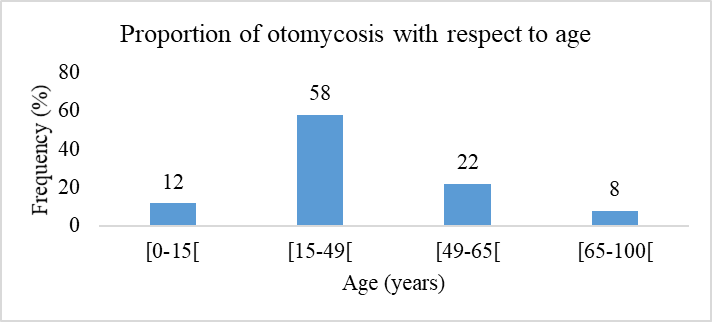Mycologic Epidemiology and Antifungal Susceptibility Patterns of Otomycosis in Yaounde, Cameroon: A Cross Sectional Study Revealing Candida albicans Dorminance and Nystatin Sensitivity
Article Information
Stanley Nkemnji Awungafac*, 1, Laure Ngando Moudoute2, Elvis Tambe Akem3, Paul Nkemtendong Tolefac4, Alexis Ndjolo5
1Pasteur Center of Cameroon, Cameroon
2Department of Microbiology, Faculty of Medicine and Biomedical Sciences, University of Yaounde I, Yaounde, Cameroon
3Epicentre, Paris
4Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Yaoundé, Cameroon
5Centre International de Recherche Chantal Biya (CIRCB) de Yaoundé, Cameroon.
*Corresponding author: Stanley Nkemnji Awungafac, Pasteur Center of Cameroon, Cameroon.
Received: 11 January 2024; Accepted: 19 January 2024; Published: 03 February 2024
Citation: Awungafac SN, Ngando ML, Akem, ET, Tolefac PN, & Ndjolo A. Mycologic epidemiology and antifungal susceptibility patterns of otomycosis in Yaounde, Cameroon: A cross-sectional study revealing Candida albicans dominance and nystatin sensitivity. Fortune Journal of Health Sciences. 7 (2024): 37-43.
View / Download Pdf Share at FacebookAbstract
Introduction: Otomycosis, or fungal otitis externa, has typically been described as fungal infection of the external auditory canal, with infrequent complications involving the middle ear. With an estimated global prevalence of 10%, a wide range of pathogens are implicated, which could have a significant impact on treatment outcomes in immunosuppressive conditions and the concurrent use of topical antibiotics/steroid preparations. Considering the limited data on this disease in Cameroon and the impact of various factors, including significant population growth and change, climate variations, and an increase in the number of patients, the epidemiology of otomycosis may have undergone substantial alterations over the years. There is therefore a need to characterize the mycologic epidemiology and susceptibility patterns for better treatment outcomes.
Objectives: We aimed to describe the mycological profiles and antifungal susceptibility patterns in patients with otomycosis in Yaounde, as well as to investigate potential factors associated with otomycosis in Cameroon.
Materials and Methods: We carried out a cross-sectional study on patient samples received between November 2020 to August 2021 in Yaounde University Teaching Hospital (CHUY) and Yaounde Central Hospital (HCY). Patients’ socio-demographic and clinical data were obtained. Samples were analyzed for their mycologic profiles using Sabouraud-chloramphenicol agar and antifungal susceptibility testing was carried out using the Disk and macrodilution in liquid mileu methods. Statistical analyses were carried out using SPSS version 23.0, and p-values <0.05 considered statistically significant. Results: The following fungi were isolated: Candida albicans (38%), Aspergillus niger (26%), Candida spp (22%), and Aspergillus fumigatus (14%). Nystatin demonstrated high sensitivity to most tested microorganisms, with 86% exhibiting sensitivity. Swimming and excessive ear cleaning were identified as the only two factors associated with otomycosis.
Conclusion: The most predominant fungus isolated was Candida albicans. Nystatin exhibited the highest sensitivity among other antifungals. Factors associated with otomycosis included swimming and excessive ear cleaning.
Keywords
Otomycosis, Mycological profiles, Antifungal Susceptibility patterns
Article Details
1. Introduction
Otomycosis or fungal otitis externa has typically been described as a fungal infection of the external auditory canal, auricle, and less frequently the middle ear [1]. It is estimated that approximately 10% of the world’s population will develop otitis externa at some point in their life [2,3]. A vast array of pathogens can lead to otitis externa amongst which are most commonly bacteria, fungi, and other pathogens. Fungal otitis externa (otomycosis) remains a prominent cause of otitis externa with less classical treatment options as the more common bacterial infections. Globally, the prevalence of otomycosis varies greatly by geographical region, sex, and age amongst other factors with a current global prevalence ranging between about 9-25% [4]. Although this disease is rarely life-threatening, it can present a challenging and frustrating situation for the otologist and patients due to long-term treatment and high rate of recurrence with some studies reporting recurrence rates up to about 7.3% [5]. Common complications include hearing loss, tympanic membrane perforations, and invasive temporal bone infection.
