Meningoradiculitis and Immune Mediated Myositis in a Patient with Lyme disease: A Case Report of a Probably Underestimated Association
Article Information
Andra Ezaru*,1,2, Nicolae Grecu1,2, Luisa Villa1,3, Jacques Durant4, Michele Cavalli1, Sabrina Sacconi1, Angela Puma1,5
1Université Côte d’Azur, Peripheral Nervous System and Muscle Department, CHU Nice, 30 Voie Romaine, 06000 Nice, France
2Department of Neurology, University Emergency Hospital, 169 Splaiul Independentei, 050098 Bucharest, Romania
3Université Côte d’Azur, Pathology Department, CHU Nice, France
4Université Côte d’Azur, Infectious Disease Department, CHU Nice, 30 Voie Romaine, 06000 Nice, France
5UMR7370 CNRS, LP2M; Labex ICST; Université Nice Côte d’Azur, Faculty of Medicine, Nice, France
*Corresponding author: Andra Ezaru, Côte d´Azur University, Peripheral Nervous System and Muscle Department, CHU Nice, 30 Voie Romaine, 06000 Nice, France
Received: 27 September 2021; Accepted: 06 October 2021; Published: 01 November 2021
Citation: Andra Ezaru, Nicolae Grecu, Luisa Villa, Jacques Durant, Michele Cavalli, Sabrina Sacconi, Angela Puma. Meningoradiculitis and immune mediated myositis in a patient with Lyme disease: a case report of a probably underestimated association. Archives of Clinical and Biomedical Research 5 (2021): 856-861.
View / Download Pdf Share at FacebookAbstract
We describe the association of cervical meningoradiculitis and focal immune mediated myositis secondary to Borrelia infection in a 62 year old immune competent patient. Diagnosis was made by lumbar puncture, nerve conduction studies and muscle magnetic resonance imaging (MRI). Cerebrospinal fluid (CSF) analysis found evidence of an inflammatory process with elevated production of Borrelia IgG antibodies. Deltoid muscle biopsy detected the presence of spirochetal DNA with immunohistochemical changes corresponding to dermatomyositis. The clinical – pathological associations made in this case can help clarify the intricate processes that take place on a cellular level in patients with Lyme disease.
Keywords
Lyme disease; Meningoradiculitis; Immune mediated myositis; Dermatomyositis
Article Details
1. Introduct?on
Lyme disease (LD) is a multisystem infectious disease that affects the skin, joints, heart and both the central and peripheral nervous system. The classically described stages of Lyme infection are early localized, early disseminated and late disseminated. Erythema migrans, the hallmark of infection, occurs in the early phase but the majority of patients don’t report this phenomenon. Migratory mono or oligo-arthralgia occurs weeks after inoculation, as well as nervous system involvement, as a sign of early germ dissemination [1]. While in the United States of America the most widespread agent is Borrelia burgdorferi (Bb) sensu stricto which causes arthritis, in Europe, Borrelia garinii (Bg) and Borrelia afzelii are more frequently involved. The neurotropism of Bg accounts for the high frequency of neurological forms particularly in France [2]. Neurologic manifestation ranging from central nervous system involvement with encephalomyelitis and meningitis to peripheral nervous system involvement with cranial nerve palsies, radiculitis, mononeuritis multiplex and polyneuropathy occur in 10 to 15 % of infected individuals.
Muscle involvement is less well documented. Whereas generalized myalgia is a common occurrence in the early stages of infection, myositis seems to be rare and only anecdotal reports exist of immune mediated myositis associated with LD. The concomitant involvement of muscle and nerve is even more uncommon.
We report the case of a patient with menigoradiculitis and myositis secondary to Bg infection. Muscle biopsy showed the presence of spirochetal DNA and inflammatory infiltrates as well as immunological changes typically found in dermatomyositis.
This is to our knowledge the first report of simultaneous involvement of muscle and nerve in an immune competent patient with LD with pathological evidence of a subjacent immune mechanism. It opens the opportunity to better characterize the changes that occur on a molecular level in certain infections.
