Multifaceted Functions of Prokineticins in Reproductive Cancers and Proposed Associated Therapies
Article Information
Constance Collet1,2,3#, Roland Abi Nahed1,2,3#, Kevin Gemy1,2,3,4, Wael Traboulsi5, Nicolas Lemaitre1,2,3, Pierre-Adrien Bolze6,7, Pascale Hoffmann8, Mohamed Benharouga2,3,4, Nadia Alfaidy1,2,3*
#Authors contributed equally
1Institut National de la Santé et de la Recherche Médicale, Unité 1036, Grenoble, France
2University Grenoble-Alpes, 38000, Grenoble, France
3Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Biosciences and Biotechnology Institute of Grenoble, Grenoble, France
4Centre National de la Recherche Scientifique, Unité Mixte de Recherche 5249, Laboratoire de Chimie et Biologie des Métaux, Grenoble, France
5Georgetown University Medical Center, Pre-Clinical Science Building, GD01/office number, 3900 Reservoir Rd NW, Washington DC 20007
6University of Lyon 1, University Hospital Lyon Sud, Department of Gynecological Surgery and Oncology, France
7French Reference Center for Gestational Trophoblastic Diseases, University Hospital Lyon Sud, 165, Chemin du Grand Revoyet, 69495 Pierre Bénite, France
8University Hospital of Grenoble, Department of Obstetrics and Gynaecology, and Laboratoire d’Aide à la Procréation-CECOS, La Tronche, France
*Corresponding Author: Dr. Nadia Alfaidy, Unité INSERM U1036. Laboratoire BCI –Biosanté, CEA Grenoble 17, rue des Martyrs, 38054 Grenoble cedex 9, France
Received: 29 June 2020; Accepted: 17 August 2020; Published: 02 October 2020
Citation: Constance Collet, Roland Abi Nahed, Kevin Gemy, Wael Traboulsi, Nicolas Lemaitre, Pierre-Adrien Bolze, Pascale Hoffmann, Mohamed Benharouga, Nadia Alfaidy. Multifaceted Functions of Prokineticins in Reproductive Cancers and Proposed Associated Therapies. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 408-423.
View / Download Pdf Share at FacebookAbstract
Prokineticin 1 and prokineticin 2 are two secreted proteins that belong to the prokineticin family. The two ligands act via two G protein-coupled receptors, PROKR1 and PROKR2 to induce cell’s proliferation, migration, invasion and permeability. They mostly act on epithelial, endothelial and immune cells. Deregulations in the expression of the ligands and/or the receptors of this family have been associated with both tumor and non-tumor pathologies of the reproductive system. In these pathologies, prokineticins have been reported to be associated with strong angiogenic and inflammatory activities. While their direct involvement in some threatening reproductive non-tumor pathologies such as kallmann syndrome, polycystic ovaries, preeclampsia and fetal growth restriction are well established, their role and their consideration as potential targets in the tumors of the reproductive system are stil debated. This review will address the multifaceted roles of prokineticins and their receptors in reproductive cancers. Especially, the review will address the role of the prokineticin system in two female cancers, the ovarian cancer and the gestational cancer, Choriocarcinoma. The two types of cancer differentially express the prokineticin ligands at their early developmental stages, leading to different overall responses to be adapted when antagonisation of the prokineticin system is proposed. The review will summarize recent advances in the understanding of the prokineticin system’s involvement in the reproductive cancers, discuss its multifaceted roles in relation to prokineticin’s actions and proposes associated therapies.
Keywords
Prokineticin; Reproductive cancer; Targeted therapies; Immune system; Prokineticin antagonists
Prokineticin articles, Reproductive cancer articles, Targeted therapies articles, Immune system articles, Prokineticin antagonists articles
Prokineticin articles Prokineticin Research articles Prokineticin review articles Prokineticin PubMed articles Prokineticin PubMed Central articles Prokineticin 2023 articles Prokineticin 2024 articles Prokineticin Scopus articles Prokineticin impact factor journals Prokineticin Scopus journals Prokineticin PubMed journals Prokineticin medical journals Prokineticin free journals Prokineticin best journals Prokineticin top journals Prokineticin free medical journals Prokineticin famous journals Prokineticin Google Scholar indexed journals Reproductive cancer articles Reproductive cancer Research articles Reproductive cancer review articles Reproductive cancer PubMed articles Reproductive cancer PubMed Central articles Reproductive cancer 2023 articles Reproductive cancer 2024 articles Reproductive cancer Scopus articles Reproductive cancer impact factor journals Reproductive cancer Scopus journals Reproductive cancer PubMed journals Reproductive cancer medical journals Reproductive cancer free journals Reproductive cancer best journals Reproductive cancer top journals Reproductive cancer free medical journals Reproductive cancer famous journals Reproductive cancer Google Scholar indexed journals Targeted therapies articles Targeted therapies Research articles Targeted therapies review articles Targeted therapies PubMed articles Targeted therapies PubMed Central articles Targeted therapies 2023 articles Targeted therapies 2024 articles Targeted therapies Scopus articles Targeted therapies impact factor journals Targeted therapies Scopus journals Targeted therapies PubMed journals Targeted therapies medical journals Targeted therapies free journals Targeted therapies best journals Targeted therapies top journals Targeted therapies free medical journals Targeted therapies famous journals Targeted therapies Google Scholar indexed journals Immune system articles Immune system Research articles Immune system review articles Immune system PubMed articles Immune system PubMed Central articles Immune system 2023 articles Immune system 2024 articles Immune system Scopus articles Immune system impact factor journals Immune system Scopus journals Immune system PubMed journals Immune system medical journals Immune system free journals Immune system best journals Immune system top journals Immune system free medical journals Immune system famous journals Immune system Google Scholar indexed journals Prokineticin antagonists