Nanofat, Microfat, Platelet Rich Plasma (PRP), Microneedling and Fractional CO2 Laser: A Regenerative Multimodality Approach and Protocol in Scar Management
Article Information
Aeshah Mandili1, Hazim Hamed S Alsadi2, Sherif Khamis3,4, Ziyad Alharbi3,4*
1Department of Surgery, King Faisal Specialist Hospital and Research Centre, Jeddah, Saudi Arabia
2College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
3Plastic Surgery & Burn Unit, Dr Soliman Fakeeh Hospital, Jeddah, Saudi Arabia
4Clinical Sciences Department, Fakeeh College for Medical Sciences, Jeddah, Saudi Arabia
*Corresponding Author: Ziyad Alharbi, Clinical Sciences Department, Fakeeh College for Medical Sciences, Jeddah, Saudi Arabia.
Received: 30 May 2022; Accepted: 17 June 2022; Published: 08 July 2022
Citation: Aeshah Mandili, Hazim Hamed S Alsadi, Sherif Khamis, Ziyad Alharbi. Nanofat, Microfat, Platelet Rich Plasma (PRP), Microneedling and Fractional CO2 Laser: A Regenerative Multimodality Approach and Protocol in Scar Management. Archives of Clinical and Medical Case Reports 6 (2022): 511-516
View / Download Pdf Share at FacebookAbstract
Background: Scars are the natural end-result of healing process following skin injury that extends to the reticular dermis. Recently, a worldwide effort was undertaken to investigate the regenerative potential of adipose tissue grafting, platelet rich plasma, and microneedling technique. Only few studies have shown its effect on scars. It is hypothesized that the Adipose-Derived Stem Cells (ADSCs) and numerous growth factors contained in the lipoaspirate as well as in the Platelet Rich Plasma (PRP) that contribute to the skin and scar remodeling. The purpose of this study was to evaluate the clinical regenerative effect of using combined well-arranged different collaborative modalities including: autologous microfat, nanofat graft, platelet rich plasma and microneedling followed by fractional CO2 laser on scars.
Methods: This is retrospective analysis that was conducted in KAMC, Saudi Arabia by the same surgeon. Overall, eight patients were treated using autologous microfat, nanofat graft, then three sittings of PRP with microneedling technique treating the scars, followed by session of fractional CO2 Laser. Evaluation was based on physician clinical assessment reflecting significant improvement exactly three months post-treatment.
Results: All eight patients had significant improvement of scar appearance with good satisfactory outcome regarding the quality of scars.
Conclusion: This case series has demonstrated the potential safety and efficacy of the combination therapy of autologous microfat, nanofat graft, platelet rich plasma, microneedling and fractional laser for the treatment of scars.
Keywords
Scars; Microfat; Nanofat; Platelet Rich Plasma; Microneedling; Hyperpigmentation; Adipose-Derived Mesenchymal Stem Cells (AD-MSCs)
1. Introduction
Scars are normal end result after skin injuries that extend to the reticular dermis. It is a natural process of healing after skin damage where fibrous tissue replaces normal skin after injury [1]. The resultant change in appearance can negatively affect body image and self-confidence. Those with scars are more prone to the development of depression, anxiety, feelings of shame, and aggression [2] As well as its impact in reducing social interactions. Scars can result from many causes such as trauma, burns, surgeries and infections [3-4]. Many treatments have been used before for treating and decreasing the risk of scar formation. Injection or topical application of corticosteroids onto pathologic scars is considered an effective treatment method for keloids and hypertrophic scars [5]. Beside atrophy or hypertrophy of scars, pigmentation changes are common complications after specific treatments, like hypopigmentation in scars can also occur in cryotherapy, and radiotherapy scar treatment that may involve multiple sessions and can entail unwanted cellular apoptosis and necrosis. Scars are also often treated with less invasive silicone dressings and pressure garments. [6]. Laser therapy of hypertrophic scars may improve their appearance yet nevertheless; laser technology is limited by high recurrence rates and skin discoloration [7]. Finally, surgery may be an option to correct overly conspicuous scars and includes scar revision, dermabrasion, or skin grafting (8]. Recently, a worldwide effort was undertaken to investigate the regenerative potential of adipose tissue grafting. Adipose tissue is rich of mesenchymal stem cells; Adipose-Derived Mesenchymal Stem Cells (AD-MSCs), microvascular fragments, growth factors and cytokines, which are critically important for revascularization and the enhancement of angiogenesis and decrease fibrosis [9,10]. A study done in 2014 revealed that in post liposuction, freshly isolated uncultured AD-MSCs has the ability to be involved in the cellular enrichment of making collagen and elastin matrices [11]. Adipose grafting techniques has undergone significant changes over time to improve the quality of the lipoaspirate and the survival of the fat graft after implantation [12]. As a result, the original dermal-fat grafts and microlipoinjection techniques [13, 14]. Have been advanced by current techniques of microfat and nanofat, (15,16) which are showing promising results. In several studies, Micro-Autologous Fat Transplantation (MAFT) has demonstrated its feasibility in facial rejuvenation, volumizing and contouring [17]. Tonnard, et al, described the method of Nanofat, which is the material obtained after harvesting fat via liposuction which go through mechanical emulsification through small-bore luer lock connectors, followed by cellular filtration through a 500-mm filter. This quick process allows isolation of the Stromal Vascular Fraction (SVF) as well as some nonviable adipocyte cell components. Tonnard, et al, suggested that Nanofat graft could be cheaper and simpler alternative to the traditional transplantation of AD-MSCs for epithelial tissue regeneration and skin rejuvenation [17]. Jordan Rihani has emphasized injection of nanofat combined with microfat seems to offer improvement in skin texture as well as structural volumization [21]. A recent comparative study evaluated the efficacy of nanofat and Platelet-Rich Plasma (PRP) infiltration alone and combined with fractional CO2 laser resurfacing to improve atrophic scars of the face from March 2014 to June 2015, 30 patients with atrophic acne scars on the cheeks were selected for this study. In conclusion Subcutaneous infiltration with nanofat and PRP seems to be effective to improve atrophic scars, either alone or combined with fractional CO2 laser resurfacing [18]. A split face comparative study of microneedling with PRP Versus microneedling with Vitamin C done on Thirty patients with post acne atrophic facial scars offered four sittings of microneedling with PRP on one side and microneedling with vitamin C on other side of the face at an interval of 1 month [19]. Out of 30 patients, 23 achieved reduction in scarring by one or two grades. Overall results were better with microneedling and PRP than microneedling with vitamin C [19]. Another split-face comparative study microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne. The study included 35 patients with mild to severe post acne atrophic scar. All the patients received four sequential treatments of skin microneedling alone on the right side of the face and skin microneedling followed by topical application of PRP on the left side of the face with an interval of 3 weeks. There was a significant improvement in the degree of scar severity before and after treatment on both the sides. Regarding patient’s satisfaction grades, there was a significant improvement after both treatment modalities with insignificant differences between both treatment modalities [20]. In this study, we tried to concentrate in collaborative effect and merge the possible synergistic regenerative capabilities for each modality together
2. Materials and Methods
This retrospective case series was conducted in Saudi Arabia. Patients treated and followed up over a period of 6 months. Overall, eight patients 4 male and 4 female patients with different scar types, in the age group of 25-45 years were selected. All Patients treated using one sitting of autologous microfat, nanofat graft, then three sittings of PRP with microneedling technique treating only the scars and leaving normal skin untreated, followed by session of fractional CO2 laser. Evaluation was based on physician clinical assessment and patient satisfaction with marked improvement post-treatment.