Several predisposing factors have been described, including humid climate, presence of cerumen, instrumentation of the ear, increased use of topical antibiotics/steroids preparations, immunocompromised host, patients who have undergone open cavity mastoidectomy, and those wearing hearing aids with occlusive ear molds [6]. In Sub-Saharan Africa, the prevalence of otomycosis is even higher at 39.6%, as described by several studies, and is favored by several factors such as the increase in number of immunocompromised patients, the humid climate and increased antibiotic and steroid abuse [7]. Few studies however have been carried out in Cameroon to date on this subject. In 1996, Lohdoue et al described a 6.09% prevalence in Yaoundé [8] with very little updates carried out on this subject in the country. We set an objective to describe the mycological profile and antifungal susceptibility testing in patients with otomycosis in Yaounde.
2. Methodology
We carried out a cross-sectional study from November 2020 to August 2021. The study sites included Yaounde University Teaching Hospital (CHUY) and Yaounde Central Hospital (HCY) where samples were collected and analyzed. The study population was a pool of samples at the study sites. Samples with incomplete information such as identification were excluded. One hundred samples were obtained through convenient sampling.
2.1 Study materials
2.1.1 Specimen collection material
- Sterile cotton swab
- Sterile distilled water
2.1.2 Materials for mycologic analysis
- Sabouraud + chloramphenicol culture medium
- Petri dishes, cotton blue, and other Laboratory reagents, equipment, and personnel
- Optic microscope
- Antifungal disks
2.2 Procedure
2.2.1 Specimen collection
- Ear specimens were collected from the external auditory canal using sterile cotton after signing an informed consent form at the ENT (Ear, Nose, and Throat) services of HCY and CHUY
- Specimens were collected in duplicate for each ear.
- The specimens were immediately transported to the bacteriology laboratory of CHUY for analysis within two hours. The macroscopic exam was done to know the ordour, colour and consistency.
- Afterwards, the specimens were inoculated on Sabouraud-chloramphenicol
2.2.2 Inoculation and incubation
- Specimen from the two ear was inoculated on the culture medium Sabouraud-chloramphenicol which had initially undergone sterility checks.
- Inoculation was done by gentle streaks on the surface of the sabouraud dextrose agar.
- After inoculation, the inoculated culture media were incubated at 37° for 1 to 5 days.
- After incubation collect for the wet mount
2.3 Wet mount
The specimen collected with the cotton swab was mounted on a new glass slide containing a drop of sterile normal saline. The mounted slide was examined with low magnification. The morphologic characteristics of the fungus, if present are recorded; presence of hyphae, and conidia distribution amongst others.
2.3.1 Observation of culture growth
- Each day, the incubated media will be analyzed macroscopically for growth
- Specimens that produce growth were described (color, texture, borders)
- Specimens that fail to produce growth within 5 days were considered negative and discarded
2.3.2 Microscopy of growth colonies
- Two drops of Lactophenol Cotton Blue were placed on the slide.
- Two colonies of fungal culture were added to the drops of Lactophenol Cotton Blue.
- The fungal colony and lactophenol cotton blue were mixed using forceps. A coverslip was placed on top of the preparation. The mixture was observed under the microscope at objective 40X. When yeasts were observed the test of filamentation was done, when the filamentous fungus was observed, its image was correlated with those in the reference atlas for identification.
2.3.3 Germ tube tests for identification of yeasts
- The Germ tube test was used to identify yeast isolates. One colony of the yeast isolated from the culture was incubated in 5ml animal serum for 4 hours at 37°C.
- After incubation, microscopy of a drop of the incubated mixture was done at low magnification
- Microscopy that reveals germ tubes were considered positive for C. albicans. A germ tube is an appendage that is half the width and 3-4 times the length of the yeast cell that produced it. There is no point of constriction at the origin of the germ tube from the yeast cell.
2.3.4 Antifungal Susceptibility Testing
The antifungal susceptibility was done by the disc diffusion method for yeasts following the CASFM 2021 method. The macro-dilution method in liquid milieu was used for other species of filamentous fungi following the procedure described [44]. The antifungal susceptibility was determined by the disc diffusion method on an agar medium. The fungal inoculum was prepared from 24-hour culture and the turbidity was adjusted to 0.5McFarland standard which corresponds to a suspension of approximately 1 to 2×108 CFU/ml. Sabouraud chloramphenicol agar plates were then inoculated with a swab. Fifteen minutes later, the antifungal discs were deposited on the surface of the agar. The whole was left at room temperature for 15 minutes for pre-diffusion and then incubated at 37°C for 24 to 72 hours. Inhibition zones were measured using calipers.
