Nasal Carriage Rate of Staphylococcus Aureus and Risk Factors among Healthcare Workers and Attendants of Neonatal Intensive Care Unit in a Tertiary Care Centre in Bangladesh
Article Information
Sadeka Choudhury Moni1, Md. Nazmus Sihan2, Debashish Saha3, Shahana Akter4, M. A. Mannan5*, Mohammod Shahidullah6
1Associate Professor, Department of Neonatology, BSMMU, Dhaka, Bangladesh
2Resident Physician, Department of Paediatrics, Cumilla Medical College Hospital, Cumilla, Bangladesh
3Consultant, Department of Neonatology, BSMMU, Dhaka, Bangladesh
4Medical Officer, Department of Neonatology, BSMMU, Dhaka, Bangladesh
5Professor, Department of Neonatology, BSMMU, Dhaka, Bangladesh
6Professor, Department of Neonatology, BSMMU, Dhaka, Bangladesh
*Corresponding Author: Dr. Sadeka Choudhury Moni, Associate Professor, Department of Neonatology, Room No: 214, Block-C, Bangabandhu Sheikh Mujib Medical University, Shahbag, Dhaka, Bangladesh
Received: 11 March 2022; Accepted: 19 March 2022; Published: 24 March 2022
Citation:
Sadeka Choudhury Moni, Md. Nazmus Sihan, Debashish Saha, Shahana Akter, M. A. Mannan, Mohammod Shahidullah. Nasal Carriage Rate of Staphylococcus Aureus and Risk Factors among Healthcare Workers and Attendants of Neonatal Intensive Care Unit in a Tertiary Care Centre in Bangladesh. Journal of Pediatrics, Perinatology and Child Health 6 (2022): 188-199.
View / Download Pdf Share at FacebookAbstract
Introduction: Newborn acquires Staphylococcus aureus including methicillin resistant Staphylococcus aureus (MRSA) from adult sources. There are limited data on Staphylococcus aureus carriage rate among health care workers (HCWs) and other adult contacts in Bangladesh.
Objective: The objective of the study was to determine the frequency of Staphylococcus aureus nasal colonization among HCWs and mothers/caregivers attending neonatal intensive care unit (NICU) of Bangabandhu Sheikh Mujib Medical University (BSMMU), to evaluate antibiotic sensitivity pattern of identified organism and to study the risk factors of carriage among the study groups.
Methods: This is a cross sectional observational study conducted in NICU, BSMMU, Dhaka, Bangladesh from July 2017 to December 2018 among health care workers, other staffs and mothers/family members with access to NICU. The inclusion criteria were all doctors, nurses and other staffs of NICU and mothers/family members caring the admitted newborn and consented to participate in the study. Nasal swab was collected aseptically using sterile cotton swab by the trained research assistant from both the nostrils following standard procedure and was sent immediately to microbiology lab for isolation and identification of Staphylococcus strain following standard procedure and drug susceptibility testing. IBM SPSS software package version 20 was used to analyse all collected data.
Results: Nasal swab were tested from 250 participants; 49.6% were mothers/family members and 50.4% were HCWs of different categories. Staphylococcus aureus was isolated in 18.4% of the participants. Among HCWs Staphylococcus carriage rate was 20.6% and among mother/family members carriage rate was 16.1%. There was no MRSA isolate. The isolated organisms are least sensitive to commonly used oral antibiotics, the present study did not find any risk factors for colon
Keywords
Nasal Carriage, Staphylococcus Aureus, MRSA, Health Care Workers (HCWs), Mother/Caregiver, Bangladesh
Article Details
Introduction
Staphylococcus aureus, a commensal of human skin and mucosae is the second most common pathogen responsible for health-care associated infections (HAIs) in newborn that accounts for high morbidity, mortality, and healthcare-associated costs [1]. Methi-cillin resistant Staphylococcus aureus (MRSA) has emerged as a virulent pathogen causing significant increase in late-onset infections in premature and critically ill infants in the U.S. neonatal intensive care units (NICUs) [2]. Vancomycin Resistant Staphylo-coccus Aureus (VRSA) is also an emerging problem in health care associated infections in hospital. Newborn acquires Staphylococcus aureus including MRSA from adult sources because many healthy people may carry it as a part of the normal micro flora associated with the nose, throat, perineum or skin [3]. The most frequent carriage site is the vestibulum nasi (or anterior nares), which serves as reservoir for the spread of the pathogen [4, 5]. In about 20% to 80% of the human population, anterior nares is the site of S aureus colonization [6]. Various proteins and many cell surface components help this bacteria to establish solid interactions with nasal epithelial cells [7] and thereby transforms into persistent carriage state. Acquisition of asymptomatic nasal carriage of Staph aureus and subsequent transformation into persistent nasal carrier potentially disperse the organisms into the environment around them. Recent acquisition of such carriage leads to clinical S. aureus infection, both for the carrier and for other individuals [8, 9]. Persistent carriers are considered as key source of infection in intensive care unit (ICU) patients [10].