2.1. Treatment Technique
At baseline, for eligible patients, screening and informed consent were obtained pre-operatively. Information on patient demographics and scar characteristics was collected, more than half of the treated patients were above 35 years (5/8; 62.5%). By gender, Half of cases were Male, and half were female (4/8; 50.0%). Majority of the cases were Saudis (7/8; 87.5%) and only one patient (1/8; 12.5%) was non-Saudi. Burn was the cause of the scar in (37.5%) of patients followed by Surgery and severe acne (25%). Most of the scars were located in the Face (6/8; 75.0%). All patients had atrophic scars (100%) and most of them had the scars for more than5 years (5/8; 62.5%). Only three patients (37.5%) complained of a mild pain after the initial procedure which was alleviated after paracetamol 500 mg. None of the patients had any signs of infection. Demographics and clinical data of the patients were demonstrated in (Table 1).
|
Variables |
Number (%) |
|
|
Age |
≤35 years |
3 (37.5%) |
|
>35 years |
5 (62.5%) |
|
|
Nationality |
Saudi |
7 (87.5%) |
|
Non-Saudi |
1 (12.5%) |
|
|
Gender |
Male |
4 (50.0%) |
|
Female |
4 (50.0%) |
|
|
Trauma |
1 (12.5%) |
|
|
Scar Cause |
Surgery |
2 (25.0%) |
|
Severe Acne |
2 (25.0%) |
|
|
Burn |
3 (37.5%) |
|
|
Face |
6 (75.0%) |
|
|
Scar Site |
Wrist |
1 (12.5%) |
|
Foot |
1 (12.5%) |
|
|
Scar Type |
Atrophic |
8 (100,0%) |
|
Hypertrophic |
0 (0.00) |
|
|
Scar Duration |
≤ 5 years |
3 (37.5%) |
|
> 5 years |
5 (62.5%) |
|
|
Treatment Side effect |
Mild Pain |
3 (37.5%) |
|
Infection |
0 (0.00) |
|
Table 1: Demographics and clinical data of the patients.
Areas of scars as well as areas of fat harvesting were marked Intra-operatively. Fat harvesting was based upon patient’s common location of fat deposition, usually tend to be in bilateral thigh and hip region. Preoperative planning and assessment allow volumes that will be set aside for microfat injections and nanofat injections. After setting aside the appropriate volumes of the harvested microfat to be injected, the remainder undergoes a mechanical isolation process to become nanofat.
2.2. Microfat and Nanofat Injection (1st Stage Treatment)
2.2.1. Local Tumescent Infiltration Anesthesia
Tumescent solution is mixed using a 100-mL bag of 0.9% normal saline with 2 mL of 1% plain lidocaine and 0.1 mL of 1:1000 epinephrine. Tumescent is infiltrated using a tumescent infusion cannula affixed to a 60-mL syringe, and the fluid is instilled and equally distributed between both sides. It is recommended that the tumescent solution sit for 15 minutes after injection and before harvest.
2.3. Scar Release
Before Microfat and nanofat injections, simple manual surgical release or freeing of fibrosis done intraoperatively at scar site to relieve the tension and decrease the contracture of the scar, to help improve scar pliability and helps render lengthy linear scars irregular and less discernable (Figure 2).
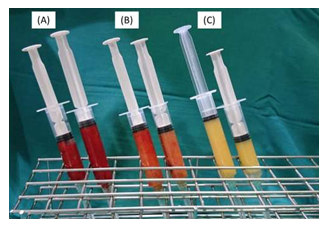
Figure 1: (A) Harvested fat before removal of suparanatant and infranatant. (B) Microfat. (C) nanofat.
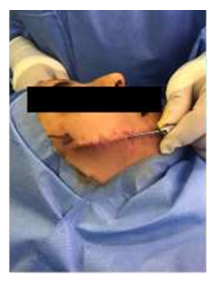
Figure 2: Simple manual surgical release or feeling of fibrosis done intraoperatively at scar site.
