Metabolic Impact of Immune-Suppressor Cells in Cancer Patients
Article Information
Masahiko Shibata1,2,3,4*, Atsushi Inukai2,5, Daigo Yoshimori2,5, Mai Ashizawa2,3, Takahiro Nakajima2,3, Makoto Takada4,6, Takashi Yazawa2,3,4, Kousaku Mimura3, Norio Inoue2,3,4, Takafumi Watanabe7, Kazunoshin Tachibana8, Satoshi Muto9, Koji Kono1,3,4, Shungo Endo10, Seiichi Takenoshita11
1Department of Comprehensive Cancer Treatment at Aizu, Fukushima Medical University, Japan
2Department of Surgery, Cancer Treatment Center, Aizu Chuo Hospital, Japan
3Department of Gastrointestinal Tract Surgery, Fukushima Medical University, Japan
4Aizu Oncology Consortium, Japan
5Department of Surgery, Nippon Medical School, Japan
6Department of Surgery, Bange Kousei General Hospital, Japan
7Department of Obstetrics and Gynecology, Fukushima Medical University, Japan
8Department of Breast Surgery, Fukushima Medical University, Japan
9Department of Chest Surgery, Fukushima Medical University, Japan
10Department of Colorecto-anal Surgery, Aizu Medical Center, Fukushima Medical University, Japan
11Fukushima Medical University, Japan
*Corresponding Author: Masahiko Shibata, Departments of Gastrointestinal Tract Surgery and Comprehensive Cancer Treatment and Research at Aizu, Fukushima Medical University, Fukushima, Japan
Received: 02 March 2022; Accepted: 09 March 2022; Published: 15 March 2022
Citation: Masahiko Shibata, Atsushi Inukai, Daigo Yoshimori, Mai Ashizawa, Takahiro Nakajima, Makoto Takada, Takashi Yazawa, Kousaku Mimura, Norio Inoue, Takafumi Watanabe, Kazunoshin Tachibana, Satoshi Muto, Koji Kono, Shungo Endo, Seiichi Takenoshita. Metabolic Impact of Immune-Suppressor Cells in Cancer Patients. Journal of Surgery and Research 5 (2022): 134-144.
View / Download Pdf Share at FacebookAbstract
Immune checkpoint inhibitors (ICIs) are not equally effective for all patients, regardless type of cancer. Immune-suppressor cells, including regulatory T (Treg) cells, tumor associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), and their metabolic pathways in the tumor microenvironment (TME) play important roles in resistance to ICIs. Although Treg cells, TAMs and MDSCs play significant roles in immunosuppression in the TME, these cells are very important in the orchestration of metabolism such as angiogenesis and production of indoleamine 2,3-dioxygenase (IDO) and nitric oxide (NO) towards tumor escape, progression and expansion. Cancer immunotherapies tailored with metabolic characterizations such as parameters of angiogenesis, inflammation or obesity may be needed for the establishment of a successful treatment modality in the immunotherapy era.
Keywords
Tumor associated macrophages (TAMs), Regulatory T (Treg) cells, Myeloid-derived suppressor cells (MDSCs), Cancer immunotherapy, immunosuppression
Tumor associated macrophages (TAMs) articles; Regulatory T (Treg) cells articles; Myeloid-derived suppressor cells (MDSCs) articles; Cancer immunotherapy articles; immunosuppression articles
Tumor associated macrophages articles Tumor associated macrophages Research articles Tumor associated macrophages review articles Tumor associated macrophages PubMed articles Tumor associated macrophages PubMed Central articles Tumor associated macrophages 2023 articles Tumor associated macrophages 2024 articles Tumor associated macrophages Scopus articles Tumor associated macrophages impact factor journals Tumor associated macrophages Scopus journals Tumor associated macrophages PubMed journals Tumor associated macrophages medical journals Tumor associated macrophages free journals Tumor associated macrophages best journals Tumor associated macrophages top journals Tumor associated macrophages free medical journals Tumor associated macrophages famous journals Tumor associated macrophages Google Scholar indexed journals Regulatory T (Treg) cells articles Regulatory T (Treg) cells Research articles Regulatory T (Treg) cells review articles Regulatory T (Treg) cells PubMed articles Regulatory T (Treg) cells PubMed Central articles Regulatory T (Treg) cells 2023 articles Regulatory T (Treg) cells 2024 articles Regulatory T (Treg) cells Scopus articles Regulatory T (Treg) cells impact factor journals Regulatory T (Treg) cells Scopus journals