From several studies it is evident that elimination of carriage in the anterior nares, incidence of S. aureus infections get reduced [11]. Nasal carriage of MRSA is reported to vary between 6.3 and 17.8% in the general population and between 18.2 and 43.8% in health care workers [12, 13]. Certain host factors, such as the obesity , HIV infection, diabetes with need for dialysis, chronic diseases like granuloma-tosiss with polyangiitis (formerly known as Wege-ner’s granulomatosis), rheumatoid arthritis, skin and soft tissue infections atopic dermatitis, and recurrent furunculosis have been related with an increased carriage rate [14]. Transmissions of Staphylococcus aureus including MRSA from health care workers to newborn admitted in NICU results in nosocomial sepsis [15]. Screening and eradication of MRSA from colonized healthcare workers have been recognized and recommended as an important part of a compre-hensive infection control policy for this organism [16]. Therefore, transmission from person to person and from health care worker to newborn patients is a health care concern of present time. There are limited data regarding Staphylococcal infection outbreak in neonatal care unit and Staphylococcus aureus carriage rate among health workers in developing countries. Staphylococcal particularly MRSA infecti-ons are associated with increased mortality and costs for the healthcare systems in developed countries [17]. For developing countries its impact seems to be worse as outbreak investigation capability by bacterial identification and antimicrobial suscep-tibility testing is limited and there is financial constraint to treat severe health care associated MRSA infections [18]. For Bangladesh neonatal sepsis shares a major cause of neonatal death [19]. Health care providers and mothers/caregivers having contact with newborns harbouring Staphylococcus aureus could be an important source of such infection. Most of the NICUs in Bangladesh allows visit by mother/caregiver to NICU as per policy. There is no adequate information on routine screen-ing of Staph aureus and MRSA, VRSA nasal colonization among health care providers working in NICU. Therefore the prevalence of Staphylococcus aureus and MRSA, VRSA nasal colonization in health care and mother/caregiver provider needs to be determined before instituting measures to prevent the transmission of such pathogens. Isolation & identify-cation Staphylococcus strain and determining the sensitivity pattern of the isolated organisms will also allow use of rational antibiotic in case of suspected infection and thus contribute to reduce neonatal mortality and morbidity.
The objective of the study was to determine the frequency of Staphylococcus aureus including MRSA & VRSA nasal colonization among health care providers and mothers/caregivers attending NICU of Bangabandhu Sheikh Mujib Medical University (BSMMU), to evaluate antibiotic sensitivity pattern of identified organism and to study the risk factors of Staphylococcus aureus carriage among the study groups.
2. Methodology
This is a cross sectional observational study carried out in the NICU, BSMMU, Dhaka, Bangladesh from July 2017 to December 2018 among health care workers, other staffs and mothers/family members having access to NICU during the study period. The inclusion criteria were all doctors, nurses and other staffs of NICU and mothers/family members caring the admitted newborns and consented to participate in the study. The exclusion criteria were those not giving consent and suffering from respiratory tract infection at the time of sample collection. The study protocol was approved by institutional review board (IRB), BSMMU. Nasal swab was collected by the trained research assistant from both the nostrils. Universal aseptic procedure was followed while collecting the sample. Two sterile cotton swab sticks one for each nostril were used for sample collection. Before starting collecting sample patients identify-cation no and the side of the nostril from which sample is collected is marked with a permanent marker on the surface of the test tube containing the swab stick. Each swab stick is moistened with sterile saline and then inserted to a depth of approximately one cm in each nostril; swab stick was then rotated five times over the inner wall of the ala and nasal septum. After collection the swab stick is returned to the container test tube sealed with cotton wool and sent immediately to the microbiology laboratory for inoculation in blood & chocolate agar media.