2.4. Fat Harvisting And Mechanical Isolation Method
Fat harvesting is performed using 60-mL BD syringes with a Tonnard fat harvester cannula with 1-mm side ports. Approximately 40 to 50 mL of aspirate is typically harvested per side using manual suction, assisted by the use of a “Johnnie Lok” to maintain adequate negative pressure. After harvest of the fat, the donor site is closed using a simple Steri-Strip closure of the entry point. Syringes are allowed to settle by gravity allowing separation of fat from supranatant and infranatant, which are discarded (Figure 1A). The 2 syringes now contain microfat given the presence of viable fat cells harvested from small-bore cannulas. Approximately 24 mL of microfat is transferred to 3-mL syringes for the microfat injection (Figure 1B). The remaining fat is set aside for continued processing into nanofat. Mechanical processing of nanofat for isolation of AD-MSCs, is performed using the Tulip nanofat system. A set of luer lock connectors is used to emulsify the fat, first with a 2.4-mm luer lock for a total of 40 passes, then a 1.2-mm luer lock for another 40 passes. Any fibrous tissue that obstructs the passage through the luer lock is extracted to prevent clogging of the filter in the following step. The emulsified fat takes on a finer texture and lighter color (Figure 1C). The final step involves a single pass through the nanotransfer filter allowing the final isolation of ADSVF without surviving adipocytes. This nanofat is also transferred to 3-mL syringes for injection. The nanofat is capable of being injected through small blunt microcannulas (25- or 27- gauge) or 30-gauge needles for superficial intradermal injections.
2.5. Injection of Microfat
Microfat provides the structural support and volumization for scar depression. It is injected through 3-mL syringes using a 2-inch 0.7-mm injection cannula. An 18-gauge needle is adequate to create entry points for passage of the 0.7-mm cannula. Injection of microfat was performed in a deep fashion along scar until appropriate volume identified.
2.6. Injection of Nanofat
The nanofat is capable of being injected through small blunt microcannulas (25- or 27- gauge) or 30-gauge needles for superficial intradermal injections of depressed scars. Typical volumes of nanofat are 8 to 10 mL per scar. Injection was performed until a yellowish discoloration of the skin showed up after that gentle digital spreading was done to spread it in all directions. Fat injections should be performed carefully using a retrograde threading technique of the cannula to avoid any intravascular complications.
2.7. Platelet-Rich Plasma with Microneedling (2nd Stage Treatment)
This stage of treatment was done as outpatient basis; the patient visited the clinic and underwent the procedure in minor procedure room under complete aseptic condition. This session of PRP and microneedling repeated 3 times with 4 weeks in between.
2.8. Platelet-Rich Plasma Preparation
Ten to twenty milliliter of fresh blood is collected from the median cubital vein into sodium citrate vacutainers under aseptic condition. The tubes are rotated in a centrifuge machine at 1500 revolutions per minute for 6 min. The first centrifugation called “soft spin” separates the blood into three layers, lowermost RBC layer (55% of total volume), topmost acellular plasma layer called platelet poor plasma (PPP, 40% of total volume), and an intermediate PRP layer (5% of total volume) called the “buffy coat.” Buffy coat with PPP is collected with the help of Finn pipette in another test tube. This tube is again centrifuged at 2500 revolution per minute for 15 min called “hard spin.” This allows the platelets (PRP) to settle at the bottom of the tube. The upper layer containing PPP is discarded and the lower layer of PRP is collected in another clean tube. The platelet concentrate is loaded in 1 mL insulin syringes containing calcium chloride (9 parts of PRP and 1 part calcium chloride) as an activator and made ready for injections into the regions of scars (Figure 3). The above process is carried out under Laminar Air Flow Hood to maintain sterility and asepsis.
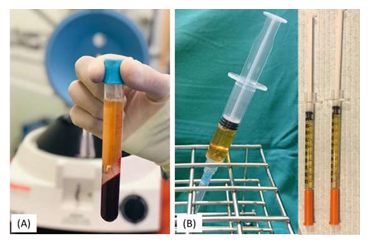
Figure 3: (A) Platelet-rich plasma after first centrifugation. (B) Platelt-rich plasma ready for injection.
2.9. Microneedling Method
Patient’s skin was first cleaned with ethyl alcohol followed by ether to remove all oils on skin surface. Topical anesthetic cream was then applied to skin under occlusion for 30 minutes. An automated microneedling device (Dermapen) with twelve microneedles of 0.25 mm to 2.5 mm length was used. The instrument consists of a rechargeable hand-piece with disposable needles at one end, which uses a motor to drive the movement of the needles. (Figure 4) Then with the help of prepared PRP syringe, PRP is spreading over treatment area under proper aseptic precaution in the clinic. All patients had sessions of PRP and microneedling and were administered every 4 weeks for 3 months post microfat and nanofat injections.

Figure 4: Automated microneedling device.
2.10. Fractional Non-Ablative Laser (3rd Stage Treatment)
This stage of treatment after finishing the sessions of PRP, patient referred to specialized laser center, where obtain one session of ablative CO2 laser (wave length 10.6 μm) using standard parameters, the common parameters used (Density 50 spots /cm2, power 30 watt, Tip 120 μm spot size beam with static operation mode). The CO2 non ablative fractional works with the patented Controlled Chaos Technology (CCTTM), this technology increases safety, fewer post-operative discomfort and a faster healing process, these parameters can be tuned gently according to patient skin type and scar characteristics. One patient needed additional sessions with QS - 1064 to improve pigmentation and tattoo effect in the scar
2.11. Follow-Up Assessment
All patients were evaluated at 4-week intervals for three months post treatment. High-resolution photographs of scars used for documentation in each visit.
3. Results
Eight patients were evaluated in this retrospective case series. By the end of 3 months of treatment, all eight patients had significant improvement of scar appearance with good satisfactory outcome and reduction in scar size. (Figures 5-7). The results of our study demonstrate that autologous lipofilling following three sittings of PRP with microneedling technique represents an excellent technique for enhancing the general appearance of scars on the face and hand.
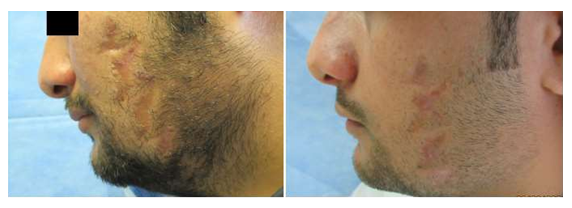
Figure 5: Male patient with left face scar after z-plasty surgery. (A) Before the treatment. (B) The same patient by the end of the third month of the treatment showing very significant improvement and reduction in scar size.