Regulatory T (Treg) cells PubMed journals Regulatory T (Treg) cells medical journals Regulatory T (Treg) cells free journals Regulatory T (Treg) cells best journals Regulatory T (Treg) cells top journals Regulatory T (Treg) cells free medical journals Regulatory T (Treg) cells famous journals Regulatory T (Treg) cells Google Scholar indexed journals Myeloid-derived suppressor cells (MDSCs) articles Myeloid-derived suppressor cells (MDSCs) Research articles Myeloid-derived suppressor cells (MDSCs) review articles Myeloid-derived suppressor cells (MDSCs) PubMed articles Myeloid-derived suppressor cells (MDSCs) PubMed Central articles Myeloid-derived suppressor cells (MDSCs) 2023 articles Myeloid-derived suppressor cells (MDSCs) 2024 articles Myeloid-derived suppressor cells (MDSCs) Scopus articles Myeloid-derived suppressor cells (MDSCs) impact factor journals Myeloid-derived suppressor cells (MDSCs) Scopus journals Myeloid-derived suppressor cells (MDSCs) PubMed journals Myeloid-derived suppressor cells (MDSCs) medical journals Myeloid-derived suppressor cells (MDSCs) free journals Myeloid-derived suppressor cells (MDSCs) best journals Myeloid-derived suppressor cells (MDSCs) top journals Myeloid-derived suppressor cells (MDSCs) free medical journals Myeloid-derived suppressor cells (MDSCs) famous journals Myeloid-derived suppressor cells (MDSCs) Google Scholar indexed journals Cancer immunotherapy articles Cancer immunotherapy Research articles Cancer immunotherapy review articles Cancer immunotherapy PubMed articles Cancer immunotherapy PubMed Central articles Cancer immunotherapy 2023 articles Cancer immunotherapy 2024 articles Cancer immunotherapy Scopus articles Cancer immunotherapy impact factor journals Cancer immunotherapy Scopus journals Cancer immunotherapy PubMed journals Cancer immunotherapy medical journals Cancer immunotherapy free journals Cancer immunotherapy best journals Cancer immunotherapy top journals Cancer immunotherapy free medical journals Cancer immunotherapy famous journals Cancer immunotherapy Google Scholar indexed journals immunosuppression articles immunosuppression Research articles immunosuppression review articles immunosuppression PubMed articles immunosuppression PubMed Central articles immunosuppression 2023 articles immunosuppression 2024 articles immunosuppression Scopus articles immunosuppression impact factor journals immunosuppression Scopus journals immunosuppression PubMed journals immunosuppression medical journals immunosuppression free journals immunosuppression best journals immunosuppression top journals immunosuppression free medical journals immunosuppression famous journals immunosuppression Google Scholar indexed journals immunotherapy era articles immunotherapy era Research articles immunotherapy era review articles immunotherapy era PubMed articles immunotherapy era PubMed Central articles immunotherapy era 2023 articles immunotherapy era 2024 articles immunotherapy era Scopus articles immunotherapy era impact factor journals immunotherapy era Scopus journals immunotherapy era PubMed journals immunotherapy era medical journals immunotherapy era free journals immunotherapy era best journals immunotherapy era top journals immunotherapy era free medical journals immunotherapy era famous journals immunotherapy era Google Scholar indexed journals chemokines articles chemokines Research articles chemokines review articles chemokines PubMed articles chemokines PubMed Central articles chemokines 2023 articles chemokines 2024 articles chemokines Scopus articles chemokines impact factor journals chemokines Scopus journals chemokines PubMed journals chemokines medical journals chemokines free journals chemokines best journals chemokines top journals chemokines free medical journals chemokines famous journals chemokines Google Scholar indexed journals tumor microenvironment articles tumor microenvironment Research articles tumor microenvironment review articles tumor microenvironment PubMed articles tumor microenvironment PubMed Central articles tumor microenvironment 2023 articles tumor microenvironment 2024 articles tumor microenvironment Scopus articles tumor microenvironment impact factor journals tumor microenvironment Scopus journals tumor