The isolated microbial colonies was identified in the microbiology lab and drug sensitivity was tested using standard technique. Methicillin resistant (MRSA) and vancomycin resistant (VRSA) strains was identified by oxacillin/cefoxitin & vancomycin disk diffusion methods respectively. For collection of other relevant data, a structured questionnaire was constructed and informations were gathered by interviewing the participants. The primary outcome was frequency of carrier of Staphylococcus aureus, MRSA, VRSA and other organisms among health care providers and mothers/family members. The participants were considered positive for nasophar-yngeal carriage of Staphylococcus aureus if sample from one or both of the nostrils yielded growth of Staphylococcus aureus. Baseline characters of health care workers and mothers/family members of NICU, risk factors of Staphylococcus aureus, MRSA and VRSA carriage among the study groups and antibiotic sensitivity pattern in study samples were collected.
2.1 Data editing, processing, analysis and presentation
Data were analyzed using IBM SPSS software package version 20.0 (IBM Corp, Armonk, New York, USA). Prevalene/carriage rate was calculated in percentage. Categorical varibles were expressed as proportion and quantitative variables as mean ± SD. Comparisons between groups for categorical varia-bles were performed using the χ2-test. Student’s t-test was used to compare two groups for normally distributed quantitative data. The significance of the obtained results was judged at the 5% level.
3. Results
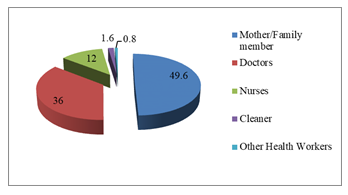
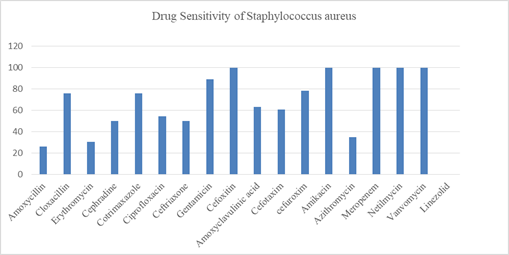
There were 250 participants in this study (Figure-1); majority (49.6%) were mothers/family members followed by doctors (36%), nurses (12%), cleaners (1.6) and others (0.8%). Female outnumbered the male participants (78.8% Vs 21.2%). Mean age (±SD) was 31.86 years ±9.001 with minimum age 20 years and maximum 62 years; maximum (51.6%) belonged to 20-30 years age category. Mean (±SD) body mass index was 23.73 ± 3.12 with prevalence of obesity among 14 participants (5.6%). History of smoking/tobacco consumption was observed among 21(8.4%) participants (Table-1). Staphylococcus aureus was isolated from one or both anterior nares among 46 (18.4%) out of 250 participants (Table-2). Among 126 HCWs Staphylococcus isolated in 26 (20.6%) and among 124 mother/family members carriage rate was 16.1% (20 out of 124). Twenty doctors (22.2%) out of 90, 4 nurses (13.3%) out of 30, 1(25%) cleaner out of 4, 1(50%) other category staff out of 2 and 20 mothers/family members out of 124 were found positive for nasal carriage of Staphy-lococcus aureus and all were Methicillin Sensitive Staphylococcus aureus (MRSS). No participants in the study group carried MRSA or VRSA strain. Antibiotic sensitivity pattern (Figure-2) showed that 100% of isolated Staphylococcus aureus strains were sensitive to meropenem, amikacin, netilmycin, vanc-omycin and linezolid. About 80-90% of the isolated strains were sensitive to gentamicin, cefuroxime, cloxacillin and cotrimoxazole. Sensitivity to ceftria-xone, cefotaxim, ciprofloxacin and cefradine was demonstrated in around 50 to 60% of isolates. Amoxycillin, erythromycin and azithromycin were least sensitive to the isolates. Only one participant of mother/family member category had isolate of Acinetobacter species which was multidrug resistant being sensitive only to colistin. The frequency of risk factors associated with nasal carriage of Staphylo-coccus aureus were studied between carriers and non-carriers. Medical disorder was considered as risk factor if hypertension, diabetes mellitus chronic kidney disease, bronchial asthma/COPD and other category was present in isolation or in combination. Oral, inhaled or topical nasal steroid was considered as immunosuppressive medication. Hand washing with soap water at least 3 times daily was taken as hand washing habit. Bivariate analysis did not find any statistically significant association between risk factors and nasal carriage status among the participants.