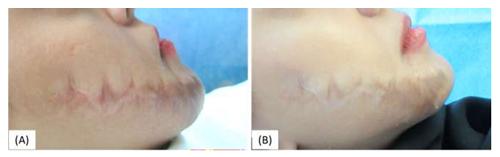
Figure 6: Female patient with right face scar after z-plasty surgery (A) Before the treatment. (B) The same patient by the end of the third month of the treatment showing marked improvement
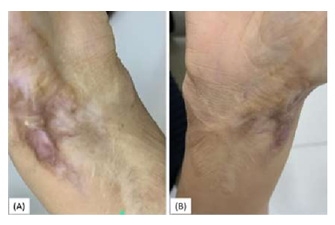
Figure 7: Female patient with right wrist scar because of burn (A) before the treatment (B) The same patient by the end of the third month of the treatment showing significant improvement.
4. Discussion
The advanced researches in regenerative therapy introduced new concepts in scar management and highlighted the importance of utilization of these researches on clinical basis. Although microfat, nanofat grafting, PRP and microneedeling have been reported to improve scars individually as presented in the literature, to date a limited body of evidence exists regarding their use in combination. To our best knowledge, no previous studies evaluated the regenerative effect of using the combination of autologous micofat graft, nanofat graft, autologous PRP and microneedling, followed by CO2 non ablative fractional laser. This study aimed to evaluate the clinical regenerative effect on scars after using these combined modalities (autologous microfat, nanofat graft, platelet rich plasma, microneedling and fractional CO2 in well-arranged sequential stages and timing. The results of our study demonstrate that autologous lipofilling following three sittings of PRP with microneedling technique represents an excellent technique for enhancing the general appearance of scars on the face and hand. As well as beneficial procedure for patients with volume loss in reconstructive and cosmetic surgery. Fat grafting offers numerous benefits for patients who would like effective skin rejuvenation and enhancement such as long-lasting results with no allergy testing and reduction in the appearance of fine lines, wrinkles, and depressions in skin. Keep in mind patients should be aware that results will take period of time with multiple sittings. In this study, all eight patients had significant improvement of scar appearance with good satisfactory outcome and reduction in scar size with no complications only mild pain (3/8; 37.5%).
In the other hand, many alternative noninvasive methods used in treating scars, with its pros and cons, with variable degrees of evidenced base medicine categorization, like laser, fillers. Fractional non-ablative lasers as solo modality for scar treatment has its benefits but many complications reported as regard, pain, pigmentation disorders and heat effect. The first fractionated laser used and the one most studied is the 1550-nm non-ablative fractionated device that uses an erbium doped fiber laser (Fraxel Restore [Reliant Technologies, San Diego, CA]) [24]. Al Harithy et al [24]. Reported during treatment patients experience pain requiring pre-treatment topical anesthesia and cooling of the skin surface during treatment. Post-treatment side effects vary based on used treatment setting. In general, mild erythema and edema is experienced 1 to 3 days post-treatment followed by bronzing and slight scaling of the skin. Alster et al. [25] reports a 25 % to 50 % improvement in atrophic acne scars appearance after a single treatment with the 1550 nm erbium-doped fiber laser and a 51 % to 75 % improvement in 87 % of patients who received at least three treatments. Also, injectable fillers such as hyaluronic acid have recently become popular, these materials are not used in all patients due to their expense, the necessity of repeat injections and the possibility of an allergic reaction [17]. As it has various of complications that can occur early include Erythema, Pain, Edema and Hypersensitivity reaction. Delayed that include Infection, nodule/abscess and foreign body granuloma [23]. Limitations of this study are the fact that it is a retrospective, nonrandomized case series. Patients were not compared to a control group that was treated with other injectables or surgery. The evaluation of the results was performed clinically and based on the patient’s satisfaction.
5. Conclusion
This study demonstrates the potential efficacy and safety of the combination therapy of autologous Microfat, Nanofat graft, platelet rich plasma with microneedling followed by non-ablative fractional laser for the treatment of scars. Further research and studies must continue in such fields and this will be one of our spectrum to search more betterment for scar management.
References
- Vincent AG, Kadakia S, Barker J, et al. Management of Facial Scars. Facial Plast Surg 35 (2019): 666-671.
- Ngaage M, Agius M. The Psychology of Scars: A Mini-Review. Psychiatr Danub 30 (2018): 633-638.
- Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 6 (2018): 4.
- Lighthall JG, Fedok FG. Treating scars of the chin and perioral region. Facial Plast Surg Clin North Am 25(2017): 55-71.
- Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg 38 (1966): 209-218.
- Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg 31 (2007): 468- 492.
- Nouri K, Vidulich K, Rivas MP. Lasers for scars: a review. J Cosmet Dermatol 5 (2006): 14- 22.
- Semra Uyulmaz, Nadia Sanchez Macedo, Farid Rezaeian, et al. Nanofat Grafting for Scar Treatment and Skin Quality Improvement, Aesthetic Surgery Journal 38 (2018): 421-428.
- Kucharzewski M, Rojczyk E, Wilemska-Kucharzewska K, et al. Novel trends in application of stem cells in skin wound healing. European Journal of Pharmacology 843 (2019): 307-315.
- Cai L, Johnstone B, Cook T, et al. IFATS Collection: Human Adipose Tissue-Derived Stem Cells Induce Angiogenesis and Nerve Sprouting Following Myocardial Infarction, in Conjunction with Potent Preservation of Cardiac Function. Stem Cells 27 (2009): 230-237.
- Alharbi Z, Almakadi S, Opla?nder C, et al. Intraoperative use of enriched collagen and elastin matrices with freshly isolated adipose-derived stem/stromal cells: a potential clinical approach for soft tissue reconstruction. BMC surgery 14 (2014): 10.