microenvironment PubMed journals tumor microenvironment medical journals tumor microenvironment free journals tumor microenvironment best journals tumor microenvironment top journals tumor microenvironment free medical journals tumor microenvironment famous journals tumor microenvironment Google Scholar indexed journals multiple metabolic regulation articles multiple metabolic regulation Research articles multiple metabolic regulation review articles multiple metabolic regulation PubMed articles multiple metabolic regulation PubMed Central articles multiple metabolic regulation 2023 articles multiple metabolic regulation 2024 articles multiple metabolic regulation Scopus articles multiple metabolic regulation impact factor journals multiple metabolic regulation Scopus journals multiple metabolic regulation PubMed journals multiple metabolic regulation medical journals multiple metabolic regulation free journals multiple metabolic regulation best journals multiple metabolic regulation top journals multiple metabolic regulation free medical journals multiple metabolic regulation famous journals multiple metabolic regulation Google Scholar indexed journals
Article Details
Introduction
Immune checkpoint inhibitors (ICIs) have been developed, and now leading to a change in the entire therapeutic algorithm in many malignant diseases. However, ICIs are not equally effective in all cancer patients, and their response rates have been reported to be different among patients [1-4]. Moreover, there are patients who had extremely progressive diseases after treatment with ICIs [5,6]. Although the mechanisms of resistance are complicated and have not yet been clarified, host factors involving immune-suppressor cells such as regulatory T (Treg) cells, tumor associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) and their metabolic pathways in the tumor microenvironment (TME) play important roles in resistant to ICIs [7-9]. Metabolic changes are frequently associated with advancement of malignant diseases, and malnutrition is frequently observed as cancer cachexia, which is a multi-factorial metabolic disorder; it is characterized by hypoproteinemia, a significant reduction of body weight, and disturbance of multiple metabolic pathways, resulting in an imbalance of multiple metabolic regulation [10]. Although causation of cancer cachexia is complicated, host immune factors regulated by a variety of cytokines and chemokines have been reported to be important candidates, as well as multiple immunocompetent cells. Systemic inflammation has been reported to be another essential causative condition of cachexia that induces inflammatory immune reactions resulting in accumulation and proliferation of MDSCs, and is now used as diagnostic criteria of cancer cachexia [11-13]. We have reported that immune function, measured by PHA-stimulated proliferation of lymphocytes (stimulation index: SI), is decreased along with the advancement of cancer, and is significantly inversely correlated with nutritional parameters such as rapid turnover protein (RTP) including prealbumin, retinol binding protein and transferrin. Circulating numbers of MDSCs were significantly elevated in patients with various types of cancer, and these levels were significantly inversely correlated with SIs and RTP levels. Moreover, systemic inflammation measured using a patient’s neutrophil-lymphocyte ratio (NLR) have been shown to be associated with levels of MDSCs, as well as inversely to both SIs and RTP levels [14-17]. Although it is well known that Treg cells, TAMs and MDSCs play significant roles in immune suppression in the TME, these cells are also very important in the orchestration of metabolism in TME such as angiogenesis and production of indoleamine 2,3-dioxygenase (IDO) and nitric oxide (NO) towards the escape, progression and expansion of tumor cells. In the present review, we describe the pathological consideration and metabolic characterizations of immune-suppressor cells in TME. Moreover, these immune suppressor cells recently are a point of focus as one of the metabolic regulators in several non-malignant conditions, such as obesity, diabetes, and pregnancy, and these interesting findings are also described in this review.