|
Variables |
Values |
|
Male N (%) |
53 (21.2) |
|
Female N (%) |
197 (78.8) |
|
Age (mean±SD) (min-max) |
31.86 ± 9.001 (20-62) |
|
Age (median IQR) |
30 |
|
Age (mode) |
28 |
|
Age category |
|
|
20-30 N (%) |
129 (51.6) |
|
31-40 N (%) |
84 (33.6) |
|
41-50 N (%) |
21 (8.4) |
|
≥51 N (%) |
16 (6.4) |
|
BMI(mean ± SD) |
23.73 ± 3.12 |
|
Obesity N (%) |
14 (5.6%) |
|
Smoking/tobacco consumption (n %) |
21 (8.4%) |
Table 1: Baseline characteristics of the participants (N=250).
|
Participants Category |
Staphylococcus aureus (MSSA) Positive N (%) |
MRSA Positive N (%) |
VRSA Positive N (%) |
|
Doctors (N=90) |
20 (22.2%) |
0 |
0 |
|
Nurses (N=30) |
04 (13.3%) |
0 |
0 |
|
Cleaners (N=4) |
01 (25%) |
0 |
0 |
|
Others (N=2) |
01 (50%) |
0 |
0 |
|
Caregivers (N=124) |
20 (16.1%) |
0 |
0 |
|
Total (N=250) |
46 (18.4%) |
0 |
0 |
Table 2: Staphylococcus aureus status among the participants (N=250).
|
Factors |
Staphylococcus aureus positive N=46 |
Staphylococcus aureus negative N=204 |
p-value |
|
Male female |
12 (24.4) 34 (75.6) |
43 (20.3) 161 (79.7) |
0.46 |
|
Age category 20-30 31-40 41-50 >50 |
20 (44.4) 18 (40.0) 5 (11.1) 2 (4.4) |
110 (53.2) 65 (32) 16 (7.9) 149 (6.9) |
0.624 |
|
Obesity Yes No |
2 (4.4) 44 (95.6) |
11 (5.4) 193 (94.6) |
0.79 |
|
Categories of participant Doctors Nurse Cleaner Mother/Other caregiver Others |
20 (44.4) 04 (8.9) 01 (2.2) 19 (42.2) 01 (2.2) |
70 (34) 26 (12.8) 03 (1.5) 105 (51.2) 01 (0.5) |
0.42 |
|
Medical Illness Yes No |
21 (44.4) 25 (55.6) |
79 (38.4) 125 (61.6) |
0.547 |
|
Intake of Steroid/ Immunosuppressive drugs Yes No |
01 (2.2) 45 (97.8) |
10 (4.9) 194 (95.1) |
0.384 |
|
H/O taking antibiotics in preceding 4 weeks Yes No |
11 (24.4) 35 (75.6) |
64 (31) 140 (69) |
0.383 |
|
Hospital admission in preceding 1 month Yes No |
9 (20) 37 (80) |
66 (32.5) 138 (67.5) |
0.099 |
|
Daily Bathing Habit Yes No |
42 (91.1) 04 (8.9) |
170 (84.2) 34 (15.8) |
0.237 |
|
Hand washing Habit Yes No |
38 (84.4) 08 (15.6) |
158 (77.3) 46 (22.7) |
0.23 |
|
Practice of ablution Yes No |
29 (62.2) 17 (37.8) |
107 (52.2) 97 (47.8) |
0.332 |
Table 3: Risk factors associated with nasopharyngeal carriage of Staphylococcus aureus (N=250).