articles Prokineticin antagonists Research articles Prokineticin antagonists review articles Prokineticin antagonists PubMed articles Prokineticin antagonists PubMed Central articles Prokineticin antagonists 2023 articles Prokineticin antagonists 2024 articles Prokineticin antagonists Scopus articles Prokineticin antagonists impact factor journals Prokineticin antagonists Scopus journals Prokineticin antagonists PubMed journals Prokineticin antagonists medical journals Prokineticin antagonists free journals Prokineticin antagonists best journals Prokineticin antagonists top journals Prokineticin antagonists free medical journals Prokineticin antagonists famous journals Prokineticin antagonists Google Scholar indexed journals cell’s proliferation articles cell’s proliferation Research articles cell’s proliferation review articles cell’s proliferation PubMed articles cell’s proliferation PubMed Central articles cell’s proliferation 2023 articles cell’s proliferation 2024 articles cell’s proliferation Scopus articles cell’s proliferation impact factor journals cell’s proliferation Scopus journals cell’s proliferation PubMed journals cell’s proliferation medical journals cell’s proliferation free journals cell’s proliferation best journals cell’s proliferation top journals cell’s proliferation free medical journals cell’s proliferation famous journals cell’s proliferation Google Scholar indexed journals epithelial articles epithelial Research articles epithelial review articles epithelial PubMed articles epithelial PubMed Central articles epithelial 2023 articles epithelial 2024 articles epithelial Scopus articles epithelial impact factor journals epithelial Scopus journals epithelial PubMed journals epithelial medical journals epithelial free journals epithelial best journals epithelial top journals epithelial free medical journals epithelial famous journals epithelial Google Scholar indexed journals endothelial articles endothelial Research articles endothelial review articles endothelial PubMed articles endothelial PubMed Central articles endothelial 2023 articles endothelial 2024 articles endothelial Scopus articles endothelial impact factor journals endothelial Scopus journals endothelial PubMed journals endothelial medical journals endothelial free journals endothelial best journals endothelial top journals endothelial free medical journals endothelial famous journals endothelial Google Scholar indexed journals immune cells articles immune cells Research articles immune cells review articles immune cells PubMed articles immune cells PubMed Central articles immune cells 2023 articles immune cells 2024 articles immune cells Scopus articles immune cells impact factor journals immune cells Scopus journals immune cells PubMed journals immune cells medical journals immune cells free journals immune cells best journals immune cells top journals immune cells free medical journals immune cells famous journals immune cells Google Scholar indexed journals polycystic ovaries articles polycystic ovaries Research articles polycystic ovaries review articles polycystic ovaries PubMed articles polycystic ovaries PubMed Central articles polycystic ovaries 2023 articles polycystic ovaries 2024 articles polycystic ovaries Scopus articles polycystic ovaries impact factor journals polycystic ovaries Scopus journals polycystic ovaries PubMed journals polycystic ovaries medical journals polycystic ovaries free journals polycystic ovaries best journals polycystic ovaries top journals polycystic ovaries free medical journals polycystic ovaries famous journals polycystic ovaries Google Scholar indexed journals
Article Details
1. Prokineticin Family
In mammals, the prokineticin family is composed of two members, the prokineticin 1 (PROK1) also called EG-VEGF (Endocrine Gland-derived Vascular Endothelial Growth Factor) and the prokineticin 2 (PROK2) also called Bv8 [1, 2]. EG-VEGF is transcribed from the prok1 gene composed of three exons located on chromosome 1 in 1p21 [3, 4]. The gene that transcribes PROK2 is located on the chromosome 3 at 3p13.
Prokineticins and their receptors are expressed in various tissues such as ovary, testis, adrenal gland, placenta, uterus, brain, digestive tract and bone marrow [5-7]. In many organs prokineticin’s expression has been shown to be dynamic throughout physiological processes such as, circadian rhythm [8] menstrual cycle [9] and throughout pregnancy [10, 11]. In these systems, prokineticins have been shown to be regulated by local effectors and/or endocrine hormones. In the ovaries PROK1 is regulated by b-hCG (human Chorionic Gonadotropin), estrogen and FSH [12-14]. In the placenta PROK1 has been shown to be regulated by hypoxia, hCG and PPARg [10, 15, 16].
The prokineticins are secreted protein that act through their binding to two G protein-coupled receptors (GPCRs) [1, 2], the prokineticin receptor 1 (PROKR1) and the prokineticin receptor 2 (PROKR2). The prokineticin receptors were discovered simultaneously by three research teams [1, 2]. They belong to the GPCR family, with seven transmembrane domains that interact with heterotrimeric G proteins. PROKR1 and PROKR2 were initially called GPR73A and B, then receptors of prokineticin 1 and prokineticin 2, respectively. They share 85% homology in their amino acid sequence, but differ in their N-terminal parts [1, 2, 17].
Strong similarities were found in their transmembrane domains, suggesting similarities in their mechanisms of activation [17]. In human, the prokr1 and prokr2 genes include two exons and are located on two different chromosomes. The prokr2 gene is located on chromosome 2 at 2q14 and prokr1 gene is located on chromosome 20 at 20p13 [1]. The two prokineticins act as powerful ligands for both PROKR1 and PROKR2 receptors. Interestingly, differential and tissue-specific expressions were reported for the two receptors. PROKR2 is abundantly expressed in the brain and testes, while PROKR1 is mainly expressed in peripheral tissues such as the spleen, prostate, pancreas, heart and blood cells. In the reproductive system, high levels of both receptors have been reported in the testis, ovary, endometrium and placenta [6, 9, 12, 18, 19].