1.1 Tumor Associated Macrophages (TAMs, Figure 1)
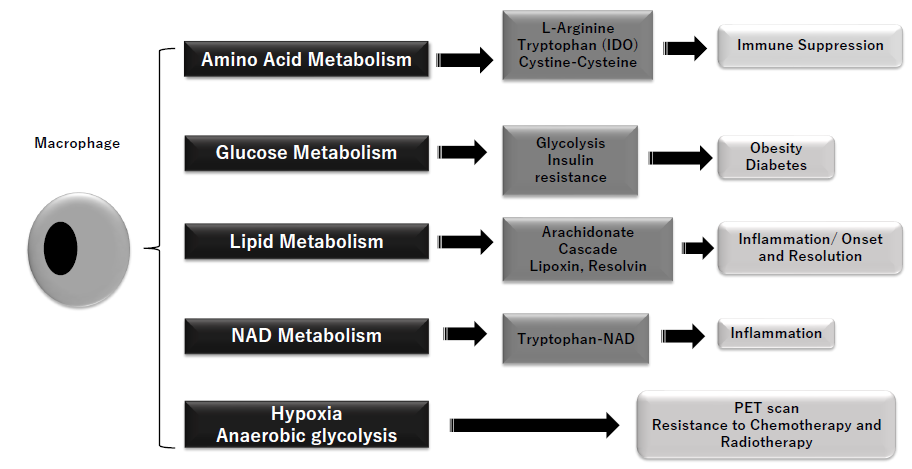
Figure 1: Metabolic action of macrophage
Arginine metabolism (to ornithine and urea) is regulated by macrophages, cystine/cysteine metabolism and tryptophan metabolism with IDO (indoleamine 2,3-dioxygenase) are substantial parts of the immunosuppression related to MDSC (myeloid-derived suppressor cells). Stimulated macrophages (M1) show metabolic shift to anaerobic glycolysis and insulin resistance, and lead to obesity and diabetes. Macrophages are important regulator of inflammation and its resolution. M1 macrophages regulate COX (cyclooxygenase) 1/COX2, leukotriene A4, and thromboxane A, while M2 does arachidonate, COX1 and PGE (prostaglandin)s. Thus, macrophages play important roles in activation and resolution of inflammation. Nicotinamide adenine dinucleotide (NAD), terminal metabolite of tryptophan, shows significant induction of inflammatory cytokines. Macrophages-related hypoxia and anaerobic glycolysis in tumor tissues, typically referred as Warburg’s effect is used in PET scan and introduce resistances to chemotherapy and radiotherapy.
Multiple phenotypes of macrophages have long been studied and recently recognized as the result of different microenvironmental stimuli [18]. TAMs have been reported to be closely associated with the regulation of variable important biological pathways related to malignant characterization, including local tumor expansion, tumor cell growth, resistance to chemotherapy and immunotherapy, metastasis, angiogenesis and immunosuppression [19-23]. Biswas and Mantovani reported two distinct phenotypes of macrophages, and named M1 and M2 [24]. M1 macrophages are proinflammatory macrophages, induced by γ interferon and Toll-like receptor (TLR) ligands, and characterized by the production of proinflammatory cytokines, inducible nitric oxide synthase (iNOS), reactive oxygen species (ROS), polarization of T-helper cell 1 (Th1) responses, and anti-tumor activity. On the other hand, M2 macrophages are induced by IL-4 and IL-13, weaken inflammation, and promote both tumor progression and immunosuppression. A major fraction of macrophages is reported to polarize to M2 during tumor progression. Lipid metabolism is reported to be essential because lipids are important for phagocytosis of macrophages as energy sources and for regulating the membrane fluidity necessary for the phagocytosis mechanism. Macrophages are associated with onset and restoration of inflammation as sources of lipid mediators [25]. M1 macrophages induce cyclooxygenase-2 (COX2) through control of COX1 and the production of leukotriene A4 and thromboxane A, while M2 macrophages upregulate arachidonate 15-lipoxygenase and COX1 [26]. Macrophages also affect the production of prostaglandin E, and can thus profoundly alter their lipid profiles as well as the production of lipid mediators involved in the activation and restoration of inflammation. Type 2 diabetes is a common disorder that causes an increase of blood glucose levels, and has become a global epidemic and a source of huge social and economic costs. Metabolic changes driven by overnutrition and resultant obesity may appear, and resistance to insulin is reported to be closely associated with chronic inflammation [27]. In the adipose tissue of obese individuals, monocytes are recruited and differentiated into M1 macrophages, and may play important immunological roles involved in inflammation [28]. An insulin resistance is established by the orchestration of cells through inflammation [29]. TAMs are characterized by their high expression of M2 markers, and it has been reported that TAMs show high glycolytic activity with high lactate secretion, which is similar to the metabolic features of M1 macrophages [30]. Therefore, although M2 macrophages play important roles in immunosuppression and tumor progression in TME, a differential metabolic regulation of macrophages that is associated with tumors may exist. Hypoxia and aerobic glycolysis are well-known to be resistant factors for antitumor therapies such as chemotherapy and radiotherapy [31,32]. Aerobic glycolysis, known as The Warburg effect, is a form of modified cellular metabolism found in cancer cells, which tends to favor fermentation over the aerobic respiration pathway that most other cells of the body prefer [33]. The Warburg effect is diagnostically the basis for the PET (positron emission tomography) scan, in which an injected radioactive glucose analog is detected at higher concentrations in malignant tumors than in non-cancerous tissues [34]. Jeong et al. reported that TAMs can contribute to tumor hypoxia and glycolysis and the production of TNF-α by TAMs is one of the mechanisms of high glycolysis in cancer cells [35]. It is also reported that intracellular nicotinamide adenine dinucleotide (NAD), which is the final product of tryptophan metabolism, works as a regulator of inflammatory cytokines including TNF-α and IL-6 [24].