4. Discussion
A total of 46 S. aureus isolates were recovered from a total of 250 participants (18.4%, 46/250), with no isolates showing methicillin resistance. Nasal carriage rate detection from this study is 18.4% which include health care workers (HCWs) and mothers/attendants. Among the 126 health care workers (HCWs) coloninization rate was 20.63% and for attendants the carrier rate was 16.12%. This carrier rate among HCWS is in line with other internationally reported findings (19.80–48 %) [20, 21]. However some reports from neighboring countries showed a lower carrier rate 15.7%-12.67% [22, 23]. Staphylococcus carrier rate was low among the attendants in comparison to other studies where rate ranged 23.4% [24] and 25.3% [25].
In this study the study population included only the female attendant which might explain the low carrier rate in this study. This study did not find any carrier for MRSA or VRSA strain among HAWs and attendants. MRSA carrier rate among health care workers (HCWs) shows wide variability among different published reports ranging 5.8 to 17.8% [26, 27]. It was also very low (0.3%) among community residents [24, 25]. Varying rates for MRSA carriage by HCWs are also reported in Pakistan 1.49% [28] and other neighbouring country like India (39.7%) [28, 29]. The variation of this estimated prevalence may be due to difference in sampling, choice of anatomic site for culture to assess colonization, the study setting, and the methodology used for MRSA culture processing as well as local infection control standards and the local prevalence of MRSA. It should also be noted that body sites other than nasopharynx notably the oropharynx and even the inguinal area are considered as primary sites with higher prevalence of colonization [30]. Culture survey of the nares alone therefore greatly under-estimate the prevalence of S. aureus colonization. Indeed, many individuals with colonization in the throat or on the skin over the inguinal area did not show any colonization in the nasopharynx [31]. Some studies have demonstrated that S. aureus, and specifically the methicillin-resistant S. aureus (MRSA) genetic background USA300 can be found elsewhere on the body (reviewed in reference [30].
In another study it was found that asymptomatic individuals are likely to carry methicillin susceptible staphylococcus aureus (MSSA) strain than skin and soft tissue infected (SSTI) patients (85% vs 38%); MRSA strain carrier was higher among SSTI (62% vs 15%). But samples taken at the same time from inguinal region showed higher proportions of isolate to be MRSA strain; 63% in infected patients and 26% in non-infected patients [32]. In this study, among the health care workers S.aureus carriage rate was highest among doctors (22.2%). This finding was similar with some other published study [22], where the carriage rate was comparable 20.8 % in Nepal. Rate of carriage is lower than doctors among nurses (13.3%). But Khanal R et al. and Rajaduraipandi K et al. reported higher carriage rate for S. aureus particularly MRSA among nurses than doctors [22, 29]. Number of other categories HCWs were less and estimated carriage rate is comparable with other study [29]. Highest carriage rate (51.6%) was observed in 20-30 years category. This finding is self-explanatory if the mean age of the participants is considered. Due to inclusion of only female caregiver and all the nursing staffs being female, female participants outnumbered the male in this study.
This study did not find any association of staphylococcus carrier with different risk factors. But factors having significant association with Staphyl-ococcus carriage were male sex, nursing occupation, 4-6 years of working duration in some other study [33]. As most of the participants in this study were female, the highest estimated carriage rate was more among female although gender is not an important risk factor in this study. In another study also sex did not affect the carriage rate [34]. Certain hast factors such as the underlying conditions or diseases are associated with nasal colonization of S.aureus; obesity, HIV infection, diabetic patient on dialysis, skin soft tissue infection, atopic dermatitis, recurrent furunculosis, chronic diseases like granulomatosis with polyangitis, rheumatoid arthritis [35]. For healthy subject smoking was not found consistently as an important risk factors associated with S.aureus carriage [36]. The susceptibility testing of S aureus isolates revealed 100% sensitivity for Meropenem, Netilmycin, Vancomycin and Linezolid, moderate sensitivity for Gentamicin (89.1%), Cloxacillin (76%), Cotrimaxazole (76%), Cefuroxim (78.3%), Ciprofloxacin (54.3%), Cefotaxim (60.9%), Cefra-dine (50%), Ceftriaxone (50%). Lowest sensitivity was observed for Amoxycillin (26%), Erythromycin (30.4%) and Azythromycin (34.8%). Therefore, from this study findings oral medication cloxacillin and cotrimaxazole, ciprofloxacin can be recommended for decolonization of S.aureus carriers. For empiric therapy of possible staphylococcal disease Netilm-ycin, Gentamicin cloxacilllin can be recommended as first line therapy.