- Bellini E, Grieco MP, Raposio E. The science behind autologous fat grafting. Annals of medicine and surgery 24 (2012): 65-73.
- Fournier PF. Facial recontouring with fat grafting. Dermatol Clin 8 (1990): 523-537.
- Asken S. Facial liposuction and microlipoinjection. J Dermatol Surg Oncol 14 (1988): 297-305.
- Tonnard P, Verpaele A, Peeters G, et al. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 132 (2013): 1017-1026.
- Lindenblatt N, van Hulle A, Verpaele A, et al. The role of microfat grafting in facial contouring. Aesthet Surg J 35 (2015): 763-771.
- Chou CK, Lee SS, Lin TY, et al. Micro-autologous Fat Transplantation (MAFT) for Forehead Volumizing and Contouring. Aesthetic plastic surgery 41 (2017): 845-855.
- Tenna S, Cogliandro A, Barone M, et al. Comparative Study Using Autologous Fat Grafts Plus Platelet-Rich Plasma with or Without Fractional CO2 Laser Resurfacing in Treatment of Acne Scars: Analysis of Outcomes and Satisfaction With FACE-Q. Aesthetic Plast Surg 41 (2017): 661-666.
- Chawla S. Split Face Comparative Study of Microneedling with PRP Versus Microneedling with Vitamin C in Treating Atrophic Post Acne Scars. Journal of cutaneous and aesthetic surgery 7 (2014): 209-212.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat 29 (2018): 281-286.
- Wagh Milind S, Varun Dixit. Tissue expansion: Concepts, techniques and unfavourable results.” Indian journal of plastic surgery: official publication of the Association of Plastic Surgeons of India vol 46 (2013): 333-48.
- Antonyshyn O, Gruss JS, Mackinnon SE, et al. Complications of soft tissue expansion. Br J Plast Surg May 41 (1988): 239-250.
- Funt David, Tatjana Pavicic. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches.” Clinical, cosmetic and investigational dermatology vol 6 (2013): 295-316.
- Al Harithy R, Pon K. Scar Treatment with Lasers: A Review and Update. Curr Derm Rep 1 (2012): 69-75.
- Alster TS, Tanzi EL, Lazarus M. The Use of Fractional Laser Photothermolysis for the Treatment of Atrophic Scars. Dermatologic Surgery 33 (2007): 295-299.
Article Details
1. Introduction
Scars are normal end result after skin injuries that extend to the reticular dermis. It is a natural process of healing after skin damage where fibrous tissue replaces normal skin after injury [1]. The resultant change in appearance can negatively affect body image and self-confidence. Those with scars are more prone to the development of depression, anxiety, feelings of shame, and aggression [2] As well as its impact in reducing social interactions. Scars can result from many causes such as trauma, burns, surgeries and infections [3-4]. Many treatments have been used before for treating and decreasing the risk of scar formation. Injection or topical application of corticosteroids onto pathologic scars is considered an effective treatment method for keloids and hypertrophic scars [5]. Beside atrophy or hypertrophy of scars, pigmentation changes are common complications after specific treatments, like hypopigmentation in scars can also occur in cryotherapy, and radiotherapy scar treatment that may involve multiple sessions and can entail unwanted cellular apoptosis and necrosis. Scars are also often treated with less invasive silicone dressings and pressure garments. [6]. Laser therapy of hypertrophic scars may improve their appearance yet nevertheless; laser technology is limited by high recurrence rates and skin discoloration [7]. Finally, surgery may be an option to correct overly conspicuous scars and includes scar revision, dermabrasion, or skin grafting (8]. Recently, a worldwide effort was undertaken to investigate the regenerative potential of adipose tissue grafting. Adipose tissue is rich of mesenchymal stem cells; Adipose-Derived Mesenchymal Stem Cells (AD-MSCs), microvascular fragments, growth factors and cytokines, which are critically important for revascularization and the enhancement of angiogenesis and decrease fibrosis [9,10]. A study done in 2014 revealed that in post liposuction, freshly isolated uncultured AD-MSCs has the ability to be involved in the cellular enrichment of making collagen and elastin matrices [11]. Adipose grafting techniques has undergone significant changes over time to improve the quality of the lipoaspirate and the survival of the fat graft after implantation [12]. As a result, the original dermal-fat grafts and microlipoinjection techniques [13, 14]. Have been advanced by current techniques of microfat and nanofat, (15,16) which are showing promising results. In several studies, Micro-Autologous Fat Transplantation (MAFT) has demonstrated its feasibility in facial rejuvenation, volumizing and contouring [17]. Tonnard, et al, described the method of Nanofat, which is the material obtained after harvesting fat via liposuction which go through mechanical emulsification through small-bore luer lock connectors, followed by cellular filtration through a 500-mm filter. This quick process allows isolation of the Stromal Vascular Fraction (SVF) as well as some nonviable adipocyte cell components. Tonnard, et al, suggested that Nanofat graft could be cheaper and simpler alternative to the traditional transplantation of AD-MSCs for epithelial tissue regeneration and skin rejuvenation [17]. Jordan Rihani has emphasized injection of nanofat combined with microfat seems to offer improvement in skin texture as well as structural volumization [21]. A recent comparative study evaluated the efficacy of nanofat and Platelet-Rich Plasma (PRP) infiltration alone and combined with fractional CO2 laser resurfacing to improve atrophic scars of the face from March 2014 to June 2015, 30 patients with atrophic acne scars on the cheeks were selected for this study. In conclusion Subcutaneous infiltration with nanofat and PRP seems to be effective to improve atrophic scars, either alone or combined with fractional CO2 laser resurfacing [18]. A split face comparative study of microneedling with PRP Versus microneedling with Vitamin C done on Thirty patients with post acne atrophic facial scars offered four sittings of microneedling with PRP on one side and microneedling with vitamin C on other side of the face at an interval of 1 month [19]. Out of 30 patients, 23 achieved reduction in scarring by one or two grades. Overall results were better with microneedling and PRP than microneedling with vitamin C [19]. Another split-face comparative study microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne. The study included 35 patients with mild to severe post acne atrophic scar. All the patients received four sequential treatments of skin microneedling alone on the right side of the face and skin microneedling followed by topical application of PRP on the left side of the face with an interval of 3 weeks. There was a significant improvement in the degree of scar severity before and after treatment on both the sides. Regarding patient’s satisfaction grades, there was a significant improvement after both treatment modalities with insignificant differences between both treatment modalities [20]. In this study, we tried to concentrate in collaborative effect and merge the possible synergistic regenerative capabilities for each modality together
2. Materials and Methods
This retrospective case series was conducted in Saudi Arabia. Patients treated and followed up over a period of 6 months. Overall, eight patients 4 male and 4 female patients with different scar types, in the age group of 25-45 years were selected. All Patients treated using one sitting of autologous microfat, nanofat graft, then three sittings of PRP with microneedling technique treating only the scars and leaving normal skin untreated, followed by session of fractional CO2 laser. Evaluation was based on physician clinical assessment and patient satisfaction with marked improvement post-treatment.