1.2 Regulatory T (Treg) cells (Figure 2)
Treg cells control immune reactions to various types of antigens to maintain immune homeostasis. In cancer, Treg cells are involved in tumor development, progression and expansion by suppressing antitumor immunity. Treg cells keep peripheral immune tolerance and work to prevent autoimmune disease on tissue injury when intracellular antigens are exposed to the immune system that may lead to memory of central thymic selection [36]. In cancer, the suppressive properties of Treg cells can also be established and tumors obtain mechanisms to escape from immune surveillance of a tumor bearing host. The suppression mechanisms have been reported to be variable, including actions to dendritic cells through cytotoxic lymphocyte antigen-4 (CTLA-4), production of inhibitory cytokines, and expression of immune checkpoint molecules such as CTLA-4, inducible T-cell co-stimulator (ICOS), and lymphocyte activation gene-3 (LAG-3) [37]. Metabolic characterization in the TME is determined by a depletion of glucose, glutamine and tryptophan and enrichment of lactic acid and kynurenines [38]. There are metabolic differences between CD8+ T cells and Treg cells. CD8+ T cells utilize aerobic glycolysis primarily whereas Treg cells use oxidative phosphorylation (OXPHOS), which is another mechanism of immune suppression and resultant tumor growth in the TME. IDO is an essential enzyme in the kynurenine pathway of tryptophan metabolism. Depleted levels of tryptophan in the tumor microenvironment and cause T cell dysfunction [39]. The IDO-dependent catabolism subsequently induces the generation of Treg cells through inhibition of IL-6 production by DCs [40]. Recent investigations have revealed that T cell responses are suppressed by nutritional conditions of the TME, such as glucose, amino acids and fatty acid. Furthermore, hypoxia is: commonly seen in tumor tissue and HIF-1 alpha; upregulated in hypoxia; a negative regulator of Treg cells differentiation; and essential for suppression activity of Treg cells [41,42].
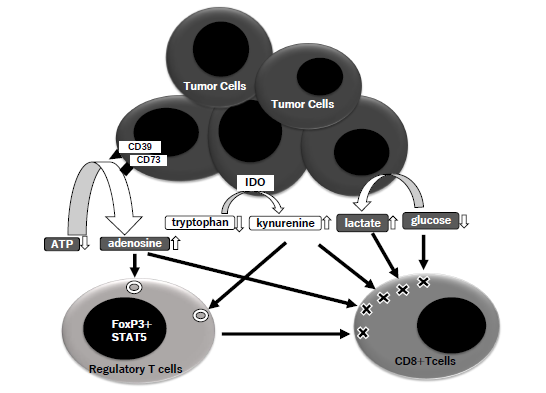
Figure 2: Metabolic actions of Treg.
The molecules of CD39 and CD73 on tumor cells metabolize extracellular ATP to adenosine, and IDO (indoleamine 2,3-dioxygenase) does tryptophan to kynurenine. Tumor cells consumes glucose and induce accumulation of lactate. The steps of these metabolism in TME (tumor microenvironment) induce an enhancement of Treg and suppression of CD8+T cells.
1.3 Myeloid-derived suppressor cells (MDSCs, Figure 3)
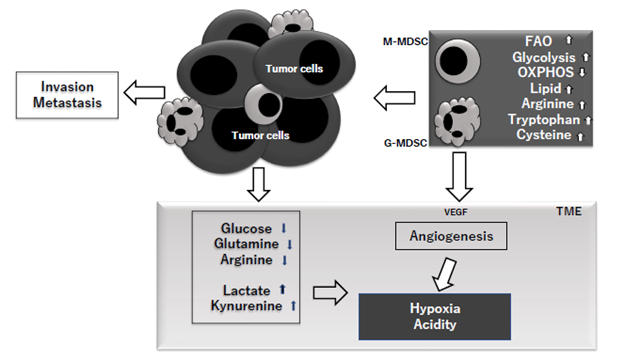
Figure 3: MDSC in the TME (tumor microenvironment) upregulate fatty acid oxidation (FAO), glycolysis and downregulate oxidative phosphorylation (OXPHOS). They also increase accumulations of lipid, tryptophan and cysteine and in TME, the concentrations of glucose, glutamine and arginine are decreased, while those of lactate and kynurenine are increased. VEGF (vascular endothelial growth factor) produced by tumor cells, MDSC and other cells including Treg is produced and lead to angiogenesis which enhance hypoxia and resultant acidity. These entire metabolic background can enhance tumor invasion and metastasis.
