Myocardial Infarction Associated with Regional Myocardial Flow Deficit and Normal Coronary Arteries in a Patient with COVID-19: A Case Report
Article Information
Joel E Money MD2, Nishant K Sekaran MD1, C Michael Minder MD1, Viet T Le, PA-C1,3, Steve M Mason, PA-C1, Joseph B Muhlestein MD1,2, Kirk U Knowlton MD1, Jeffrey L Anderson MD1,2*
1Intermountain Medical Center, Intermountain Heart Institute, Salt Lake City, Utah, USA
2University of Utah School of Medicine, Salt Lake City, Utah, USA
3Rocky Mountain University of Health Professions, Provo, Utah, USA
*Corresponding Authors: Jeffrey L Anderson, MD, Intermountain Medical Center, Building 4, 6th floor, Murray, UT, 84107, USA
Received: 06 November 2020; Accepted: 17 November 2020; Published: 23 November 2020
Citation: Joel E Money, Nishant K Sekaran, C Michael Minder, Viet T Le, Steve M Mason, Joseph B Muhlestein, Kirk U Knowlton, Jeffrey L Anderson. Myocardial Infarction Associated with Regional Myocardial Flow Deficit and Normal Coronary Arteries in a Patient with COVID-19: A Case Report. Cardiology and Cardiovascular Medicine 4 (2020): 729-735.
View / Download Pdf Share at FacebookAbstract
Myocardial injury is increasingly recognized as a complication in patients with COVID-19, but the pathophysiological basis remains uncertain. Here we present a case of non-ST-segment elevation myocardial infarction associated with small vessel or microvascular dysfunction as determined by multimodality imaging in a patient with COVID-19.
Keywords
Cardiac MRI; COVID-19; Microvascular thrombosis; MINOCA; Myocardial infarction; Perfusion imaging; Positron emission tomography
Article Details
Abbreviations:
CCTA: coronary CT angiography; CMR: cardiac magnetic resonance imaging; CT: computed tomography; ECG: electrocardiogram; LVEF: left ventricular ejection fraction; MI: myocardial infarction; MINOCA: MI in the absence of obstructive coronary artery disease; NSTEMI: non-ST-segment elevation myocardial infarction; PET: positron emission tomography.
1. Introduction
COVID-19 is a multiorgan disease. In addition to involving the lungs, it also can affect the heart, kidney, brain, gastrointestinal tract, liver, and skin. Myocardial injury is increasingly recognized as a complication of COVID-19, but its pathophysiologic basis remains poorly characterized [1]. Here we report a case of a patient with COVID-19 who experienced non-ST-segment elevation myocardial infarction associated with reversible microvascular dysfunction as determined by multimodality imaging.
2. Case Presentation
A 59-year-old man with a past medical history including diabetes mellitus type 2, hypertension, and class III obesity was diagnosed by PCR with COVID-19 after close contact with his spouse, who also was infected and required hospitalization. After 2-weeks of progressive outpatient illness, he was admitted to hospital with increasing shortness of breath and diagnosed with COVID-19 pneumonia. After 3 days of inpatient care, fever, dyspnea and acute kidney injury had resolved (without the need for supplemental oxygen), and he was discharged without medications. Two days later he presented to the emergency department after waking up with severe substernal chest pressure. Vital signs were notable for a blood pressure of 176/86 mmHg, a heart rate of 68 bpm, a temperature of 36.5C, and normal oxygen saturation on room air. Physical exam was notable for faint upper lobe crackles with an otherwise normal cardiovascular examination. Sublingual nitroglycerin was administered in the emergency department and led to relief of chest pain.
2.1 Investigations
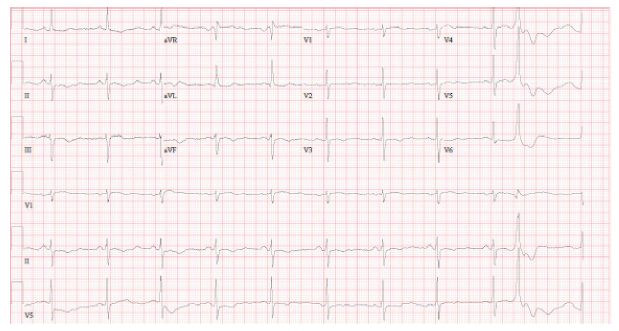
ECG on presentation revealed dynamic T-wave inversions in leads V3-V5 as well as in leads II, III, and aVF (Figure 1). Initial cardiac troponin-I was elevated at 0.10 ng/mL, which increased to 8.06 ng/mL, peaked at 19.03 ng/mL, then trended down to 14.26 ng/mL. Chest CT angiography showed no pulmonary embolism. A transthoracic echocardiogram revealed basal/mid septal wall motion abnormalities corresponding to a septal perforator vascular distribution with an LVEF of 55% (Supplemental Video 1).