Several studies have been carried out to determine the affinity of the two ligands to their receptors. It clearly appears that PROK2 exhibits higher affinity for PROKRs (EC50 of 4.5 ± 0.8 nmol/L for PROKR1 and 6.4 ± 1.3 nmol/L for PROKR2) [20], whereas PROK1 exhibits a moderate affinity for both receptors (EC50 of 27.6 ± 8.2 nmol/L for PROKR1 and 52.2 ± 16.4 nmol/L for PROKR2). Distinct expression patterns of the prokineticins and their receptors have been reported in various tissues, providing the cue for their tissue-specific biological functions [5-7]. In addition, the differential pattern of expression of G-proteins plus the multiple types of G-proteins that can couple to these receptors further increase the functional complexity of the system. PROK1 and PROK2 bind to PROKR1 and PROKR2, which can be coupled to Gi, Gs and Gq to activate MAPK/Akt, induce cAMP accumulation and calcium mobilization [20].
2. Prokineticins and Diseases
Prokineticins were first identified as potent agents stimulating the muscle contraction of the gastrointestinal tract [14, 21]. Later, PROK1 was shown to mediate proliferation and differentiation of the enteric neural crest cells during the enteric nervous system (ENS) development [22]. Since their discovery, prokineticin proteins have been associated with two key biological functions, angiogenesis, including vascular remodeling, and inflammation [6, 23, 24]. Over the last two decades, research articles on the prokineticins have either studied their role in angiogenesis and neovascularization or in inflammation [6, 23, 24]. However, few studies have addressed combined actions of the prokineticins in a given physiological or pathological process.
A determinant factor that links the biology of prokineticins with vascular remodeling is the hypoxic environment which develops upon a vascular damage and which is known to up-regulate prokineticins. Importantly, both PROK1 and PROK2 promoter regions have response elements for the hypoxia inducible factor (HIF-1) [6, 23, 24]. In several studies, prokineticins have been reported as potent angiogenic factors and their receptors are highly expressed in both microvascular and macrovascular endothelial cells [6, 23].
PROK1 was reported as a potent angiogenic factor that promotes angiogenesis in various steroidogenic glands [4, 8, 10]. In relation to inflammatory processes, both ligands were reported to activate this process. However, unlike PROK1, PROK2 is more associated with this process as it is expressed in the bone marrow. PROK2 is also reported as the preferential prokineticin expressed by immune cells as it acts as a potent chemoattractant for monocytes and neutrophils [24, 25]. It also induces survival, differentiation and activation of the granulocytic and monocytic lineages [26]. To fulfil the angiogenic and inflammatory functions, prokineticins and their receptors have been reported to be involved in the control of multiple processes including proliferation, differentiation, invasion and migration of the target cells. These effects are mainly represented by endothelial and epithelial cells for the angiogenic processes and by immune cells such as monocytes, macrophages and neutrophils for the inflammatory processes [26, 27].
While, the prokineticin system has been reported to be associated with numerous diseases, its association with the reproduction-associated pathologies is the most investigated [13, 28-30]. Since their discovery, multiple studies highlighted the direct involvement of the prokineticin system in the development of severe reproduction pathologies, such as the polycystic ovarian syndrome (PCOS), the congenital diseases, named Kallmann syndrome, the Hirschsprung disease [30-32] and multiple pregnancy-associated pathologies [6, 10, 16, 33-35]. Nevertheless, the majority of the studies in relation to the reproductive system emanated from their involvement in placental development, during pregnancy. In relation to pregnancy pathologies, PROK1 constitutes the master member. In fact, strong evidences established the direct involvement of PROK1/PROKRs system in physiological and pathophysiological aspects of pregnancy; these include clinical, in vitro, ex vivo, and in vivo studies [6, 10, 16, 33-36]. Through its receptors PROKR1 and PROKR2, PROK1 controls the proliferation and invasion of trophoblast cells to ensure the establishment of the fetomaternal circulation, along with direct effect on intravillous vasculature to ensure vascular branching and angiogenesis [6, 10, 11]. Beside its physiological effect, PROK1 and its receptors have been reported to be causative and/or compensatory factors in most of the threatening pathologies of pregnancy, such as preeclampsia and fetal growth restriction [37,38]. Also, PROK1 has been designated as a new noninvasive biomarker of successful embryo implantation and oocyte competence in conventional in vitro fertilization-embryo transfer [34, 39, 40].
2.1 Prokineticin’s and Reproductive Tumor Diseases
Due to their wide spectrum of actions, notably as potent angiogenic and immunoregulatory factors and to the fact that both PROK1 and PROK2 are activated by the most potent environmental cue of angiogenesis and tumorigenesis, the oxygen tension; investigators have searched for the PROKs/PROKRs system involvement in cancer development in a variety of tissues [4, 22, 36, 41-49]. While numerous studies reported deregulations of PROK1, PROK2 and/or their receptors in a wide range of cancers, few studies brought evidences for their direct involvement in cancer development and progression [22, 36, 41, 46, 50].
Table 1: Reports an updated list of the different type of tumors that were investigated in relation to the potential involvement of the prokineticin family in their growth, progression and/or aggressiveness.
As exemplified through this listing, the prokineticin system appears to be deregulated in many types of cancers. However the involvement of the ligands and/or their receptors is tightly dependent on the type of cancer, on its severity and its localization within the affected tissue. Because most of the examined cancers in relation to the prokineticin system emanate from descriptive studies involving cohorts enrolled at advanced stages of cancer, it is difficult to conclude on whether the deregulation of PROK system is a cause or consequence of the cancer. Nevertheless, most of the data confirmed the deregulation of prokineticins at these stages, suggesting their tight association with cancer’s aggravation. In these cases, their deregulations should rather be considered as consequences of the disease.
