Myocardial Fatty Acid-Glucose Fuel Balance as Target to Treat Cardiac Diseases
Article Information
Jan FC Glatz1*, Miranda Nabben1,2, Joost JFP Luiken1
1Department of Genetics and Cell Biology, Faculty of Health, Medicine and Life Sciences, Maastricht University, and Department of Clinical Genetics, Maastricht University Medical Center+, Maastricht, the Netherlands
2CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, the Netherlands
*Corresponding Authors: Jan FC Glatz, Department of Genetics and Cell Biology, FHML, Maastricht University, P.O. Box 616, 6200 MD Maastricht, the Netherlands
Received: 17 September 2020; Accepted: 24 September 2020; Published: 29 September 2020
Citation: Jan FC Glatz, Miranda Nabben, Joost JFP Luiken. Myocardial Fatty Acid-Glucose Fuel Balance as Target to Treat Cardiac Diseases. Cardiology and Cardiovascular Medicine 4 (2020): 584-590.
View / Download Pdf Share at FacebookAbstract
The currently most prevalent cardiac diseases, diabetic cardiomyopathy and hypertrophic heart failure, each associate with a chronic change in energy substrate utilization towards a single type of substrate, i.e., fatty acids or glucose, respectively. Recent experimental studies suggest that proper cardiac contractile performance is dependent on a finely tuned balance between the utilization of these two substrates. Furthermore, re-balancing myocardial fuel supply (fatty acids versus glucose) appears an effective treatment option in cardiac disease.
Keywords
Cardiometabolic disease; Cardio-myopathy; Cluster of differentiation 36; GLUT4; Metabolic modulation
Article Details
1. Cardiac disease and cardiac substrate metabolism
Most cardiac diseases are known to associate with marked changes in myocardial substrate utilization [1]. Moreover, in recent years compelling evidence has been published that cardiometabolic alterations can be a primary cause for ventricular contractile dysfunction and chronic cardiac disease. The latter notion applies to both diabetes-related heart failure (diabetic cardiomyopathy) and pressure overload-induced or hypertrophic heart failure, currently the two main types of myocardial dysfunction [2-4]. The majority of patients with diabetes develop cardiac dysfunction and eventually die from cardiovascular diseases. A hallmark in these patients is that fuel selection, which in the healthy heart, principally relies on the uptake of a mixture of (long-chain) fatty acids and glucose, shifts towards the utilization of merely fatty acids only. Such full dependence on fatty acids for myocardial energy provision is accompanied by excessive lipid storage in cardiac myocytes, which in turn, elicits contractile dysfunction [5, 6].
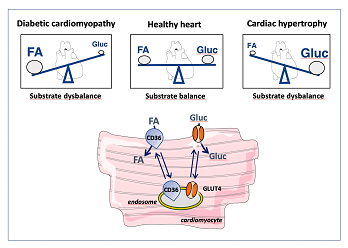
Pressure overload-induced heart failure is characterized by an impaired contractile function and a chronic fuel shift towards the predominant use of glucose. Conversely, several studies have reported that a primary substrate switch towards the predominant utilization of either fatty acids or glucose (for instance, as seen in case of an inborn error of metabolism) is accompanied with aberrant control of cardiac metabolism and cardiac contractile dysfunction [7, 8]. These observations indicate that chronic fuel shifts towards a single type of substrate are intimately linked with cardiac dysfunction, and suggest that interventions aimed at re-balancing such tilted energy substrate preference towards an appropriate mix of substrates may restore cardiac contractile function (reviewed in [9]) (Figure 1).
2. Rate-governing step in myocardial substrate utilization
Long-chain fatty acids and glucose are the main substrates for myocardial energy provision, with lactate, ketone bodies and amino acids being proper alternatives but under normal conditions contributing only to a minor extent [1]. The regulation of myocardial fatty acid and glucose metabolism has been studied in detail to reveal in both cases a pivotal role for the myocellular uptake process. Specifically, the rate-governing kinetic step in fatty acid utilization is CD36-mediated transsarcolemmal uptake. Regulation of the rate of fatty acid uptake is accomplished by recycling of the membrane protein CD36 between an intracellular storage pool (endosomes) and the sarcolemma, which process is affected by, for instance, changes in contraction and changes in the presence of insulin [10].
Similarly, glucose uptake by cardiomyocytes is dependent on the presence of glucose transporters GLUT1 and GLUT4 in the sarcolemma. While GLUT1 is constitutively present in the sarcolemma to maintain basal uptake rates, GLUT4 recycles between endosomes and the sarcolemma thereby increasing and adjusting the rate of glucose uptake to desired levels [11, 12]. As a result, cardiac fatty acid and glucose utilization are determined largely by the presence in the sarcolemma of membrane proteins CD36 and GLUT4, respectively (Figure 1).