MDSCs are observed in most patients with cancer, where MDSCs infiltrating cancer tissue orchestrate many immunocompetent cells toward immunosuppression and resultant cancer escape. Prosperous studies on MDSCs have been performed in cancer patients during past decade and reported that MDSCs is the biggest obstacle for successful cancer immunotherapies [43]. The induction and expansion of MDSCs is clearly associated with chronic inflammation in cancer patients. Although normal inflammatory response after infectious diseases or trauma is self-limiting, the chronic inflammation in cancer patients develops different characterizations including immunosuppression, hypoxia and tumor progression [44]. In addition to developing solid status of immune suppression via induction of other immune suppressing cells such as Treg cells, MDSCs induce strong metabolic alterations toward immunosuppression and tumor expansion in the TME. It was reported that MDSCs produce reactive oxygen species (ROSs), which are not only toxic to tumor-infiltrating lymphocytes but also proliferate MDSCs [44]. An increased production of ROS upregulates the expression of VEGF (vascular endothelial growth factor) on MDSCs, and suppressed production of ROS induces the differentiation of MDSCs to macrophages or DCs. MDSCs also produce reactive nitrogen species (RNS) and nitric oxide (NO), and increased levels of NO induce the expression levels of cyclooxygenase 2 (COX-2) and HIF-1alpha, resulting in high production of prostaglandin-E2 (PGE2) and VEGF. PGE2 has been reported to upregulate the expressions of IDO, IL-10, and arginase. The decreased levels of L-arginine by arginase produced by MDSCs can be a cause of apoptosis of T lymphocytes via inducible nitric oxide synthase (iNOS) and block T cell activation [45]. MDSC in the TME have been reported to show upregulation of fatty acid oxidation (FAO), glycolysis, increased uptake of lipid, arginine, tryptophan and cysteine, and decreased oxidative phosphorylation (OXPHOS). It has been reported that, in TME, the levels of glucose, glutamine and arginine are decreased and those of lactate and kynurenine are increased. Thus, the TME became thus to be hypoxic and acidic [46]. It has been reported that, in cancer patients, elevated body mass index (BMI) is unexpectedly associated with longer survival, the so called “obesity paradox” [47]. However, there are solid data that tumors grow more rapidly and the survival is worse in obese patients than in non-obese patients. Moreover, excess weight is associated with increased cancer risk, morbidity and mortality in young adult patients with cancer [48]. MDSCs are frequently found in adipose tissue with the presence of pro-inflammatory mediators such as IL-6, IL-1β, TNF-α and PGE2, which are major inducers of MDSCs [49]. Obesity is frequently associated with metabolic dysfunction such as increased blood sugar levels. It has been demonstrated that obesity-driven MDSCs protect against an increase of glucose levels and insulin tolerance [49]. It is well known that chronic inflammation is closely related to aging. Franceschi reported that increased proinflammatory status is a distinctive feature of aging, and named chronic inflammation via continuous antigenic load and stress “inflamm-aging” [50]. Aging-associated pathogenesis such as atherosclerosis, Alzheimer’s disease, osteoporosis and diabetes mellitus are closely related to chronic inflammation. Since the numbers of MDSCs in bone marrow, peripheral blood, spleen and lymph nodes increase with age [51,52], increased immunosuppression and carcinogenesis among older individuals may be caused by inflamm-aging-driven MDSCs. Myelopoiesis increases along with aging, and its process has been reported to be regulated by TGF-β produced by MDSCs. Salminen et al. reported that this age-related growth of MDSCs may result in immunosenescence and destruction of host tissue appearing in older individuals [53,54]. As beneficial functions of MDSCss, one important point regarding pregnancy may be picked up in this review. MDSCs facilitate implantation and protect the allogeneic early embryo from immune-mediated rejection [55]. The insufficient tolerance can lead to severe complications such as preterm birth and fetal growth retardation and therefore tolerance is important during pregnancy. Although maternal-fetal tolerance was studied and helper T 2 cells (Th2 cells) were the major factor, another complicated mechanism involving MDSC has recently been reported to exist [56,57]; it was reported that the number of MDSC is significantly increased in the circulating blood of pregnant women compared to non-pregnant women, and especially high in the placenta in comparison with maternal and fetal blood [56,57]. The mechanisms of immune tolerance involving MDSCs are: inhibition of T-cell function via expression of arginase, iNOS, and IDO; polarization of Th2-response [58,59], and inhibition of NK cell function via downregulation of NKG2D receptor in NK cells.