2.1. Treatment Technique
At baseline, for eligible patients, screening and informed consent were obtained pre-operatively. Information on patient demographics and scar characteristics was collected, more than half of the treated patients were above 35 years (5/8; 62.5%). By gender, Half of cases were Male, and half were female (4/8; 50.0%). Majority of the cases were Saudis (7/8; 87.5%) and only one patient (1/8; 12.5%) was non-Saudi. Burn was the cause of the scar in (37.5%) of patients followed by Surgery and severe acne (25%). Most of the scars were located in the Face (6/8; 75.0%). All patients had atrophic scars (100%) and most of them had the scars for more than5 years (5/8; 62.5%). Only three patients (37.5%) complained of a mild pain after the initial procedure which was alleviated after paracetamol 500 mg. None of the patients had any signs of infection. Demographics and clinical data of the patients were demonstrated in (Table 1).
|
Variables |
Number (%) |
|
|
Age |
≤35 years |
3 (37.5%) |
|
>35 years |
5 (62.5%) |
|
|
Nationality |
Saudi |
7 (87.5%) |
|
Non-Saudi |
1 (12.5%) |
|
|
Gender |
Male |
4 (50.0%) |
|
Female |
4 (50.0%) |
|
|
Trauma |
1 (12.5%) |
|
|
Scar Cause |
Surgery |
2 (25.0%) |
|
Severe Acne |
2 (25.0%) |
|
|
Burn |
3 (37.5%) |
|
|
Face |
6 (75.0%) |
|
|
Scar Site |
Wrist |
1 (12.5%) |
|
Foot |
1 (12.5%) |
|
|
Scar Type |
Atrophic |
8 (100,0%) |
|
Hypertrophic |
0 (0.00) |
|
|
Scar Duration |
≤ 5 years |
3 (37.5%) |
|
> 5 years |
5 (62.5%) |
|
|
Treatment Side effect |
Mild Pain |
3 (37.5%) |
|
Infection |
0 (0.00) |
|
Table 1: Demographics and clinical data of the patients.
Areas of scars as well as areas of fat harvesting were marked Intra-operatively. Fat harvesting was based upon patient’s common location of fat deposition, usually tend to be in bilateral thigh and hip region. Preoperative planning and assessment allow volumes that will be set aside for microfat injections and nanofat injections. After setting aside the appropriate volumes of the harvested microfat to be injected, the remainder undergoes a mechanical isolation process to become nanofat.
2.2. Microfat and Nanofat Injection (1st Stage Treatment)
2.2.1. Local Tumescent Infiltration Anesthesia
Tumescent solution is mixed using a 100-mL bag of 0.9% normal saline with 2 mL of 1% plain lidocaine and 0.1 mL of 1:1000 epinephrine. Tumescent is infiltrated using a tumescent infusion cannula affixed to a 60-mL syringe, and the fluid is instilled and equally distributed between both sides. It is recommended that the tumescent solution sit for 15 minutes after injection and before harvest.
2.3. Scar Release
Before Microfat and nanofat injections, simple manual surgical release or freeing of fibrosis done intraoperatively at scar site to relieve the tension and decrease the contracture of the scar, to help improve scar pliability and helps render lengthy linear scars irregular and less discernable (Figure 2).

Figure 1: (A) Harvested fat before removal of suparanatant and infranatant. (B) Microfat. (C) nanofat.

Figure 2: Simple manual surgical release or feeling of fibrosis done intraoperatively at scar site.
2.4. Fat Harvisting And Mechanical Isolation Method
Fat harvesting is performed using 60-mL BD syringes with a Tonnard fat harvester cannula with 1-mm side ports. Approximately 40 to 50 mL of aspirate is typically harvested per side using manual suction, assisted by the use of a “Johnnie Lok” to maintain adequate negative pressure. After harvest of the fat, the donor site is closed using a simple Steri-Strip closure of the entry point. Syringes are allowed to settle by gravity allowing separation of fat from supranatant and infranatant, which are discarded (Figure 1A). The 2 syringes now contain microfat given the presence of viable fat cells harvested from small-bore cannulas. Approximately 24 mL of microfat is transferred to 3-mL syringes for the microfat injection (Figure 1B). The remaining fat is set aside for continued processing into nanofat. Mechanical processing of nanofat for isolation of AD-MSCs, is performed using the Tulip nanofat system. A set of luer lock connectors is used to emulsify the fat, first with a 2.4-mm luer lock for a total of 40 passes, then a 1.2-mm luer lock for another 40 passes. Any fibrous tissue that obstructs the passage through the luer lock is extracted to prevent clogging of the filter in the following step. The emulsified fat takes on a finer texture and lighter color (Figure 1C). The final step involves a single pass through the nanotransfer filter allowing the final isolation of ADSVF without surviving adipocytes. This nanofat is also transferred to 3-mL syringes for injection. The nanofat is capable of being injected through small blunt microcannulas (25- or 27- gauge) or 30-gauge needles for superficial intradermal injections.