As regards the determination of MIC, 1ml of Sabouraud broth + chloramphenicol was introduced into six test tubes, afterward a stock solution of 139.2ug/ml of antifungal was prepared, and a series of double dilution was done in the culture medium until obtention of a series of concentration varying from 69.6ug/ml to 2.17ug/ml. Next, 1ml of inoculum prepared at 1 to 2×108 CFU/ml was introduced into each tube until obtention of a series of concentrations of antifungal ranging from 34.8ug/ml to 1.08ug/ml. Simultaneously, one case-control tube containing Sabouraud and the inoculum and a negative case-control containing only the culture media were prepared under the same conditions. The tubes were incubated for 24-72 hours at 37°C. The Minimum Inhibitory Concentration (MIC) corresponds to the least concentration of antifungal capable of inhibiting all visible growth was determined after adding almar blue. Alamar Blue reduction may signify an impairment of cellular metabolism and is not necessarily specific to the interruption of electron transport and mitochondrial dysfunction. This change from oxidized to reduced state allows flexibility of detection where measurements can be quantitative as colorimetric and/or fluorometric readings (the latter being more sensitive) or qualitative as a visible change in color indicating the presence or absence of viable cells [45].
2.4 Data entry and statistical analysis
Data was collected in Microsoft Excel 2016 and analyzed using SPSS version 23. Continuous variables were described with measures of central tendency (mean, median, mode) and dispersion measures (variance and standard deviation) stating their 95% confidence intervals. Qualitative variables were described with frequencies and proportions and differences between proportions, verified using the independent Chi-square test. P-values of <0.05 were considered statistically significant.
2.4.1 Ethical considerations
The research proposal was submitted to the Institutional Review Board of the Faculty of Medicine and Biomedical Sciences, for an ethical clearance. Research authorization was obtained from the directorates of the institutions.
3. Results
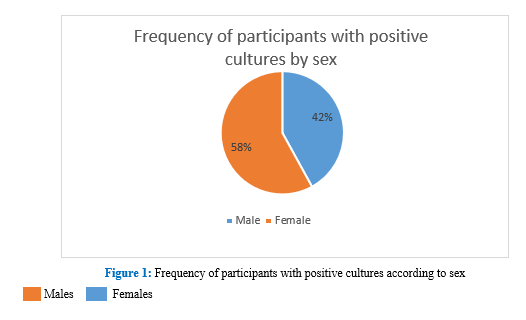
Samples were taken from 100 participants, of whom 50 samples showed positive cultures for fungi. Of the 50 patients with otomycosis, 58.0% (29/50) were female,
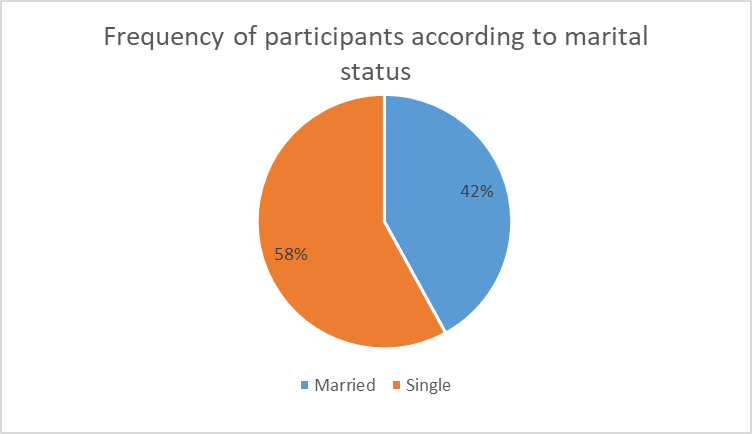
The distribution of patients according to marital status showed that 42% of patients were married and 58% were single, Figure 2.