2. Conclusions and Future Directions
Metabolic impacts in the TME play a crucial, although not fully understood, role in resistance to ICIs. As described above, a wide variety of metabolic characterizations associated with Treg cells, TAMs and
MDSCs in the TME exist. Among them, hypoxia, angiogenesis and chronic inflammation are important host factors to be considered when therapeutic approaches combined with ICIs are tried. Moreover, cancer immunotherapies tailored with metabolic characterizations such as parameters of hypoxia, inflammation or obesity may be needed for the establishment of successful treatment modalities against cancer in the immunotherapy era.
Conflict of interest
All of the authors declare that there is no conflict of interest regarding this article.
Funding statement
This research did not receive any specific funding from any commercial, public and non-profit organization.
Acknowledgements
Authors wish to thank the secretary, Mrs. Hideko Taguchi for excellent help in preparation of this article.
References
- Albittar AA, Alhalabi O, Glitza Oliva IC. Immunotherapy for melanoma. Adv Exp Med Biol 1244 (2020): 51-68.
- Siefker-Radtke AO, Apolo AB, Bivalacqua TJ, et al. Immunotherapy with checkpoint blockade in the treatment of urothelial carcinoma. J Urol 199 (2018): 1129-1142.
- Bair SM, Mato A, Svoboda J. Immunotherapy for the treatment of Hodgkin lymphoma: An evolving paradigm. Clin Lymphoma Myeloma Leuk 18 (2018): 380-391.
- Borghaei H, Langer CJ, Gadgeel S, et al. 24-month overall survival from KEYNOTE-021 cohort: Pemetrexed and carboplatin with or without pembrolizumab as first -line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol 14 (2019): 124-129.
- Champiat S, Cercle L, Ammari S, et al. Hyperprogressive disease is a new pattern in progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23 (2017): 1920-1928.
- Tay C, Qian Y, Sakaguchi S. Hyper-progressive disease: The potential role and consequences of T-regulatory cells foiling anti-PD-1 cancer immunotherapy. Cancers (Bael) 13 (2020): 48.
- Giannone G, Ghisoni E, Genta S, et al. Immuno-metabolism and microenvironment in cancer: Key players for immunotherapy. Int J Mol Sci 21 (2020): 4414.
- Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci 110 (2019): 2080-2089.
- Grzywa TM, Sosnowska A, Matryba P, et al. Myeloid cell-derived arginase in cancer immune response. Front Immunol 11 (2020): 938.
- Ni J, Zhang L. Cancer cachexia: Definition, staging and emerging treatments. Cancer Management Res 12(2020) 5597-5605.
- Fearon KC, Voss AC, Hustead DS. Cancer Cachexia Study Group. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 83 (2006): 1345-1350.
- Evans WJ, Morley JE, Argiles J, et al. Cachexia: new definition. Clin Nutr 27 (2008): 793-799.
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 12 (2011): 489-495.
- Gonda K, Shibata M, Ohtake T, et al. Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, inflammation, and poor prognosis in patients with breast cancer. Oncol Lett 14 (2017): 1766-1774.
- Nakamura I, Shibata M, Gonda K, et al. Serum levels of vascular endothelial growth factor are increased and correlate with malnutrition, immunosuppression involving MDSCs and inflammation in patients with cancer of the digestive system. Oncology Lett 5 (2013): 1682-1686.
- Gonda K, Shibata M, Shimura T et al. Serum soluble interleukin-2 receptor is increased in malnourished and immunosuppressive patients with gastric and colorectal cancer: Possible influence of myeloid-derived suppressor cells. World J Oncol 3 (2012): 158-164.
- Ohki S, Shibata M, Gonda K, et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep 28 (2012): 453-458.
- Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev 262 (2014): 36-55.
- Ye H, Zhou Q, Zheng S, et al. Tumor associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis 9 (2018): 453.