2.2 Diagnosis and management
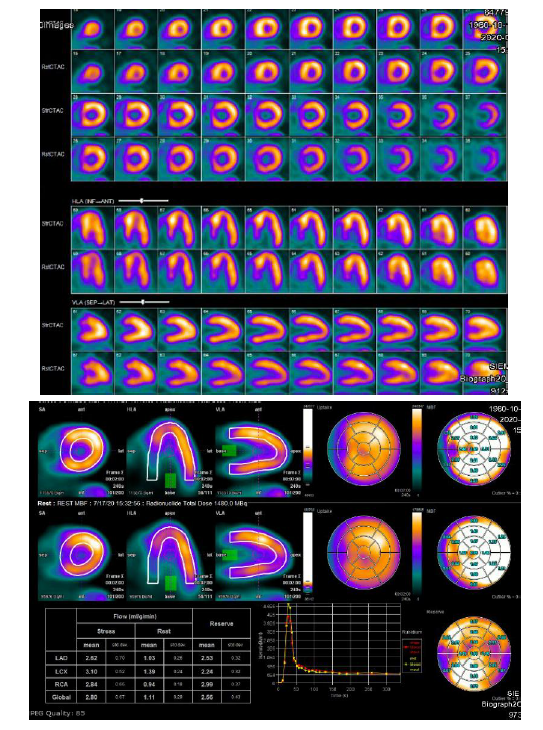
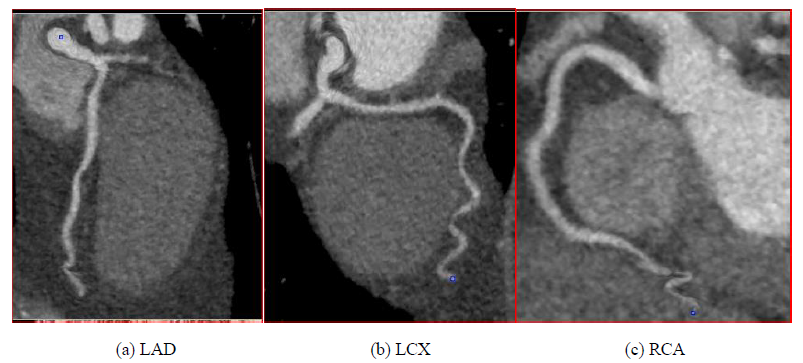
Based on clinical presentation, imaging, ECG, and laboratory testing, a diagnosis of NSTEMI was made. The patient was treated with aspirin, a loading dose of clopidogrel, therapeutic dosing of enoxaparin, carvedilol, and atorvastatin. By the next day, chest pain had resolved, he was feeling well, and he insisted on discharge. Enoxaparin was stopped and the other medications continued. An appointment was made for outpatient Rb-82 Positron Emission Tomography (PET) myocardial perfusion stress testing. Cardiac PET performed one week later demonstrated a small to medium sized fixed perfusion defect in the basal/mid aspect of the septal wall (Figure 2), corresponding to echocardiographic wall motion abnormalities. Coronary CT angiography was performed 11 days later and revealed no evidence of obstructive epicardial coronary artery disease or coronary calcification (Figure 3).
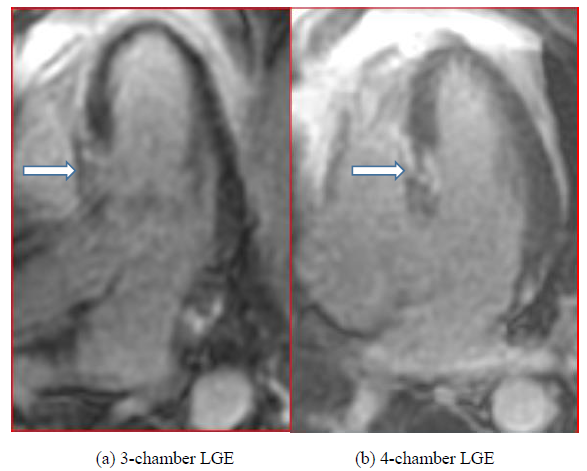
Convalescent regadenoson stress cardiac MRI was performed at 6 weeks. This revealed residual septal hypokinesis from the base to the mid left ventricle with otherwise normal left ventricular wall motion. Rest and stress first-pass gadolinium cine sequences demonstrated normal perfusion. However, late gadolinium enhancement imaging (at 10 minutes) demonstrated moderate sized patchy transmural enhancement at the base to mid segments of the interventricular septum, corresponding to the regional wall motion abnormalities (Figure 4).