Figure 1: Schematic presentation of the association of cardiac disease with a tilted fatty acid–glucose substrate balance. In the healthy heart, the contributions from (long-chain) fatty acids and glucose to energy provison are similar, but in diabetic cardiomyopathy are shifted towards fatty acids, and in cardiac hypertrophy towards glucose. Myocellular uptake of fatty acids and of glucose is governed by the presence of substrate transporters CD36 and GLUT4, respectively, which recycle between intracellular storage sites (endosomes) and the sarcolemma, as illustrated in the lower panel. FA, fatty acid; Gluc, glucose.
3. Membrane substrate transporters and cardiac disease
Given the intimate link between chronic alterations in cardiac fuel selection and cardiac disease, and the pivotal role of substrate transporters CD36 and GLUT4 in the regulation of the rate of fatty acid and glucose uptake, as outline above, it can be inferred that in cardiac disease the intracellular CD36 and GLUT4 distribution is affected, and that, furthermore, CD36 and/or GLUT4 recycling within the cardiomyocytes may form a suitable target for so-called metabolic modulation therapy, aimed at re-balancing the substrate preference of the heart in order to to restore its contractile performance. Many examples underscore this concept, mostly obtained from studies with experimental animals or with pluripotent stem cell-derived human cardiomyocytes, and hold promise for future application in patients.
A prominent example is the consumption of a high fat-containing (Western) diet and the often resulting obesity, in which condition the heart is subject to excess lipid supply. Such oversupply elicits a shift in myocardial energy provision towards an increased utilization of fatty acids at the expense of glucose [13, 14]. In experimental animal studies it has been established that this substrate switch is initiated by a rapid (within days) net tanslocation of CD36 from endosomes to the sarcolemma, which then leads to a concomitant increase in the rate of fatty acid uptake followed by a cascade of events leading to myocellular lipid accumulation, mitochondrial dysfunction, insulin resistance and contractile dysfunction [15, 16]. Absence of CD36, as is seen in null mice [17] but also in selected patients with a CD36 gene mutation [18], or blocking its activity by anti-CD36 antibodies [19], prevents all of these metabolic changes while the contractile function is maintained.
A second example is sustained pressure overload, which also leads to changes in myocardial metabolism and function. It has been documented that the first change seen is a marked increase in glucose utilization, at the expense of fatty acids, which precedes the development of left ventricular hypertrophy and contractile dysfunction [20]. This shift towards increased glucose utilization is accompanied by an increased presence of GLUT4 at the sarcolemma [21]. Selective downregulation of GLUT4 translocation in a cell model of cardiac hypertrophy [A. Sun, M. Nabben and J. Luiken, unpublished observations] or feeding a high fat-containing diet to rodents with experimentally induced cardiac hypertrophy [22] in each case elicited normalization of glucose utilization (accompanied with normalized, i.e., increased, fatty acid utilization) together with the recovery of myocardial contractile function.
4. Concluding remarks
In this short review we have outlined that energy substrate metabolism is an important parameter determining proper contractile function of the heart, and that chronic changes in substrate selection, in particular with respect to the contribution of fatty acids and glucose to myocardial energy provision, appear inseparably linked to the development of cardiac disease, and vice versa. The corollary is that a chronically altered myocardial substrate preference can be applied as early readout parameter for the development of cardiac diseases. Monitoring of substrate preference in patients could be performed by state-of-the-art magnetic resonance imaging [23].
The observations discussed in this review also indicate that the heart performs optimally when utilizing a certain mixture of fatty acids and glucose, with the notion that consuming either too little or too much of either substrate is detrimental [24]. The reason for the requirement of such balanced mixture of metabolic substrates is not known but may relate to the need of both substrates to feed subsidiary metabolic pathways (e.g., anaplerosis). Additionally, both substrates may be utilized for post-translational modification of cellular proteins thereby markedly influencing the functioning of these proteins. Finally, a mixture of substrates will help to avoid a condition of excess intracellular fatty acids (or glucose) as that would increase the risk for lipotoxicity (or glucotoxicity) [9].
Importantly, these insights provide a basis for therapy to treat cardiac diseases. Modulation of cellular energy substrate preference can be achieved by intervention in the rate-governing steps of myocardial fatty acid and glucose utilization. In the past decade the latter have been disclosed in much detail, and found to comprise membrane protein-mediated substrate uptake involving CD36 for fatty acids and GLUT4 for glucose. Studies in experimental animal models and in pluripotent stem cell-derived human cardiomyocytes have provided the first indication that applying CD36 and/or GLUT4 as target for metabolic modulation approaches is an effective strategy to re-balance myocardial substrate preference [22, 25, 26]. Now that the pivotal roles of CD36 and GLUT4 have been confirmed in patient studies [11, 18, 27], manipulating their sarcolemmal presence should be explored as treatment target for cardiac diseases also in the human setting. For this, focus should be on manipulating the subcellular recycling machinery of these membrane transporters, because the recycling of each transporter involves several specific trafficking proteins. These specific trafficking proteins could be targeted to rectify cardiac substrate uptake during cardiac diseases. Preliminary observations underscore the feasibility of such approach [28].