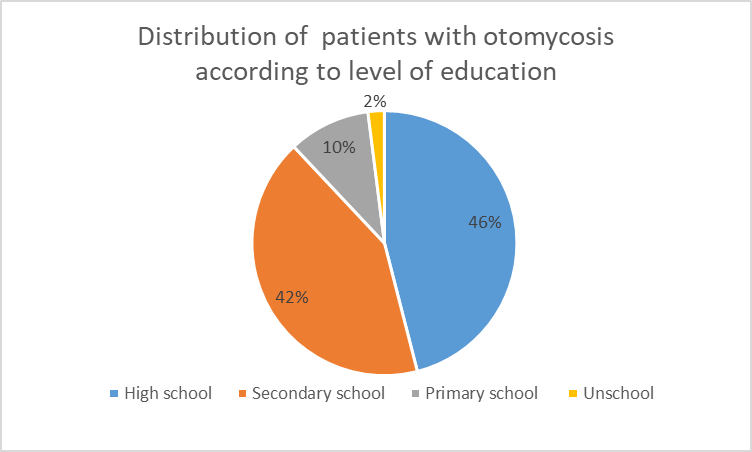
The distribution of patients with otomycosis according to level of education (Figure 3), showed that patients with high school education were the most infected (46%) followed by patients with secondary education (42%) and patients with primary education (10%). Only 2% were unschooled.
Table I represents the proportion of patients who had clinical features versus age. Pruritus was the most common symptom, followed by otalgia. There was predominance of clinical features of patients between 15 and 19 years of age.
Table 1: Frequency of symptoms by age (years)
|
[0-15] |
[15-49] |
[49-65] |
[65-100] |
Total |
|
|
Pruritus |
5(12%) |
27(66%) |
6(15%) |
3(7%) |
41(100%) |
|
Otorrhoea |
0(0%) |
6(75%) |
2(25%) |
0(0%) |
8(100%) |
|
Tinnitus |
0(0%) |
7(54%) |
4(31%) |
2(15%) |
13(100%) |
|
Otalgia |
3(8%) |
23(59%) |
9(23%) |
4(10%) |
39(100%) |
|
Biting sensation in the ear |
1(11%) |
7(78%) |
1(11%) |
0(0%) |
9(100%) |
|
Hearing loss |
0(0%) |
6(86%) |
1(14%) |
0(0%) |
7(100%) |
|
Aural fullness |
1(8%) |
6(50%) |
2(17%) |
3(25%) |
12(100%) |
Figure 4 shows the proportion of otomycosis by age. Patients aged between 15 and 49 years represented over half (58%) followed by 49-65 age group.
3.1 Mycologic profile in participant with otomycosis
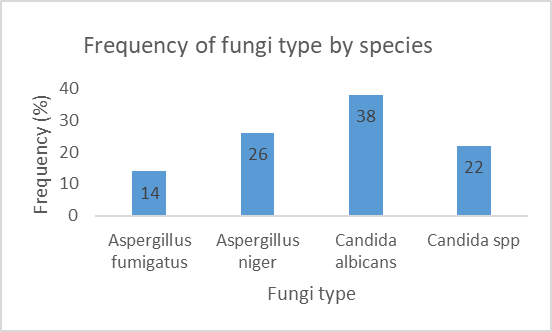
Figure 5 shows the mycologic profile of participants in the study. Candida albicans was the most predominant fungi isolated (38%).
3.2 Antifungal susceptibility profiles of fungi isolated from patients with otomycosis
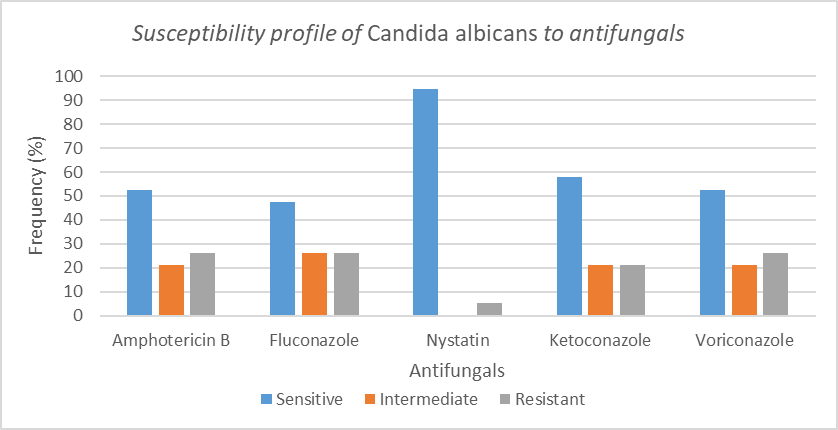
The antifungal susceptibility profile was done on all the specimens to ascertain their clinical categorization. Figure 6 represents the antifungal susceptibility of Candida albicans to the various antifungals tested.