4.1 Limitation
In this study carriers were not classified as persistent or intermittent carriers. One important limitation of present study is that mupirocin, the agent recomm-ended for eradication of staphylococcal nasal carriage, was not tested.
5. Conclusion
The Staphylococcus aureus carrier rate among health care workers and mothers/attendants of NICU, BSMMU is 18.4%, Among HCWs Staphylococcus carriage rate was 20.6% and among mother/family members carriage rate was 16.1%. None of the study sample harbored MRSA or VRSA strain. The isolated organisms are least sensitive to commonly used oral antibiotics, the present study did not find any risk factors for colonization of Staphylococcus aureus.
Acknowledgement
Sincere acknowledgement to University Grant Commission (UGC), Bangladesh for funding this research and Department of microbiology, BSMMU for doing culture sensitivity of nasal swab.
Conflict of Interest
The authors have no conflicts of interest associated with the material presented in this paper.
References
- Hocevar SN, Edwards JR, Horan TC, et al. Device-associated infections among neonatal intensive care unit patients: incidence and associated pathogens reported to the National Healthcare Safety Network, 2006-2008. Infect Control Hosp Epidemiol 33 (2012): 1200-1206.
- Lessa FC, Edwards JR, Fridkin SK, et al. Trends in incidence of late-onset methicillin-resistant Staphylococcus aureus infection in neonatal intensive care units: data from the National Nosocomial Infections Surveillance System, 1995-2004. Pediatr Infect Dis J 28 (2009): 577–581.
- Brooks GF, Butel JC, Morse SA. Normal microbial flora of the human body. In: Brooks GF, Butel JC, Morse SA, eds. Jawetz, Melnick and Adelberg's Medical Microbio-logy, 23th edition. McGraw-Hill; USA (200l): 196-200.
- Williams R.E. Healthy carriage of Staphylo-coccus aureus: its prevalence and importance. Bacteriol. Rev 27 (1963): 56-71.
- Sivaraman K, Venkataraman N, Cole A M. Staphylococcus aureus nasal carriage and its contributing factors. Future Microbiol 4 (2009): 999-1008.
- Brown A F, Leech J M, Rogers T R, et al. Staphylococcus aureus colonization: modul-ation of host immune response and impact on human vaccine design. Front. Immunol 4 (2014): 507.
- Mulcahy M E, Geoghegan J A, Monk I R, et al. Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin. PLoS Pathog 8 (2012): e1003092.
- Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol Rev 10 (1997): 505-520.
- Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis 5 (2005): 751-762.
- Nouwen J, Schouten J, Schneebergen P, et al. Staphylococcus aureus carriage patterns and the risk of infections associated with continuous peritoneal dialysis. J Clin Microbiol 44 (2006): 2233-2236.
- Boelaert JR, Van Landuyt HW, Godard CA, et al. Nasal mupirocin ointment decreases the incidence of Staphylococcus aureus bacter-aemias in haemodialysis patients. Nephrol Dial Transplant 8 (1993): 235-239.
- Pathak A, Marothi Y, Iyer RV, et al. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus in healthy preschool children in Ujjain, India. BMC Pediatr 10 (2010): 100.
- Treesirichod A, Hantagool S, Prommalikit O. Nasal carriage and antimicrobial suscep-tibility of Staphylococcus aureus among medical students at the HRH Princess Maha Chakri Sirindhorn Medical Center, Thailand: a cross sectional study J Infect Public Health 6 (2013): 196-201.