Acknowledgements
MN was supported by the Dutch Heart Foundation, Dekker grant # 2019T041.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85 (2005): 1093-1129.
- Wende AR, Brahma MK, McGinnis GR, et al. Metabolic origins of heart failure. JACC: Basic Transl Sci 2 (2017): 297-310.
- Matsuura TR, Leone TC, Kelly DP. Fueling cardiac hypertrophy. Circ Res 126 (2002): 197-199.
- Tan Y, Zhang Z, Zheng C, et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol 17 (2002): 585-607.
- Borradaile NM, Schaffer JE. Lipotoxicity in the heart. Curr Hypertens Rep 7 (2005): 412-417.
- Athithan L, Gulsin GS, McCann GP, et al. Diabetic cardiomyopathy: Pathophysiology, theories and evidence to date. World J Diabetes 10 (2019): 490-510.
- Guertl B, Noehammer C, Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol 81 (2000): 349-372.
- Das AM, Steuerwald U, Illsinger S. Inborn errors of energy metabolism associated with myopathies. J Biomed Biotechnol (2010): 1-19.
- Glatz JFC, Nabben M, Young ME, et al. Re-balancing cellular energy substrate metabolism to mend the failing heart. Biochim Biophys Acta 1866 (2002): 165579.
- Glatz JFC, Luiken JJFP. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res 59 (2018): 1084-1093.
- Klip A, McGraw TE, James DE. Thirty sweet years of GLUT4. J Biol Chem 294 (2019): 11369-11381.
- Bertrand L, Auquier Renguet E, Angé M, et al. Glucose transporters in cardiovascular system in healh and disease. Eur J Physiol 472 (2002): 1385-1399.
- Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocrinol Metabol Dis 11 (2010): 31-39.
- Ouwens DM, Diamant M, Foddor M, et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 50 (2007): 1938-1948.
- Bonen A, Jain SS, Snook LA, et al. Extremely rapid increase in fatty acid transport and intramyocellular lipid accumulation but markedly delayed insulin resistance after high fat feeding in rats. Diabetologia 58 (2015): 2381-2391.
- Liu Y, Steinbusch LKM, Nabben N, et al. Palmitate-induced vacuolar-type H+-ATPase inhibition feeds forward into insulin resistance and contractile dysfunction. Diabetes 66 (2017): 1521-1534.
- Sung MM, Koonen DP, Soltys CL, et al. Increased CD36 expression in middle-aged mice contributes to obesity-related cardiac hypertrophy in the absence of cardiac dysfunction. J Molec Med 89 (2011): 459-469.
- Tanaka T, Nakata T, Oka T, et al. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res 42 (2001): 751-759.
- Angin Y, Steinbusch LKM, Simons PJ, et al. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Bioch J 448 (2012): 43-53.
- Li J, Kemp BA, Howell NL, et al. Metabolic changes in spontaneously hypertensive rat hearts precede cardiac dysfunction and left ventricular hypertrophy. J Am Heart Assoc 8 (2019): e010926.
- Geraets IME, Glatz JFC, Luiken JJFP, et al. Pivotal role of membrane substrate transporters on the metabolic alterations in the pressure-overloaded heart. Cardiovasc Res 115 (2019): 1000-1012.
- Steinbusch LKM, Luiken JJFP, Vlasblom R, et al. Absence of fatty acid transporter CD36 protects against Western-type diet-related cardiac dysfunction following pressure overload in mice. Am J Physiol-Endocrinol Metab 301 (2011): 618-628.
- Gropler RJ, Beanlands RSB, Dilsizian V, et al. Imaging myocardial remodeling. J Nucl Med 51 (2010): 88-101.
- Kerr M, Dodd MS, Heather LC. The ‘Goldilocks zone’ of fatty acid metabolism; to ensure that the relationship with cardiac function is just right. Clin Sci 131 (2017): 2079-2094.
- Yang J, Sambandam N, Han X, et al. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res 100 (2007): 1208-1217.
- Dirkx E, van Eys GJ, Schwenk RW, et al. Protein kinase-D1 overexpression prevents lipid-induced cardiac insulin resistance. J Molec Cell Cardiol 76 (2014): 208-217.
- Melis M, Carta G, Pintus S, et al. Polymorphism rs1761667 in the CD36 gene is associated to changes in fatty acid metabolism and circulating endocannabinoid levels distinctively in normal weight and obese subjects. Front Physiol 8 (2017): 1006.
- Wang S, Wong L-Y, Neumann D, et al. Augmenting vacuolar H+-ATPase function prevents catrdiomyocytes from lipid-overload induced dysfunction. Int J Molec Sci 21 (2002): 1520.