Because most advanced and far-reaching studies on prokineticins and cancer have been investigated in the reproductive system, the following sections will report the updated investigations on prokineticins in relation to prostate, testis, ovarian and placental cancers. These subsections will report the associated deregulated member, its role in the development and /or progression of the related cancer and will propose associated therapies, when applicable.
2.1.1 Prokineticins in the prostate and testicular cancers
PROKs and their receptors are expressed in the testis and the prostate. In the prostate, PROK1 is localized in the glandular epithelial cells and PROK2 as well, as the two receptors are expressed in the prostate epithelial cells [47]. In the testis, PROK1 is predominantly expressed in testosterone-producing Leydig cells, whereas PROK2 expression is restricted to primary spermatocytes [4, 6, 10]. The PROKRs are also expressed in vascular endothelial cells in the testis [4, 13]. Importantly, adenoviral delivery of PROK1 or PROK2 to the mouse testis resulted in a potent angiogenic response [4], suggesting that PROKs contribute with other factors, such as VEGF-A, in the maintenance of the integrity and the proliferation of the blood vessels in this organ. The exact role of each of these receptors and their implication in the physiology of prostate and testis functions remain to be elucidated.
In relation to prostate cancer, the PROK1 expression has been reported to be elevated in the prostate compared to healthy individuals. These levels were correlated to the cancer’s malignancy [47]. Thus, the levels of PROK1 could be useful for prostate cancer outcome evaluations and can be used as a target for prostate cancer treatment in the future. Regarding the testis cancer, few studies investigated prokineticins involvement in the development and or progression of this cancer. In a study comparing PROK1 levels of expression in different testicular tumors, including Leydig cell tumors, seminomas and non-seminomatous germ cell tumors, it has been reported that PROK1 ispredominantly expressed in Leydig cell tumors. The PROKRs have also been reported to be expressed in the testicular endothelium, suggesting an involvement of the PROKs/PROKRs system in neoplastic testicular angiogenesis [48].
2.1.2 Prokineticins in the ovarian cancer
In the ovary, the representative and most abundant member is the PROK1, as numerous studies demonstrated that PROK2 is weakly or not expressed in the female reproductive organs [51]. PROK1, as well as its receptors have been reported to be strongly involved in physiological and pathophysiological processes of the ovary. In this organ, the expression of PROK1 fluctuates according to the ovarian cycle and is mainly associated with the secretary phase with a direct role in the maintenance of corpus luteum vascularization [12]. PROK1 is secreted during the follicular phase by granulosa cells of the primordial and primary follicles. Upon ovulation, PROK1 is secreted by granulosa lutein cells and its expression rises throughout the luteal phase with a peak during the very late stage [12]. Interestingly, PROK1 mRNA levels are also elevated in apoptotic tissues, especially during the corpus luteum regression or in the atretic follicles [18]. To fulfil this pattern of expression in the ovary, PROK1 has been reported to be controlled by a panel of key ovarian regulatory factors, such as hCG, estrogen, FSH, oxygen tension and thrombin [12]. These events being associated with inflammation, it has been demonstrated that PROK1 triggers an onsite recruitment and activation of monocytes [18]. Study of gene expression profiles comparing VEGF, the major factor of the ovarian angiogenesis, and EG-VEGF revealed that their profiles rarely overlapped and seem to exhibit complementary type of functions during the ovarian cycle [18].
Given the above-described roles for PROK1 in the angiogenic and in the inflammatory processes in the ovaries, it was expected that deregulations in the PROK/PROKR system could be associated with the development of severe ovarian diseases. In this context, several studies reported prokineticins deregulation in the Polycystic ovarian syndrom (PCOS) [53]. Moreover, implication of prokineticins has also been investigated in ovarian cancer.
Ovarian cancer is the 5th cause of cancer deaths in women and it has the highest rate of death among gynecological cancers [54]. Malignant tumors that originate from the epithelium account for more than 90% of ovarian cancers. Among these is the high grade serous ovarian cancer; it accounts for nearly 70% of all ovarian cancers and is considered as the most threatening one [55]. Despite substantial survival benefits, chemotherapies are highly toxic and numerous patients develop resistance within few months after the first treatment. Therefore, there is still a need for efficient and less harmful treatment for this cancer [55].
Despite the fact that PROK1 is no longer detectable after menopause in the ovaries, some studies reported PROK1 positive cells at the tumors' periphery [56]. Two other studies reported significant correlations between PROK1 increased expression and the aggressiveness of the cancer, suggesting its potential consideration as a therapeutic target for this cancer [41,46]. On the contrary, Zhang et al. 2003 demonstrated that PROK1 mRNA levels decreased in the later stages of ovarian tumor development compared to premenopausal ovaries or early stages carcinomas [49]. Furthermore, in the same study, it was reported that PROK1 was secreted by the tumor microenvironment and especially by tumor-infiltrating T cells [49]. Importantly, the observation that PROK1 was mainly expressed by immune infiltrating cells in the ovary has been substantiated by other studies demonstrating that prokineticins could not only be expressed by tumor cells but also by immune cells in the tumor microenvironment [57]. Altogether, these findings strongly suggest that the balance between the angiogenic and the pro-inflammatory functions of the prokineticins in a given cancer may grandly contribute to the development and or progression of that cancer. While the role of the PROKs in the angiogenic process that accompany the early stages of ovarian cancer is strongly proposed, their expression by the local immune cells suggests that these proteins could be considered as specific targets to attenuate inflammatory associated reactions in the ovarian cancer.