2.5. Injection of Microfat
Microfat provides the structural support and volumization for scar depression. It is injected through 3-mL syringes using a 2-inch 0.7-mm injection cannula. An 18-gauge needle is adequate to create entry points for passage of the 0.7-mm cannula. Injection of microfat was performed in a deep fashion along scar until appropriate volume identified.
2.6. Injection of Nanofat
The nanofat is capable of being injected through small blunt microcannulas (25- or 27- gauge) or 30-gauge needles for superficial intradermal injections of depressed scars. Typical volumes of nanofat are 8 to 10 mL per scar. Injection was performed until a yellowish discoloration of the skin showed up after that gentle digital spreading was done to spread it in all directions. Fat injections should be performed carefully using a retrograde threading technique of the cannula to avoid any intravascular complications.
2.7. Platelet-Rich Plasma with Microneedling (2nd Stage Treatment)
This stage of treatment was done as outpatient basis; the patient visited the clinic and underwent the procedure in minor procedure room under complete aseptic condition. This session of PRP and microneedling repeated 3 times with 4 weeks in between.
2.8. Platelet-Rich Plasma Preparation
Ten to twenty milliliter of fresh blood is collected from the median cubital vein into sodium citrate vacutainers under aseptic condition. The tubes are rotated in a centrifuge machine at 1500 revolutions per minute for 6 min. The first centrifugation called “soft spin” separates the blood into three layers, lowermost RBC layer (55% of total volume), topmost acellular plasma layer called platelet poor plasma (PPP, 40% of total volume), and an intermediate PRP layer (5% of total volume) called the “buffy coat.” Buffy coat with PPP is collected with the help of Finn pipette in another test tube. This tube is again centrifuged at 2500 revolution per minute for 15 min called “hard spin.” This allows the platelets (PRP) to settle at the bottom of the tube. The upper layer containing PPP is discarded and the lower layer of PRP is collected in another clean tube. The platelet concentrate is loaded in 1 mL insulin syringes containing calcium chloride (9 parts of PRP and 1 part calcium chloride) as an activator and made ready for injections into the regions of scars (Figure 3). The above process is carried out under Laminar Air Flow Hood to maintain sterility and asepsis.

Figure 3: (A) Platelet-rich plasma after first centrifugation. (B) Platelt-rich plasma ready for injection.
2.9. Microneedling Method
Patient’s skin was first cleaned with ethyl alcohol followed by ether to remove all oils on skin surface. Topical anesthetic cream was then applied to skin under occlusion for 30 minutes. An automated microneedling device (Dermapen) with twelve microneedles of 0.25 mm to 2.5 mm length was used. The instrument consists of a rechargeable hand-piece with disposable needles at one end, which uses a motor to drive the movement of the needles. (Figure 4) Then with the help of prepared PRP syringe, PRP is spreading over treatment area under proper aseptic precaution in the clinic. All patients had sessions of PRP and microneedling and were administered every 4 weeks for 3 months post microfat and nanofat injections.

Figure 4: Automated microneedling device.
2.10. Fractional Non-Ablative Laser (3rd Stage Treatment)
This stage of treatment after finishing the sessions of PRP, patient referred to specialized laser center, where obtain one session of ablative CO2 laser (wave length 10.6 μm) using standard parameters, the common parameters used (Density 50 spots /cm2, power 30 watt, Tip 120 μm spot size beam with static operation mode). The CO2 non ablative fractional works with the patented Controlled Chaos Technology (CCTTM), this technology increases safety, fewer post-operative discomfort and a faster healing process, these parameters can be tuned gently according to patient skin type and scar characteristics. One patient needed additional sessions with QS - 1064 to improve pigmentation and tattoo effect in the scar
2.11. Follow-Up Assessment
All patients were evaluated at 4-week intervals for three months post treatment. High-resolution photographs of scars used for documentation in each visit.
3. Results
Eight patients were evaluated in this retrospective case series. By the end of 3 months of treatment, all eight patients had significant improvement of scar appearance with good satisfactory outcome and reduction in scar size. (Figures 5-7). The results of our study demonstrate that autologous lipofilling following three sittings of PRP with microneedling technique represents an excellent technique for enhancing the general appearance of scars on the face and hand.

Figure 5: Male patient with left face scar after z-plasty surgery. (A) Before the treatment. (B) The same patient by the end of the third month of the treatment showing very significant improvement and reduction in scar size.

Figure 6: Female patient with right face scar after z-plasty surgery (A) Before the treatment. (B) The same patient by the end of the third month of the treatment showing marked improvement

Figure 7: Female patient with right wrist scar because of burn (A) before the treatment (B) The same patient by the end of the third month of the treatment showing significant improvement.
4. Discussion
The advanced researches in regenerative therapy introduced new concepts in scar management and highlighted the importance of utilization of these researches on clinical basis. Although microfat, nanofat grafting, PRP and microneedeling have been reported to improve scars individually as presented in the literature, to date a limited body of evidence exists regarding their use in combination. To our best knowledge, no previous studies evaluated the regenerative effect of using the combination of autologous micofat graft, nanofat graft, autologous PRP and microneedling, followed by CO2 non ablative fractional laser. This study aimed to evaluate the clinical regenerative effect on scars after using these combined modalities (autologous microfat, nanofat graft, platelet rich plasma, microneedling and fractional CO2 in well-arranged sequential stages and timing. The results of our study demonstrate that autologous lipofilling following three sittings of PRP with microneedling technique represents an excellent technique for enhancing the general appearance of scars on the face and hand. As well as beneficial procedure for patients with volume loss in reconstructive and cosmetic surgery. Fat grafting offers numerous benefits for patients who would like effective skin rejuvenation and enhancement such as long-lasting results with no allergy testing and reduction in the appearance of fine lines, wrinkles, and depressions in skin. Keep in mind patients should be aware that results will take period of time with multiple sittings. In this study, all eight patients had significant improvement of scar appearance with good satisfactory outcome and reduction in scar size with no complications only mild pain (3/8; 37.5%).