2.3 Follow-up
At a two-month follow-up visit, the patient was clinically stable without symptoms of angina, heart failure, or recurrent cardiovascular hospitalizations. He was independent in activies of daily living without reduction in his functional status. Therapy consisted of dual anti-platelet therapy, statin, and beta blocker.
3. Discussion
Myocardial injury in patients with COVID-19 is an increasingly recognized phenomenon, with reports ranging from 34% of patients in a New York cohort with elevated cardiac biomarkers [1] to as many as 78% of patients found to have an abnormal CMR after recovery in a German cohort [2]. The etiology of myocardial injury is poorly understood but has been ascribed to a heterogeneous group of processes, including direct viral myocardial injury, lymphocytic myocarditis [3], immune-mediated injury, epicardial coronary artery occlusion, and microvascular dysfunction/microthrombi [4, 5].
SARS-CoV-2 targets not only pulmonary epithelial cells but also the vascular endothelium via the ACE-2 receptor [5]. Although microvascular dysfunction has been postulated as a potential mechanism of myocardial injury, its pathogenesis remains poorly characterized. A recent report of fulminant cardiogenic shock with ST-segment elevation MI in a patient with COVID-19 demonstrated extensive microvascular thrombi on autopsy [6]. Identifying coronary perfusion defects early during the course of COVID-19 related acute cardiac injury could help clinicians better understand the mechanisms of myocardial injury, offer important prognostic information, and potentially direct more effective therapies. However, perfusion imaging in COVID-19, including with Rb-82 PET, has not been well described in this population.
Here we showcase the use of multi-modality cardiac imaging including cardiac PET stress/rest, coronary CT angiography and cardiac MRI to phenotype COVID-19-related NSTEMI in the absence of epicardial coronary artery disease. NSTEMI was diagnosed by a classical rise and fall pattern in cardiac troponin, together with a typical clinical presentation, and further confirmed by late gadolinium enhancement in the affected region of echocardiographic and CMR wall motion abnormality. Absence of epicardial coronary occlusion qualifies this case as MINOCA associated with COVID-19 [7].
These findings indicate to us that regional myocardial ischemia, associated with reversible small branch (i.e., septal perforator) or microvascular dysfunction/ occlusion in the absence of epicardial disease or embolic risk factors, was the underlying mechanism of myocardial injury. We hypothesize that microthrombi may have been the underlying cause of the regional myocardial perfusion deficit [6], which resolved by the time of convalescent stress CMR at 6 weeks. The limitation of non-simultaneous or delayed acquisition of some of the imaging tests (e.g., echo and PET), a consequence of patient preference and COVID-19 scheduling restrictions, should not in our view impact the pathophysiologic implications.
In support of the utility of multimodality imaging in the care of COVID-19 patients with cardiovascular complications, a JACC Scientific Expert Panel recently published recommendations on this topic, including proposed applications of echocardiography, PET perfusion imaging, and cardiac MRI [8].
4. Conclusions
Myocardial injury is increasingly recognized as a complication in patients with COVID-19, but its pathophysiological basis remains poorly characterized. Here we demonstrate the complementary role of multi-modality imaging in identifying and monitoring these processes in a patient with COVID-related myocardial injury. Our findings point to cardiac small vessel or microvascular perfusion defects, and we postulate that these may be more common than previously recognized. We propose that multimodality imaging can be useful in the evaluation of cardiovascular complications in patients with COVID-19 and can expand our limited knowledge of the role of microvascular disease.
References
- Lala A, Johnson KW, Russak AJ, et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. Journal of the American College of Cardiology 76 (2020): 533-546.
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiology Jul 27 (2020): e203557.
- Siripanthong, B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 17 (2020): 1463-1471.
- Lowenstein CJ, Solomon SD. Severe COVID-19 is a Microvascular Disease. Circulation 142 (2020): 1609-1611.
- Knowlton KU. Pathogenesis of SARS-CoV-2 induced cardiac injury from the perspective of the virus. Journal of Molecular and Cellular Cardiology 147 (2020): 12-17.
- Guagliumi G, Sonzogni A, Pescetelli I, Pellegrini D, Finn AV. Microthrombi and ST-Segment-Elevation Myocardial Infarction in COVID-19. Circulation 142 (2020): 804-809.
- Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation 139 (2019): e891-e908.
- Rudski, L, Januzzi JL, Rigolin VH, et al. Multimodality Imaging in Evaluation of Cardiovascular Complications in Patients With COVID-19: JACC Scientific Expert Panel. Journal of the American College of Cardiology 76 (2020): 1345-1357.