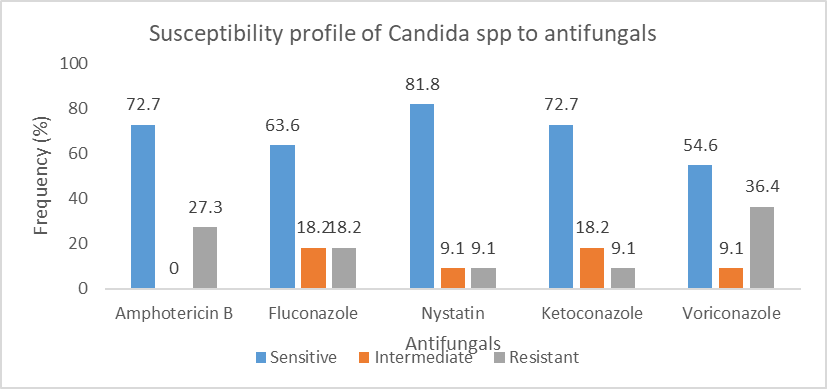
Figure 7 shows the susceptibility of candida species to the antifungals tested. Candida spp most sensitive to nystatin.
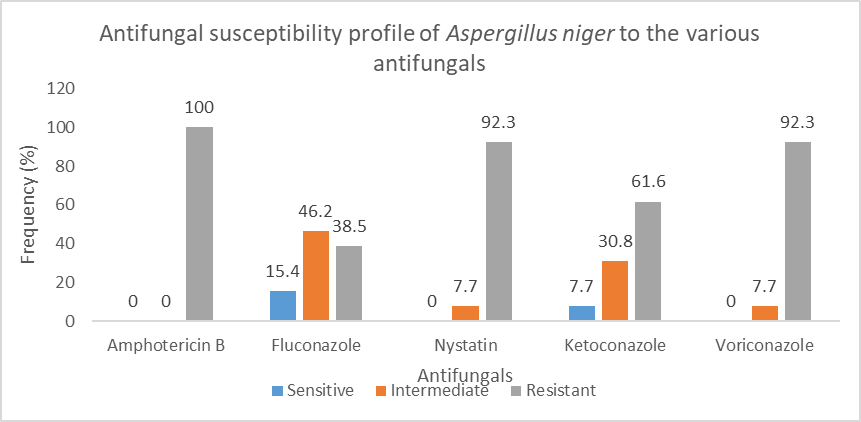
Figure 8 shows the antifungal susceptibility profile of Aspergillus niger to the various antifungals tested. Aspergillus was 100% sensitive to Amphotericin B.
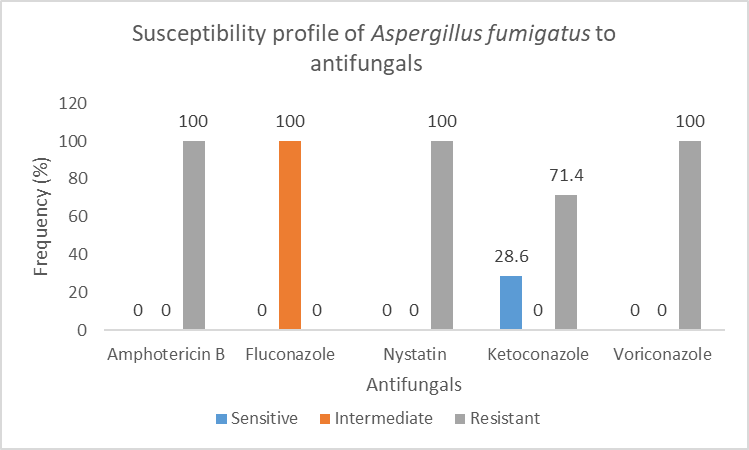
Figure 9 shows the antifungal susceptibility profile of Aspergillus fumigatus to the antifungals tested.
3.3 Potential factors associated with otomycosis infection
Table II shows a statistically significant association between swimming, excessive cleaning of ears, and otomycosis. This means that people who engage in swimming and excessive cleaning of the ears are more likely to develop otomycosis than people who do not.
Table 2: Study of the association between risk factors and otomycosis
|
Swimming |
Excessive cleaning of ears |
Prolong use of topical antibiotics |
Use of earphone |
|
|
Presence of otomycosis |
30 (30%) |
45 (45%) |
45 (45%) |
39(39%) |
|
Absence of otomycosis |
7(7%) |
25(25%) |
40 (40%) |
37 (37%) |
|
P value |
<0,0001 |
<0,0001 |
0,2623 |
11,200 |
4. Discussion
The main fungi isolated in these centres were Candida albicans 38%, Aspergillus niger 26%, Candida spp 22%, and Aspergillus fumigatus 14%. The findings were different from those reported in several other countries in terms of predominant fungi causing otomycosis. In Germany, Khaled et al reported the most common species to be Aspergilus niger (41%), Aspergilus flavus (30%) and Candida albicans only made up about 3% [46]. Still in Europe, Turkey, Ozcan et al reported in one study the most common fungi to be Aspergilus niger (44.8%) [47]. Studies in south America, Asia are similar to those in Europe. They showed Aspergilus fumigatus and Aspergilus niger as predominating organisms causing ear infections [23,48,49]. In Nigeria, Fasunla et al in their study showed that the predominant organisms were Aspergilus niger and Aspergilus fumigatus at 48.36% and 33.96% respectively [50]. As shown in these studies, the results vary from country to country. The difference in results between countries could be explained by the differences in the ecological system, hence the importance to always do an antifungal sensitivity test. Also, in one recent Brazilian study, the most frequently isolated species were C. albicans (30%), C. parapsilosis (20%), A. niger (20%), A. flavus (10%), A. fumigatus (5%), C. tropicalis (5%), Trichosporon asahii (5%) and Scedosporium apiospermum (5%) [51]. This is similar to the results of the present study.