- Chanmee T, Ontong P, Konno K, et al. Tumor associated macrophages as major players in the tumor microenvironment. Cancers 6 (2014): 1670-1690.
- Yang C, He L, He Pet al. Increased drug resistance in breast cancer by tumor associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med Oncol 32 (2015) 352.
- Mantovani A, Locati M. Macrophage metabolism shapes angiogenesis in tumors. Cell Metab 24 (2016): 653-654.
- Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab 15 (2012): 432-457.
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11 (2010): 889-896.
- Martinez FO, Gordon S, Locati M, et al. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patters of gene expression. J Immunol 177 (2006): 7303-7311.
- Mosca M, Polentarucci N, Mangano G, et al. Regulation of the microsomal prostaglandin E synthase-1 in polarized mononuclear phagocyte and its constitutive expression in neutrophils. J Leukoc Biol 82 (2007): 320-326.
- Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA 100 (2003): 7265-7270.
- Weinberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112 (2003): 1796-1808.
- McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 41 (2014): 36-48.
- De-Brito NM, Duncan-MJ, Da-Costa HC, et al. Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Biochim Biophys Acta Mol Cell Res 9 (2016): 63-78.
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13 (2008): 472-482.
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 4 (2004): 437-447.
- Warburg O. On the origin of cancer cells. Science 123 (1956): 309-314.
- Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology 231 (2004): 305-332.
- Jeong H, Kim S, Hong BJ, et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res 79 (2019): 795-806.
- Warren JS, Strayer DS. Immunopathology. In: Rubin EL, Reisner H, ed. Essentials of Rubin’s pathology. (2013): 85-86
- Tanaka A, Sakaguchi S. Targeting Treg cells in cancer immunotherapy. Eur J Immunol 49 (2019): 1140-1146.
- Singer K, Gottfried E, Kreutz M, et al. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother 60 (2011): 425-431.
- Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9 (2003): 1269-1274.
- Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 183 (2009): 2475-2483.
- Shi H, Chi H. Metabolic control of Treg cell stability, plasticity, and tissue-specific heterogeneity. Front Immunol (2019).
- Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha dependent induction of FoxP3 drives regulatory T cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA 109 (2012): E2784-2793.
- Ostrand-Rosenberg S. Myeloid-derived suppressor cells: Their role in cancer and obesity. Curr Opin Immunol 51 (2018): 68-75.
- Groth C, Hu X, Weber R, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumor progression. Brit J Cancer 120 (2019): 16-25.
- Nagaraj S, Gupta K, Pisarev V. et al. Altered recognition of antigen is a mechanism of CD8+T cell tolerance in cancer. Nat Med 13 (2007): 828-835.
- Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nature Rev Immunol 21 (2021): 485-498.
- Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer. A review. Curr Oncol Rep. 18 (2016): 56.
- Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci 1311 (2014): 57-76.
- Xia S, Shu H, Yang L, et al. Gr-1+CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem (2011) 286: 23591-23599.
- Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908 (2000): 244-254.
- Alves AS, Ishimura ME, Duarte YAO, Bueno V. Parameters of the immune system and vitamin D levels in old individuals. Front Immunol 9 (2018): 11-22
- Enioutina EY, Bareyan D, Daynes RA. A role for immature myeloid cells in immune senescence. J Immunol 186 (2011): 697-707.
- Pang WW, Price EA, Sahoo D, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Nat Acad Sci USA 108 (2011): 20012-20017.
- Salminen A, Kauppinen A, Kaarniranta K. AMPK activation inhibits the function of myeloid-derived suppressor cells (MDSC): impact on cancer and aging. J Mol Med 97 (2019): 1049-1064.
- Ostrand-Rosenberg S, Sinha P, Figley C, et al. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice. J Leukoc Biol 101 (2017): 1091-1101
- Wegmann TG, Lin H, Guilbert I, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship is successful pregnancy a TH2 phenomenon? Immunol Today 14 (1993): 353-356.
- Kostlin-Gille N, Gille C. Myeloid-derived suppressor cells in pregnancy and the neonatal period. Front Immunol 11 (2020): 584712.
- Kostlin N, Kugel H, Spring B, et al. Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses. Eur J Immunol 44 (2014): 2582-2591.
- Kostlin N, Hofstadter K, Ostermeir AL, et al. Granulocytic myeloid cells accumulate in human placenta and polarize toward a Th2 phenotype. J Immunol 196 (2016): 1132-1145.