- Adèle Sakr, Fabienne Brégeon, Jean-Louis Mège, Jean-Marc Rolain, Olivier Blin. Staphylococcus Nasal Colonization: An update on mechanisms, Epidemiology, Risk factors and Subsequent infections. Frontiers in microbiology 9 (2018): 2419.1-15
- Isaacs D, Fraser S, Hogg G, et al. Staphylococcus aureus infections in Austra-lasian neonatal nurseries. Arch Dis Child Fetal Neonatal Ed 89 (2004): 331-335.
- Fadeyi A, Bolaji BO, Oyedepo OO. Methicillin resistant Staphylococcus aureus, carriage among health care workers of critical care units in a Nigerian hospital.American Journal of Infectious Diseases 6 (2010): 18-23.
- David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23 (2010): 616-687.
- Popovich KJ, Weinstein RA, Bala Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains?. Clin Infect Dis 46 (2008): 787-794.
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385 (2015): 430-440.
- Akoua Koffi C, Dje K, Toure R. Nasal carriage of methicillin resistant Staphylo-coccus aureus among health care personnel in Abidjan (Coted’Ivoire). Dakar Med 49 (2004): 70-74.
- Citak S, Bayazit FN, Aksoy F. Nasal carriage and methicillin resistance of Staphylococcus aureus in patients and hospital staff in a tertiary referral center setting. Afr J Microbiol Res 5 (2011): 1615-1618.
- Khanal R, Sah P, Lamichhane P, et al. Nasal carriage of methicillin resistant Staphylo-coccus aureus among health care workers at a tertiary care hospital in Western Nepal. Antimicrobial Resistance and Infection Control 4 (2015): 2-5.
- Khalili MB, Sharifi-Yazdi MK, Dargahi H, et al. Nasal colonization rate of Staphylococcus aureus strains among health care service employees of teaching university hospitals in Yazd. Acta Med Iran 47 (2009): 315-317.
- Choi CS,Yin CS, Bakar AA, et al. Nasal carriage of Staphylococcus aureus among healthy adults. Journal of Microbiology, Immunology, and Infection 39 (2006): 458-464.
- Chen B, Dai X, He B, et al. Differences in Staphylococcus aureus nasal carriage and molecular characteristics among community residents and healthcare workers at Sun Yat-Sen University, Guangzhou, Southern China. BMC Infectious Diseases 303 (2015).
- Akoua Koffi C, Dje K, Toure R. Nasal carriage of methicillin resistant Staphylo-coccus aureus among health care personnel in Abidjan (Cote d’Ivoire). Dakar Med 49 (2004): 70-74.
- Cesur S, Cokca F. Nasal carriage of methicillin-resistant Staphylococcus aureus among hospital staff and outpatients. Infect Control Hosp Epidemiol 25 (2004): 169-171.
- S taphylococcal Nasal Carriage of Health Care Workers. Journal of the College of Physicians and Surgeons Pakistan 20 (2010): 439-443 439.
- Rajaduraipandi K, Mani KR, Panneerselvam K, et al. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus: a multicenter study. Indian J Med Microbiol 24 (2006): 34-38.
- McKinnell JA, Huang SS, Eells SJ, et al. Quantifyinthe impact of extranasal testing of body sites for methicillin-resistant Staphyl-ococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 34 (2013): 161-170.
- Miller LG, Eells SJ, Taylor AR, et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology . Clin Infect Dis 54 (2012): 1523-1535.
- Kumar N, David MZ, Boyle-Vavra S, et al. High Staphylococcus aureus Colonization Prevalence among Patients with Skin and Soft Tissue Infections and Controls in an Urban Emergency Department. Journal of Clinical Microbiology 15 (2015): 810-815.
- Al-Humaidan OS, El-Kersh TA, Al-Akeel RA.Risk factors of nasal carriage of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus among health care staff in a teaching hospital in central Saudi Arabia. Saudi Med J 36 (2015): 1084-1090.
- Liu C M, Price L B, Hungate B A, et al. Staphylococcus aureus and the ecology of the nasal microbiome. Sci. Adv 1 (2015): e1400216.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Frontiers in Microbiology 9 (2018): 1-15.
- Cole A L, Schmidt-Owens M, Beavis A C, et al. Cessation from smoking improves innate host defense and clearance of experimentally inoculated nasal S. aureus. Infect. Immun 86 (2018): e912-e917.