2.1.3 Prokineticins in the placental cancer
Since the first ontology study on prokineticins, published by Ferrara et al in 2001, the human placenta appeared as a strong site of PROK1/PROKRs expression [3]. Publications following this first identification confirmed the strong expression of the PROKs, especially that of PROK1 and its receptors in the human placenta [13, 29, 58]. However, most of these studies considered one gestational age that often corresponded to placentas collected at term pregnancies [29, 58]. Since 2006, more comprehensive studies [6, 10, 11, 23, 30, 33, 36, 37] reported complete profile, pattern, and regulations of the prokineticins during human pregnancy. Also, these studies investigated their involvement in the development of the most threatening pathologies of pregnancy [6, 10, 11, 30, 36, 37]. Briefly, those findings demonstrated that PROK1 was highly expressed during the first trimester of pregnancy, with the strongest localization in the syncytiotrophoblast and Hofbauer cells (placental macrophages). PROKR1 was mainly expressed in non-differentiated cells, while PROKR2 prevails in the differentiated cells, such as the syncytiotrophoblast and the extravillous trophoblasts (EVT). These studies also demonstrated that both PROKR1 and PROKR2 were expressed by placental micro- and macro-vascular endothelial cells [23]. Through its actions on PROKR1 and PROKR2, PROK1 has been reported to control trophoblast proliferation, migration and invasion. Its actions on the endothelial cells mainly concerned its effect on their proliferation and permeability [10, 11].
Because PROK1 is a circulating protein, its levels were examined throughout pregnancy and used to compare normal and pathological pregnancies. Circulating levels of PROK1 in non-pregnant women are about 50 pg/ml. These amounts increase five times during the first trimester of pregnancy to reach 250 pg/ml and significantly fall during the second and third trimesters of pregnancy to reach 70 pg/ml [23]. Importantly, deregulated PROK1 levels were observed in threatening pathologies of pregnancy such as preeclampsia and fetal growth restriction [11, 37]. In relation to these conditions, PROK1 is proposed as a new diagnostic and/or prognostic biomarker [11, 37].
Given the direct involvement of PROK1 and its receptors in the control of key aspects of placental development during the first trimester of pregnancy and its deregulation in pregnancy pathologies, the PROK1/PROKRs appeared as potential targeted proteins. While no study has yet addressed this topic in relation to pregnancy pathologies, such as preeclampsia and FGR, a recent study reported the involvement of PROK1 and its receptors in the development and progression of gestational choriocarcinoma (CC) and proposed therapeutic options through the targeting of the receptors [36].
Gestational CC is a malignant trophoblastic tumor that can develop from a normal or abnormal pregnancy [59]. Most often, this malignant lesion appears following complete (CHM) or partial hydatidiform mole (PHM), a spontaneous abortion, or an ectopic pregnancy [59]. Patients who acquire CHM or PHM have a high risk of developing CC. However, this risk is much higher for CHM (20%) compared to PHM (1.5%). It is well established that the progression of MHC towards a CC is governed by the acquisition by trophoblastic cells of a hyperproliferative character [60]. This phenomenon is reflected in a second step by the appearance of cellular clusters, which acquire a migratory and invasive phenotype. CC is a very metastatic tumor due to the invasive and intrinsic property of trophoblastic cells. Diagnosis and monitoring of CC progression are currently based on the measurement of circulating levels of b-hCG (human chorionic gonadotropin), secreted by syncytiotrophoblast cells [61].
As reported above, PROK1 acts as a novel placental growth factor which controls trophoblast proliferation, invasion and is upregulated by hypoxia and by hCG, two key parameters of tumorigenesis. Altogether, these data suggested that the PROK system might be involved in the development and or progression of this cancer. In this context our laboratory recently deciphered the role of PROK1 and its receptors in the development of this cancer through, a clinical study using a distinctive cohort of CHM and CC patients; an in vitro study using the human choriocacinoma cell line, the JEG-3; and an in vivo study using a recent animal model of CC. This study demonstrated that both placenta and circulating PROK1 levels were significantly increased in CHM and CC; that PROK1 increased JEG-3 proliferation, migration and invasion in 2D and 3D culture systems and that these observations were substantiated using the developed animal model [36]. To better characterize the type of receptor involved in these effects, non-peptide specific antagonists for PROKR1 and PROKR2 were employed [8, 62]. Importantly, our data showed that the antagonization of PROK1 receptors reduced JEG-3 tumorigenesis in vitro and in vivo and that the PROKR2 antagonist was more potent in mediating these effects [36].
Because, the actual measurements of circulating b-hCG have been reported to be associated with the generation of false positives, requiring some patients to undergo invasive and unnecessary therapeutic procedures such as chemotherapy, hysterectomy, and other surgeries [50], the study by Traboulsi & al offers the possibility to consider circulating PROK1 as potential diagnostic biomarker for CC progression. Also, this study proposes the antagonization of its receptors as potential therapy to treat gestational CC. This may provide safe and less toxic therapeutic option compared with the currently used multi-agent chemotherapies.
3. Antagonisation of the Prokineticin System as a Therapeutic Opportunity for Reproductive Cancers
The prokineticin system is equally involved in angiogenic and inflammatory processes, which are two key routes that drive cancer development and progression. In relation to angiogenesis, PROKs and especially PROK1 inhibition has been proposed as combinatory option of therapies that target the most angiogenic factor, the VEGF. This was very attempting, because the effects of both factors in regards to the angiogenic processes were somewhat complementary. Beyond this important aspect, patients treated with anti-VEGF therapies show strong refractoriness [63], suggesting that exploration of new anti-angiogenic therapies that target less ubiquitous and more tissue-specific factors such as prokineticins might lead to better results. This can be applicable for reproductive cancers that affect the ovary and the placenta.