In the other hand, many alternative noninvasive methods used in treating scars, with its pros and cons, with variable degrees of evidenced base medicine categorization, like laser, fillers. Fractional non-ablative lasers as solo modality for scar treatment has its benefits but many complications reported as regard, pain, pigmentation disorders and heat effect. The first fractionated laser used and the one most studied is the 1550-nm non-ablative fractionated device that uses an erbium doped fiber laser (Fraxel Restore [Reliant Technologies, San Diego, CA]) [24]. Al Harithy et al [24]. Reported during treatment patients experience pain requiring pre-treatment topical anesthesia and cooling of the skin surface during treatment. Post-treatment side effects vary based on used treatment setting. In general, mild erythema and edema is experienced 1 to 3 days post-treatment followed by bronzing and slight scaling of the skin. Alster et al. [25] reports a 25 % to 50 % improvement in atrophic acne scars appearance after a single treatment with the 1550 nm erbium-doped fiber laser and a 51 % to 75 % improvement in 87 % of patients who received at least three treatments. Also, injectable fillers such as hyaluronic acid have recently become popular, these materials are not used in all patients due to their expense, the necessity of repeat injections and the possibility of an allergic reaction [17]. As it has various of complications that can occur early include Erythema, Pain, Edema and Hypersensitivity reaction. Delayed that include Infection, nodule/abscess and foreign body granuloma [23]. Limitations of this study are the fact that it is a retrospective, nonrandomized case series. Patients were not compared to a control group that was treated with other injectables or surgery. The evaluation of the results was performed clinically and based on the patient’s satisfaction.
5. Conclusion
This study demonstrates the potential efficacy and safety of the combination therapy of autologous Microfat, Nanofat graft, platelet rich plasma with microneedling followed by non-ablative fractional laser for the treatment of scars. Further research and studies must continue in such fields and this will be one of our spectrum to search more betterment for scar management.
References
- Vincent AG, Kadakia S, Barker J, et al. Management of Facial Scars. Facial Plast Surg 35 (2019): 666-671.
- Ngaage M, Agius M. The Psychology of Scars: A Mini-Review. Psychiatr Danub 30 (2018): 633-638.
- Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 6 (2018): 4.
- Lighthall JG, Fedok FG. Treating scars of the chin and perioral region. Facial Plast Surg Clin North Am 25(2017): 55-71.
- Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg 38 (1966): 209-218.
- Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg 31 (2007): 468- 492.
- Nouri K, Vidulich K, Rivas MP. Lasers for scars: a review. J Cosmet Dermatol 5 (2006): 14- 22.
- Semra Uyulmaz, Nadia Sanchez Macedo, Farid Rezaeian, et al. Nanofat Grafting for Scar Treatment and Skin Quality Improvement, Aesthetic Surgery Journal 38 (2018): 421-428.
- Kucharzewski M, Rojczyk E, Wilemska-Kucharzewska K, et al. Novel trends in application of stem cells in skin wound healing. European Journal of Pharmacology 843 (2019): 307-315.
- Cai L, Johnstone B, Cook T, et al. IFATS Collection: Human Adipose Tissue-Derived Stem Cells Induce Angiogenesis and Nerve Sprouting Following Myocardial Infarction, in Conjunction with Potent Preservation of Cardiac Function. Stem Cells 27 (2009): 230-237.
- Alharbi Z, Almakadi S, Oplander C, et al. Intraoperative use of enriched collagen and elastin matrices with freshly isolated adipose-derived stem/stromal cells: a potential clinical approach for soft tissue reconstruction. BMC surgery 14 (2014): 10.
- Bellini E, Grieco MP, Raposio E. The science behind autologous fat grafting. Annals of medicine and surgery 24 (2012): 65-73.
- Fournier PF. Facial recontouring with fat grafting. Dermatol Clin 8 (1990): 523-537.
- Asken S. Facial liposuction and microlipoinjection. J Dermatol Surg Oncol 14 (1988): 297-305.
- Tonnard P, Verpaele A, Peeters G, et al. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 132 (2013): 1017-1026.
- Lindenblatt N, van Hulle A, Verpaele A, et al. The role of microfat grafting in facial contouring. Aesthet Surg J 35 (2015): 763-771.
- Chou CK, Lee SS, Lin TY, et al. Micro-autologous Fat Transplantation (MAFT) for Forehead Volumizing and Contouring. Aesthetic plastic surgery 41 (2017): 845-855.
- Tenna S, Cogliandro A, Barone M, et al. Comparative Study Using Autologous Fat Grafts Plus Platelet-Rich Plasma with or Without Fractional CO2 Laser Resurfacing in Treatment of Acne Scars: Analysis of Outcomes and Satisfaction With FACE-Q. Aesthetic Plast Surg 41 (2017): 661-666.
- Chawla S. Split Face Comparative Study of Microneedling with PRP Versus Microneedling with Vitamin C in Treating Atrophic Post Acne Scars. Journal of cutaneous and aesthetic surgery 7 (2014): 209-212.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat 29 (2018): 281-286.
- Wagh Milind S, Varun Dixit. Tissue expansion: Concepts, techniques and unfavourable results.” Indian journal of plastic surgery: official publication of the Association of Plastic Surgeons of India vol 46 (2013): 333-48.
- Antonyshyn O, Gruss JS, Mackinnon SE, et al. Complications of soft tissue expansion. Br J Plast Surg May 41 (1988): 239-250.
- Funt David, Tatjana Pavicic. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches.” Clinical, cosmetic and investigational dermatology vol 6 (2013): 295-316.
- Al Harithy R, Pon K. Scar Treatment with Lasers: A Review and Update. Curr Derm Rep 1 (2012): 69-75.
- Alster TS, Tanzi EL, Lazarus M. The Use of Fractional Laser Photothermolysis for the Treatment of Atrophic Scars. Dermatologic Surgery 33 (2007): 295-299.
