexpected intracranial extension, which indicates that MRI alone is an adequate imaging study for nasal glioma without intracranial extension. However, some authors believe CT-Scan can add value since it gives a better evaluation of the bones and reveals changes to the floor of the anterior cranial fossa, such as the enlargement of the foramen cecum, the erosion of the crista galli and the deformations of the nasal pyramid [11, 17]. If needed, the Society for Pediatric Radiology has developed the Image Gently campaign advocating for radiation dose reduction strategies. Lower mAs can be used while preserving the diagnostic quality of CT exams in order to decrease patient radiation dose [16]. However, the usefulness of CT may be of variable utility in young children, whose bone architecture is not fully formed, leading to a false interpretation of bone dehiscence [8]. Some authors claim that both MRI and CT should be done to an increased diagnostic specificity [1]. In our case we preferred to surround ourselves with all the possible precautions in order to eliminate the maximum of differential diagnoses.
Nasal gliomas should be treated by complete excision to prevent recurrence, which is described in 4-10% of cases [10], but absent in our experience. Surgery is necessary to avoid aesthetic changes such as deformities of the nasal pyramid or hypertelorism. There is controversy about the ideal age for surgery [11, 18]. We agree that for newborns, surgery may be delayed if there is no evidence of a rapid increase in tumor volume or complications such as cerebrospinal fluid leakage (CSF) or meningitis, although it´s described in the literature cases of antenatal diagnosis that have been taken care of in the first days of life [8]. During this waiting period, the child should be under constant supervision until surgery is indicated on the basis of age and physical condition. The choice of the most appropriate surgical technique is also controversial [10, 11, 19]. Some authors argue that an exploratory craniotomy before excision of the mass is safer, as this would be a reversal of all communication with the central nervous system. However, since 15-20% of nasal gliomas have a dural link [1], we believe that if the preoperative evaluation does not suggest an encephalocele and if the anterior base of the skull appears normal, a nasal approach should first be performed. A useless craniotomy with its risks can thus be avoided, although the family should be informed that this procedure may be considered necessary during surgery. Different approaches can be followed for nasal gliomas, depending on location and preference of the surgeon. The intranasal approach has aesthetic benefits, but does not always allow complete excision. The use of endoscopes may be useful for the control of the ethmoid-screened plate [1, 8]. The external approach may start with a para-latero-nasal incision, an incision on the mass, or an external rhinoplasty or other first. As these lesions lack malignant potential, nasal gliomas are treated with surgery alone. Extranasal gliomas can be excised via lateral rhinotomy, external rhinoplasty, midline nasal incision, or a bicoronal incision. Endoscopy allows for improved visualization of the cribriform plate. Minimally invasive endoscopic surgery is emerging as the mainstay for treatment of intranasal glioma. In our experience, we used different approaches depending on the case, and especially for the reported case, the parents were psychologically prepared, and there has been a conjoined decision concerning the ideal age to intervene. Although the diagnosis was made intrauterine, the child was operated at the age of 26 months. This delay in the surgical management was due to several factors: the waiting for an acceptable weight for the intervention, the stability of the general state of the patient and the absence of any vital impact on the patient (mainly aesthetic in this case). It was an external rhinoplasty approach, with pyramidal reconstruction using a conchal cartilage graft. The postoperative course was simple and without nasal deformity (Figure 4). Pathologically, nasal gliomas are red masses, with a gray, yellow or purple surface, which may be covered by skin or mucosa [4, 11]. The consistency is rubbery or firm (Figure 5).
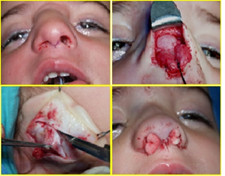
Figure 4: Surgical steps, external transcolumellar approach and reconstruction with conchal cartilage graft, at 26 months.
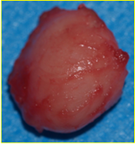
Figure 5: Surgical specimen (macroscopy).
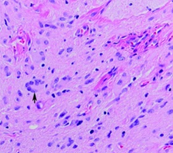
Figure 6: Histopathology demonstrating moderately cellular, well-differentiated neuroglial tissue. Large neurons, including binucleate and multinucleate forms, are shown (arrow).