In regards to the prokineticin system involvement in inflammation, growing literature is addressing their local role in regulating immune responses. To date, these studies mainly reported the involvement of PROK2 in these functions [64]. In the case of ovarian cancer, PROK2 was shown to be expressed by components of the immune system, especially the myeloid cells and macrophages [49, 63]. These cells have been reported to represent a crucial cell subpopulation responsible for the refractoriness to anti-VEGF treatment [49, 63]. These data strongly suggest that therapies targeting not only PROK1 for its pro-angiogenic effects, but also PROK2 for its mediated refractoriness responses can advance the treatment of cancers that overexpress these proteins, such as the ovarian cancer. Figure 1 reports a cartoon that illustrates these conclusions. The panel A shows an active vascularized tumor secreting, among other angiogenic and pro-inflammatory factors, the prokineticin ligands (PROK1 and PROK2) and expressing their receptors. The increased secretion of prokineticin is potentiated by the tumor hypoxic environment. The secreted prokineticins are part of the first signals that allow immune cells mobilization towards the tumor’s microenvironment. Once at the tumor site, the immune cells will in turn, contribute to the exacerbation of prokineticins secretion, inducing tumor cells proliferation, migration and invasion. Panel B shows the same phenomenon but in the presence of PROKR1 and /or PROKR2 antagonists. In this case, the prokineticin receptors will be occupied by the antagonists, which consequently leads to a significant decrease in all prokineticins’ mediated effects. In this schematic, blocking antibodies are also proposed as targeted therapy.
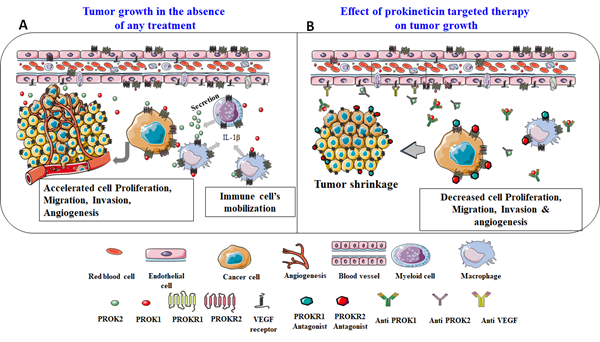
Figure 1: Reports a proposed model for tumor development in the absence or presence of targeting molecules ( antagonists or antibodies) of PROK receptors.
Importantly, several studies have shown that the use of antagonists or antibodies against PROK2 in vivo, significantly decreased tumor growth in poorly and highly vascularized cancers [49, 63]. These effects were similar to those obtained with anti-VEGF treatments and the combination of both treatments was sometimes even more efficient [49, 63]. Through the inhibition of the prokineticin signaling, the tumor vasculature is no longer expanding; it acquires necrotic properties and exhibits a decrease in the production and secretion of pro-inflammatory cytokines.
Altogether these findings, strongly propose that therapies targeting prokineticins will not only be efficient to attenuate their pro-angiogenic activities but also to decrease their exacerbation of inflammatory responses within the tumor.
4.Conclusion and Perspectives
To date, the proposed therapies that target the prokineticin system are mainly based on the antagonization of its receptors and have only been used in vitro, ex vivo and in animal models [30, 65, 66]. Although more in vivo and translational studies are still needed to consider the use of these antagonists in human, they have shown beneficial effects in in vivo models. In fact, both antagonists exhibit analgesic effects and control the nociceptive process that is mediated by inflammatory pathologies [67]. More importantly, the treatment of the gravid CC mice with antagonists allowed the maintenance of the gestation with fewer resorbed fetuses compared to non-treated mice [36]. Altogether, these data advocate a strong therapeutic potential for these two antagonists when used solely or in combination with actual anti-angiogenic or anti–inflammatory molecules.
Because growing literature demonstrate that angiogenic factors are often involved in local immunosuppressive effects in the tumor microenvironment, one can speculate that this can also be the case for prokineticins as they are strong angiogenic factors and are highly expressed by monocytes that generate myeloid dendritic cells, involved in immnossupressive effects. Thus, the anatgonistaion of the prokineticin signaling can serve to control two arms of tumor development, angiogenesis and immunosuppression.
Financial Support
We acknowledge the following sources of funding: Institut National de la Santé et de la Recherche Médicale (U1036), University Grenoble-Alpes, Commissariat à l’Energie Atomique (DSV/iRTSV/BCI), Région Auvergne-Rhône-Alpes “CLARA, Oncostarter”, Ligue Nationale contre le Cancer and Ligue Départementale (Savoie) contre le Cancer, Inserm Transfert.
Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- Lin DC, Bullock CM, Ehlert FJ, et al. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. The Journal of biological chemistry 277 (2002): 19276-19280.
- Masuda Y, Takatsu Y, Terao Y, et al. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochemical and biophysical research communications 293 (2002): 396-402.
- LeCouter J, Kowalski J, Foster J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412 (2001): 877-884.
- LeCouter J, Lin R, Tejada M, et al. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proceedings of the National Academy of Sciences of the United States of America 100 (2003): 2685-2690.
- Boulberdaa M, Urayama K, Nebigil CG. Prokineticin receptor 1 (PKR1) signalling in cardiovascular and kidney functions. Cardiovascular research 92 (2011): 191-198.
- Brouillet S, Hoffmann P, Feige JJ, Alfaidy N. EG-VEGF: a key endocrine factor in placental development. Trends in endocrinology and metabolism: TEM 23 (2012): 501-508.
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nature reviews Cancer 2 (2002): 795-803.
- Cheng MY, Lee AG, Culbertson C, et al. Prokineticin 2 is an endangering mediator of cerebral ischemic injury. Proceedings of the National Academy of Sciences of the United States of America 109 (2012): 5475-5480.
- Battersby S, Critchley HO, Morgan K, et al. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. The Journal of clinical endocrinology and metabolism 89 (2004): 2463-2469.
- Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology 147 (2006): 1675-1684.