4.2 Antifungal susceptibility testing
This study showed that nystatin was highly sensitive to most microorganisms tested with 86.6% of microorganisms being sensitive, followed by 46%, 36%, 36% for ketoconazole, amphotericin B and fluconazole respectively. Meanwhile, high resistance was noticed with voriconazole and amphotericin B at 58% and 56% respectively. In a 2020 study done in Iran, the most sensitive antifungal was terbinafine which has great activity whereas Fluconazole has low activity and 77.8% of candida were resistant to Caspofungin [52]. These antifungi were not routinely tested in our study. In a study in Germany by Ali et al, molds isolates had highest susceptibility to voriconazole whereas yeast highest sensitivity to Nystatin (88.24%) followed by Amphotericin B (82.35%) [46]. The results of sensibility to nystatin are similar to the present study. In this same study yeast was 100% resistant to terbinafine and 94.12% resistant to itraconazole [46]. The resistances are different from those described in this present study. The difference could be explained by local antifungal policies and local ecology.
4.3 Clinical presentation and associated factors
In the index study, otomycosis was more common between 15-49 years followed by 49 - 65 years than 0 - 15 years with prevalence of 58%, 22% and 12% respectively. While some series have reported otomycosis to be more common in children, in this study otomycosis was more common between adults compared to children. The results in this study are similar to those described in Iran by Prasad et al. [49]. This could be explained by the fact that adults spent more time outside and, in the farm, hence more likely to get in contact with these air-containing pathogens. In this series, these ear infections had varied clinical presentations with the most common presentations being pruritus (80%), otalgia (26%), tinnitus (26%), aural fullness (24%), and otorrhea (16%). The findings are similar to those published by Prasad et al [49] in Iran and Musa et al in Nigeria [53]. In a univariate analysis, factors associated to otomycosis included swimming and excessive ear cleanings. These factors were similar to those described by Prasad et al [49] in Iran. This could be explained by the fact that obsessive manipulation of the external ear with any hard objects such as wooden sticks or earphones to clean the ear of wax and vigorous rubbing of the ears for relief from itching (in case of otalgia due to eustachian catarrh, serous otitis, or acute otitis media) is a common practice. These practices may cause trauma (usually minor hence unnoticed) to the skin of external auditory canal and deposition of fungal conidia in the wound leading to fungal infection. Further, earphones are usually shared amongst populations hence increasing the risk of contamination.
5. Conclusion
The diagnosis of otomycosis is based on a high index of suspicion. Culture is an indispensable arm for confirmatory diagnosis and specific treatment options. Candida albicans was the most predominant followed by Aspergillus fumigatus and candida species. Nystatin was highly sensitive to most microorganisms tested, followed by ketoconazole, amphotericin B, and fluconazole respectively. Among predisposing factors were excessive cleaning of ears, and swimming.
Study Limitations
This study may not be completely representative of Cameroon, though the study area, Yaounde, receives patients coming from diverse zones. Hence, a larger study is recommended to get a broader representation. We recommend further studies be done with more advanced techniques that are more precise and accurate.
References
- Kamali Sarwestani H, Daie Ghazvini R, Hashemi SJ, Rezaie S, Gerami Shoar M, Mahmoudi S, et al. Investigation of Etiologic Agents and Clinical Presentations of Otomycosis at a Tertiary Referral Center in Tehran, Iran. Iran J Public Health 48 (2019): 331-7.
- Wiegand S, Berner R, Schneider A, Lundershausen E and Dietz A. Otitis Externa. Deutsches Arzteblatt international 116 (2019): 224-234.
- Hajioff D, MacKeith S. Otitis externa. BMJ Clin Evid 15 (2015).
- Dekhil K. Presentation pattern and fungal agents spectrum causing Otommycosis. Asian Journal of Pharmaceutical and Clinical Research 11 (2018): 408.