Histologically, these lesions are composed of islets or large clumps of glial tissue in a fibrous tissue fundus. Glia is represented by astrocytes with variable nucleus. Oligodendrocytes rarely appear. Fibrous tissue may be predominant in some cases, especially in recurrent gliomas in older children, and should not be confused with fibroids. The existence of focal calcifications are not frequent but can be observed. The anatomopathological result of our patient enters this frequent group of glial cells, with presence of active astrocytes. The evolution of this nasal glial pathology is generally favorable, especially after complete excision. However postoperative follow-up should be continued, if possible, for several years. Histopathological diagnosis is made by observation of mature glial cells, interwoven with fibrovascular connective tissue. Occasionally focal areas of calcification are also seen. The presence of leptomeninges, ependyma, or choroid plexus would support a diagnosis of encephalocele rather than a diagnosis of nasal glioma.
6. Conclusion
Nasal gliomas are rare pathologies and a source of great controversy. Their increasingly developed prenatal diagnosis could improve the quality of the management of this pathology, especially in the psychological preparation of the parents who more easily accept the management process. The technique of open rhinoplasty is the least traumatic and gives better results in the short and long term. The reconstruction of the nasal pyramid can be done with cartilage of the conch.
Conflicting Interests
The authors declare that there is no conflict of interest.
References
- Ma KH, Cheung KL. Nasal Glioma. Hong Kong Med J 6 (2006): 477-479.
- Verney Y, Zanolla G, Teixeira R, et al. Midline nasal mass in infancy: a nasal glioma case report. Eur J Pediatr Surg 11 (2001): 324-327.
- Uzunlar AK, Osma U, Yilmaz F, et al. Nasal glioma: Report of two Cases. Turk J Med Sci 31 (2001): 87-90.
- Haafiz AB, Sharma R, Faillace WJ. Congenital midline nasofrontal mass. Two case reports with a clinical review. Clin Pediatr (Phila) 9 (1995): 382-386.
- Tonni G, Lituania M, Bonasoni MP, et al. Prenatal ultrasound and histological diagnosis of fetal nasal glioma (heterotopic central nervous system tissue): report of a new case and review of the literature. Arch Gynecol Obstet 3 (2011): 55-59.
- Clarós P, Bandos R, Clarós A Jr, et al. Nasal gliomas: main features, management and report of five cases. Int J Pediatr Otorhinolaryngol 46 (1998): 15-20.
- Clarós P, Wienberg P, Clarós A, et al. Encefalocele intranasal. Anales ORL. Iber-Amer. XXII 6 (1995): 591-600.
- Ajose-Popoola O, Lin HW, Silvera VM, et al. Nasal Glioma: Prenatal Diagnosis and Multidisciplinary Surgical Approach. Skull Base Rep 1 (2011): 83-88.
- Barkovich AJ, Vandermarck P, Edwards MS, et al. Congenital nasal masses: CT and MR imaging features in 16 cases. Am J Neuroradiol 1 (1991): 105-116.
- Michaels L. Ear, nose and throat, histopathology. Springer (1987): 189-190.
- Puppala B, Mangurten HH, McFadden J, et al. Nasal glioma. Presenting as neonatal respiratory distress. Definition of the tumor mass by MRI. Clin Pediatr (PH) 1 (1990): 49-52.
- De Basio P, Scarso E, Prefumo F, et al. Prenatal diagnosis of a nasal glioma in the mid trimester. Ultrasound in Obstetrics and Gynaecology 5 (2006): 571-573.
- Grzegorczyk V, Brasseur-Daudruy M, Labadie G, et al. Prenatal diagnosis of a nasal glioma. Pediatr Radiol 40 (2010): 1706-1709.
- Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology 232 (2004): 735-738.
- Hedlung G. Congenital frontonasal masses: developmental anatomy, malformations, and MR imaging. Pediatr Radiol 36 (2006): 647-662.
- Adil E, Robson C, Perez-Atayde A, et al. Congenital nasal neuroglial heterotopia and encephaloceles: An update on current evaluation and management. The Laryngoscope 126 (2016): 2161-2167.
- Swift AC, Singh SD. The presentation and management of the nasal glioma. Int J Pediatr Otorhinolaryngol 10 (1985): 253-261.
- Reilly JR, Koopman CF, Cotton R. Nasal mass in a pediatric patient. Head Neck 5 (1992): 415-418.
- Hughes GB, Sharpino G, Hunt W, et al. Management of congenital midline nasal mass. A review, Head Neck Surg 2 (1980): 222-233.