- Hoffmann P, Saoudi Y, Benharouga M, et al. Role of EG-VEGF in human placentation: Physiological and pathological implications. Journal of cellular and molecular medicine 13 (2009): 2224-2235.
- Fraser HM, Bell J, Wilson H, et al. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. The Journal of clinical endocrinology and metabolism 90 (2005): 427-434.
- Maldonado-Perez D, Evans J, Denison F, et al. Potential roles of the prokineticins in reproduction. Trends in endocrinology and metabolism: TEM 18 (2007): 66-72.
- Ngan ES, Tam PK. Prokineticin-signaling pathway. The international journal of biochemistry and cell biology 40 (2008): 1679-1684.
- Brouillet S, Hoffmann P, Chauvet S, et al. Revisiting the role of hCG: new regulation of the angiogenic factor EG-VEGF and its receptors. Cellular and molecular life sciences: CMLS 69 (2012): 1537-1550.
- Garnier V, Traboulsi W, Salomon A, et al. PPARgamma controls pregnancy outcome through activation of EG-VEGF: new insights into the mechanism of placental development. American journal of physiology Endocrinology and metabolism 309 (2015): E357-E369.
- Soga T, Matsumoto S, Oda T, Saito T, et al. Molecular cloning and characterization of prokineticin receptors. Biochimica et biophysica acta 1579 (2002): 173-179.
- Kisliouk T, Friedman A, Klipper E, et al. Expression pattern of prokineticin 1 and its receptors in bovine ovaries during the estrous cycle: involvement in corpus luteum regression and follicular atresia. Biology of reproduction 76 (2007): 749-758.
- Tiberi F, Tropea A, Apa R, et al. Prokineticin 1 mRNA expression in the endometrium of healthy women and in the eutopic endometrium of women with endometriosis. Fertility and sterility 93 (2010): 2145-2149.
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews Immunology 10 (2010): 826-837.
- Wade PR, Palmer JM, Mabus J, et al. Prokineticin-1 evokes secretory and contractile activity in rat small intestine. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 22 (2010): e152-e161.
- Ngan ES, Lee KY, Sit FY, Poon HC, Chan JK, Sham MH, Lui VC, Tam PK. Prokineticin-1 modulates proliferation and differentiation of enteric neural crest cells. Biochimica et biophysica acta 1773 (2007): 536-545.
- Brouillet S, Hoffmann P, Benharouga M, et al. Molecular characterization of EG-VEGF-mediated angiogenesis: differential effects on microvascular and macrovascular endothelial cells. Molecular biology of the cell 21 (2010): 2832-2843.
- Negri L, Ferrara N. The Prokineticins: Neuromodulators and Mediators of Inflammation and Myeloid Cell-Dependent Angiogenesis. Physiological reviews 98 (2018): 1055-1082.
- Giannini E, Lattanzi R, Nicotra A, et al. The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proceedings of the National Academy of Sciences of the United States of America 106 (2009): 14646-14651.
- Monnier J, Piquet-Pellorce C, Feige JJ, et al. Prokineticin 2/Bv8 is expressed in Kupffer cells in liver and is down regulated in human hepatocellular carcinoma. World journal of gastroenterology 14 (2008): 1182-1191.
- Monnier J, Samson M. Prokineticins in angiogenesis and cancer. Cancer letters 296 (2010): 144-149.
- Alfaidy N, Baron C, Antoine Y, et al. Prokineticin 1 is a new biomarker of human oocyte competence: expression and hormonal regulation throughout late folliculogenesis. Biology of reproduction 101 (2019): 832-841.
- Catalano RD, Lannagan TR, Gorowiec M, et al. Prokineticins: novel mediators of inflammatory and contractile pathways at parturition? Molecular human reproduction 16 (2010): 311-319.
- Traboulsi W, Brouillet S, Sergent F, et al. Prokineticins in central and peripheral control of human reproduction. Hormone molecular biology and clinical investigation 24 (2015): 73-81.
- Cole LW, Sidis Y, Zhang C, et al. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. The Journal of clinical endocrinology and metabolism 93 (2008): 3551-3559.
- Matsumoto S, Yamazaki C, Masumoto KH, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proceedings of the National Academy of Sciences of the United States of America 103 (2006): 4140-4145.
- Alfaidy N, Hoffmann P, Boufettal H, et al. The multiple roles of EG-VEGF/PROK1 in normal and pathological placental angiogenesis. BioMed research international (2014): 451906.
- Brouillet S, Hoffmann P, Thomas-Cadi C, et al. [PROK1, prognostic marker of embryo implantation?]. Gynecologie, obstetrique & fertilite 41 (2013): 562-565.
- Evans J, Catalano RD, Morgan K, et al. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology 149 (2008): 2877-2887.
- Traboulsi W, Sergent F, Boufettal H, et al. Antagonism of EG-VEGF Receptors as Targeted Therapy for CC Progression In Vitro and In Vivo. Clinical cancer research: an official journal of the American Association for Cancer Research 23 (2017): 7130-7140.
- Brouillet S, Murthi P, Hoffmann P, et al. EG-VEGF controls placental growth and survival in normal and pathological pregnancies: case of fetal growth restriction (FGR). Cellular and molecular life sciences: CMLS 70 (2013): 511-525.
- Holloway AC, Salomon A, Soares MJ, et al. Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. American journal of physiology Endocrinology and metabolism 306 (2014): E443-E456.
- Alfaidy N, Baron C, Antoine Y, et al. Prokineticin 1 is a new biomarker of human oocyte competence: expression and hormonal regulation throughout late folliculogenesis. Biology of reproduction 101 (2019): 832-841.
- Alfaidy N, Hoffmann P, Gillois P, et al. PROK1 Level in the Follicular Microenvironment: A New Noninvasive Predictive Biomarker of Embryo Implantation. The Journal of clinical endocrinology and metabolism 101 (2016): 435-444.