- Kiakojori K, Bagherpour Jamnani N, Khafri S, Mahdavi Omran S. Assessment of Response to Treatment in Patients with Otomycosis. Iran J Otorhinolaryngol 30 (2018): 41-7.
- Anwar K, Gohar M S. Otomycosis; clinical features, predisposing factors and treatment implications. Pakistan journal of medical sciences 30 (2014): 564-567.
- Fayemiwo SA, Ogunleye VO, Adeosun AA, Bakare RA. Prevalence of otomycosis in Ibadan: a review of laboratory reports. Afr J Med Med Sci 39 (2010): 219-22.
- Lohoue Petmy J, Bengono Touré G, Founda Onana A. Etude des otomycoses à Yaoundé. Rev Laryngol Otol Rhinol (Bord) 117 (1996): 119-21.
- Prakash SB, Leelatejaswini RM, Deekshita V. A clinical and microbial study of otomycosis: an original study. Journal of Evolution of Medical and Dental Sciences 4: 12376-85.
- Abdelazeem M, Gamea A, Mubarak H, Elzawawy N. Epidemiology, causative agents, and risk factors affecting human otomycosis infections. Turkish Journal of Medical Sciences 45 (2015): 820-826.
- Hansen John. Netter’s Clinical Anatomy. 4th Edition. Amsterdam Elsevier. 13th December (2017).
- Reena A Bhatt, Mary L Windle. Ear Anatomy: Overview, Embryology, Gross Anatomy 02 January (2020).
- Babak A, Mark M, Calvin J, Guy M, Rebecca F. Master Techniques in Facial Rejuvenation. 2nd ed. Elsevier (2017): p368.
- Putz R, Pabst R. Sobotta. Atlas of Human Anatomy one volume edition: Head, Neck, Upper Limb, Thorax, Abdomen, Pelvis, Lower Limb. 14th edition. München: Churchill Livingstone (2009): 832.
- Swibel Rosenthal LH, Patadia MO and Stankiewicz JA. Otolaryngology: A Color Handbook. 1st edition. CRC Press (2016).
- Rosenfeld RM, Schwartz SR, Cannon CR, Roland PS, Simon GR, Kumar KA, et al. Clinical practice guideline: acute otitis externa executive summary. Otolaryngol Head Neck Surg 150 (2014): 161-8.
- Mattoo Da, Bhatia Dm. An observational study of the common aerobic flora of human cerumen in ghaziabad and its surroundings - a cross-sectional study. Global journal for research analysis 7 (2018): 3.
- Prasanna V, Edwin B, Kannan I. Isolation of bacteria from normal external auditory canal. Int J Med Res Rev 3 (2015): 597-00
- Shilpa R, Swamy M. Itchy Ears: Evaluation of Predisposing Factors and Treatment Outcomes 29 (2021): 66-71
- Viswanatha B & Naseeruddin K. Fungal infections of the ear in immunocompromised host: a review. Mediterranean journal of hematology and infectious diseases 3 (2011): e2011003.
- Prabhakaran P, Navin N, Srinivasan R, et al. A comparative study of efficacy of clotrimazole and fluconazole ear drops in otomycosis. J. Evolution Med. Dent. Sci 7 (2018): 2058-2061
- Kozel TR & Wickes B. Fungal diagnostics. Cold Spring Harbor perspectives in medicine 4 (2014): a019299.
- Agarwal P, Devi LS. Otomycosis in a Rural Community Attending a Tertiary Care Hospital: Assessment of Risk Factors and Identification of Fungal and Bacterial Agents. J Clin Diagn Res 11: DC14-8.
- Nazeer H, Mohiddin S, Pakanati S. MYCOLOGY OF OTOMYCOSIS IN A TERTIARY CARE TEACHING HOSPITAL. J Res Med Den Sci 3 (2015): 27.
- Borlingegowda V, Sumatha D, Vijayashree M. Otomycosis in Immunocompetent and Immunocompromised Patients: Comparative Study and Literature Review. Ear, nose, & throat journal 91 (2012): 114-21.
- Lageju N, Shahi S, Goil N. Clinical profile of Otomycosis: a hospital-based study at central terrain region of Nepal. Janaki Medical College Journal of Medical Science 3 (2016): 20.
- Pradhan B, Tuladhar N, Amatya R. Prevalence of Otomycosis in Outpatient Department of Otolaryngology in Tribhuvan University Teaching Hospital, Kathmandu, Nepal. The Annals of otology, rhinology, and laryngology 112 (2003): 384-7.
- Kotigadde, Subbannayya, Shekhar, Manisha, Thada, Nikhil, et al. Primary Otomycosis in the Indian Subcontinent: Predisposing Factors, Microbiology, and Classification. International journal of microbiology (2014): 636493.