- Balu S, Pirtea L, Gaje P, et al. The immunohistochemical expression of endocrine gland-derived-VEGF (EG-VEGF) as a prognostic marker in ovarian cancer. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie 53 (2012): 479-483.
- Benlahfid M, Traboulsi W, Sergent F, et al. Endocrine gland-derived vascular endothelial growth factor (EG-VEGF) and its receptor PROKR2 are associated to human colorectal cancer progression and peritoneal carcinomatosis. Cancer biomarkers: section A of Disease markers 21 (2018): 345-354.
- Bouzoubaa M, Benlahfid M, Sidqui M, et al. Vascular endothelial growth factor (VEGF) and Endocrine gland-VEGF (EG-VEGF) are down regulated in head and neck cancer. Clinical otolaryngology: official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery (2020).
- Heck D, Wortmann S, Kraus L, et al. Role of Endocrine Gland-Derived Vascular Endothelial Growth Factor (EG-VEGF) and Its Receptors in Adrenocortical Tumors. Hormones & cancer 6 (2015): 225-236.
- Jiang X, Abiatari I, Kong B, et al. Pancreatic islet and stellate cells are the main sources of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 in pancreatic cancer. Pancreatology: official journal of the International Association of Pancreatology 9 (2009): 165-172.
- Lozneanu L, Avadanei R, Cimpean AM, et al. Relationship between the Proangiogenic Role of Eg-Vegf, Clinicopathological Characteristics and Survival in Tumoral Ovary. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi 119 (2015): 461-465.
- Pasquali D, Rossi V, Staibano S, et al. The endocrine-gland-derived vascular endothelial growth factor (EG-VEGF)/prokineticin 1 and 2 and receptor expression in human prostate: Up-regulation of EG-VEGF/prokineticin 1 with malignancy. Endocrinology 147 (2006): 4245-4251.
- Samson M, Peale FV, Frantz G, et al. Human endocrine gland-derived vascular endothelial growth factor: expression early in development and in Leydig cell tumors suggests roles in normal and pathological testis angiogenesis. The Journal of clinical endocrinology and metabolism 89 (2004): 4078-4088.
- Zhang L, Yang N, Conejo-Garcia JR, et al. Expression of endocrine gland-derived vascular endothelial growth factor in ovarian carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research 9 (2003): 264-272.
- Sasaki S, Sasaki Y, Iino K. Recurrent gestational trophoblastic disease in a case of suspected quiescent gestational trophoblastic disease: a case report. The Journal of reproductive medicine 55 (2010): 317-320.
- Ferrara N, LeCouter J, Lin R, et al. EG-VEGF and Bv8: a novel family of tissue-restricted angiogenic factors. Biochimica et biophysica acta 1654 (2004): 69-78.
- Gao MZ, Zhao XM, Sun ZG, et al. Endocrine gland-derived vascular endothelial growth factor concentrations in follicular fluid and serum may predict ovarian hyperstimulation syndrome in women undergoing controlled ovarian hyperstimulation. Fertility and sterility 95 (2011): 673-678.
- Meng L, Yang H, Jin C, et al. miR285p suppresses cell proliferation and weakens the progression of polycystic ovary syndrome by targeting prokineticin1. Molecular medicine reports 20 (2019): 2468-2475.
- Kurian AW, Ward KC, Howlader N, et al. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 37 (2019): 1305-1315.
- Matulonis UA, Sood AK, Fallowfield L, et al. Ovarian cancer. Nature reviews Disease primers 2 (2016): 160-161.
- Maldonado-Perez D, Brown P, Morgan K, et al. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochimica et biophysica acta 1793 (2009): 1315-1324.
- Shojaei F, Wu X, Zhong C, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450 (2007): 825-831.
- Denison FC, Battersby S, King AE, et al. Prokineticin-1: a novel mediator of the inflammatory response in third-trimester human placenta. Endocrinology 149 (2008): 3470-3477.
- Froeling FE, Seckl MJ. Gestational trophoblastic tumours: an update for 2014. Current oncology reports 16 (2014): 408.
- Louwen F, Muschol-Steinmetz C, Reinhard J, et al. A lesson for cancer research: placental microarray gene analysis in preeclampsia. Oncotarget 3 (2012): 759-773.
- Esfandiari N, Goldberg JM. Heterophile antibody blocking agent to confirm false positive serum human chorionic gonadotropin assay. Obstetrics and gynecology 101 (2003): 1144-1146.
- Balboni G, Lazzari I, Trapella C, et al. Triazine compounds as antagonists at Bv8-prokineticin receptors. Journal of medicinal chemistry 51 (2008): 7635-7639.
- Shojaei F, Wu X, Qu X, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proceedings of the National Academy of Sciences of the United States of America 106 (2009): 6742-7747.
- LeCouter J, Zlot C, Tejada M, et al. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proceedings of the National Academy of Sciences of the United States of America 101 (2004): 16813-16818.
- Jacobson O, Weiss ID, Niu G, et al. Prokineticin receptor 1 antagonist PC-10 as a biomarker for imaging inflammatory pain. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 52 (2011): 600-607.
- Negri L, Maftei D. Targeting the Prokineticin System to Control Chronic Pain and Inflammation. Current medicinal chemistry 25 (2018): 3883-3894.
- Negri L, Lattanzi R. Bv8-prokineticins and their receptors: modulators of pain. Current pharmaceutical biotechnology 12 (2011): 1720-1727.
- Goi T, Nakazawa T, Hirono Y, et al. Antiprokineticin (PROKR1) monoclonal antibody suppresses angiogenesis and tumor growth in colorectal cancer Suppl 4 (2014): S665-S671.