- Koltsidopoulos P, Skoulakis C. Otomycosis with Tympanic Membrane Perforation: A Review of the Literature. Ear, Nose & Throat Journal 99 (2020): 518-521.
- Opperman CJ, Copelyn J. Aspergillus niger otomycosis in a child with chronic otitis externa. Southern African Journal of Infectious Diseases 35 (2020): 3.
- Ameye S, Adeyemo A, Eziyi J, Amusa Y. Clinical Profile of Otomycosis in a Sub-saharan African Tertiary Health Center. Otorhinolaryngology Clinics an International Journal 10 (2018): 52-5.
- Suchit R, Sindhu V Nath, Harilal P. Otomycosis in chronic otitis media. Journal of evidence based medicine and healthcare (2017): 95576.
- Singh, Mandeep, Mittal, Nalini, Kakkar, Manish, et al. Otomycosis: A Clinicomycologic Study. Ear, nose, & throat journal 79 (2000): 606-9.
- Sugui JA, Kwon-Chung KJ, Juvvadi PR, Latgé JP and Steinbach WJ. Aspergillus fumigatus and related species. Cold Spring Harbor perspectives in medicine 5 (2014): a019786.
- Latgé J-P. Aspergillus fumigatus and Aspergillosis. Clin Microbiol Rev 12 (1999): 310-50.
- Low C-Y, Rotstein C. Emerging fungal infections in immunocompromised patients F1000 Med Rep 3 (2011).
- Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 63 (2016): e1-e60.
- Taheri-Talesh N, Horio T, Araujo-Bazán L, Dou X, Espeso EA, Peñalva MA, et al. The tip growth apparatus of Aspergillus nidulans. Molecular biology of the cell 19 (2008): 1439-1449.
- Kashem SW, Kaplan DH. Skin Immunity to Candida albicans. Trends in immunology 37 (2016): 440-450.
- Kabir MA, Hussain MA, Ahmad Z. Candida albicans: A Model Organism for Studying Fungal Pathogens. ISRN microbiology (2012): 538694.
- Deepa A, Nair BJ, Sivakumar T, & Joseph AP. Uncommon opportunistic fungal infections of oral cavity: A review. Journal of oral and maxillofacial pathology: JOMFP 18 (2014): 235-243.
- Bhavan P, Rajkumar R, Subramanian R, Seenivasan DrC, Kannan S. Culture and Identification of Candida Albicans from Vaginal Ulcer and Separation of Enolase on SDS-PAGE. International Journal of Biology 18 (2009).
- McClary D. Factors Affecting the Morphology of Candida Albicans. Annals of the Missouri Botanical Garden 39 (1952): 137-164.
- Kamga H, Essama S, Grand Y, Tchuedji N, Boda M, Henri S, et al. In vitro Evaluation of Antifungal Activity of Virgin Coconut oil and White Palm Kernel Oil on Candida Species-Experimental Study. Microbiology Research Journal International 27 (2019): 1-9.
- Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel, Switzerland) 12 (2012): 12347-12360.
- Ali K, Hamed MA, Hassan H, Esmail A, Sheneef A. Identification of Fungal Pathogens in Otomycosis and Their Drug Sensitivity: Our Experience. International archives of otorhinolaryngology 22 (2018): 400-403.
- Ozcan KM, Ozcan M, Karaarslan A, Karaarslan F. Otomycosis in Turkey: predisposing factors, aetiology and therapy. J Laryngol Otol 117 (2003): 39-42.
- Aneja KR, Sharma C, Joshi R. Fungal infection of the ear: A common problem in the north eastern part of Haryana. International Journal of Pediatric Otorhinolaryngology 74 (2010): 604-7.
- SC Prasad, Subbannayya K, Manisha S, Nikhil Dinaker T, Prashanth P, et al. Primary Otomycosis in the Indian Subcontinent: Predisposing Factors, Microbiology, and Classification. International Journal of Microbiology (2014): 9.
- Fasunla J, Ibekwe T, Onakoya P. Otomycosis in western Nigeria. Mycoses 51 (2008): 67-70.
- Pontes ZBV da S, Silva ADF, Lima E de O, Guerra M de H, Oliveira NMC, et al. Otomycosis: a retrospective study. Braz j otorhinolaryngol 75 (2009): 367-70.
- Gharaghani M, Halvaeezadeh M, Ali Jalaee G, Taghipour S, Kiasat N, Zarei Mahmoudabadi A. Antifungal susceptibility profiles of otomycosis etiological agents in Ahvaz, Iran. Curr Med Mycol 6 (2020): 18-22.
- Musa TS, Bemu AN, Grema US, Kirfi AM. Pattern of otitis externa in Kaduna Nigeria. Pan Afr Med J 21 (2015).