2. Materials and methods
A 62-year-old French male was admitted to our Neuromuscular Department with one-month history of abrupt onset severe left shoulder pain, left upper limb paresthesia and left hand weakness. Medical history was unremarkable except for L5-S1 chronic radiculopathy, right eye cataract and minor thalassemia. Of note, the patient regularly practiced outdoors activities, for the most part mountain biking and trekking. The pain was partially responsive to an initial empirical treatment with 20 mg Prednisone a day and first and second-line analgesics.
Clinical examination revealed slight atrophy involving the left deltoid, supraspinatus and first interossei muscles, with left scapular winging. Moderate motor deficit at 4/5 on the medical research council scale was noted involving the left deltoid, supraspinatus, wrist flexors and extensors, finger flexors and extensors, first dorsal interosseous, and abductor pollicis brevis. Tactile hypoesthesia was present in the homolateral C8 – T1 dermatomes. The rest of the neurologic examination was unremarkable.
Standard blood work revealed mild leukocytosis in the range of 12.5 x 10^9/L, with neutrophilia at 76, 4% and no other notable abnormalities. Inflammation markers and autoimmune panel were negative.
Nerve conduction studies revealed reduced compound motor action potentials (CMAP) amplitudes in the left axillary nerve at 0.67 mV, the median nerve at 4, 5 mV and the ulnar nerve at 3.3 mV with normal sensory action potentials. Needle electromyography (EMG) found fibrillation potentials, positive sharp waves and a reduced recruitment of motor unit action potentials (MUAP) in the left first dorsal interosseous and common finger extensor muscles. Conversely, a myogenic pattern of MUAP recruitment was found in left deltoid muscle. The results were compatible with left C7 - C8 – T1 radiculitis associated with left deltoid myopathy. A muscle MRI was performed in order to assess possible involvement of other muscle groups but revealed no other abnormalities apart from left deltoid contrast enhancement. No root compression or spinal cord lesions were noted.
CSF analysis showed normal glucose levels with elevated protein at 0, 8 g/L, cellularity at 80 elements/mm3 with 96% lymphocytic predominance, elevated IgG index and intrathecal oligoclonal bands synthesis. A complementary blood panel found Bb sensu lato IgG and IgM antibodies on enzyme linked immunosorbent assay (ELISA) and subsequent Western-Blot analysis confirmed the presence of outer surface protein C bands for Bg. Subsequent CSF examination also revealed elevated synthesis of Bb sensu lato specific IgG antibodies at 130, 7 UI/ml.
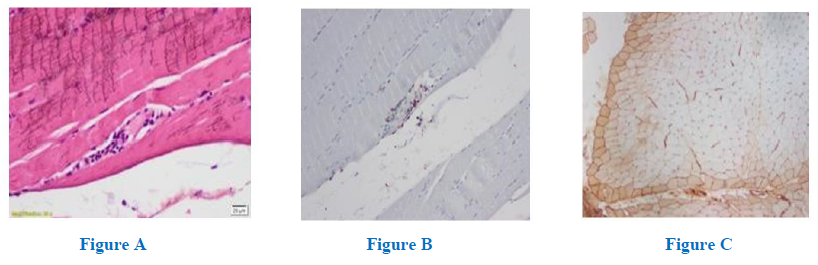
In view of the clinical and electro diagnostic studies (EDX) we proceeded with a left deltoid biopsy which revealed discrete T lymphocitic pericapillary and perifascicular infiltrates (Fig A and B) with perifascicular expression of human leukocyte antigen (HLA) type I (Fig C), with no signs of necrosis or regeneration, an anatomopathological picture characteristically seen in dermatomyositis (DM). Polymerase chain reaction (PCR) showed the presence of Bg DNA in the tissue sample.
3. Result
A diagnosis of LD related meningoradiculitis and focal immune mediated myositis was made, and the patient was started on combined antibiotic therapy with intramuscular Ceftriaxone at a dose of 2 g twice daily and Doxycycline at a dose of 20 mg daily for a total of 28 days. We added concurrent prednisolone therapy at a dose of 1mg/kg daily for the first two months with progressive improvement of distal paresthesia, shoulder pain and left arm muscle strength.
On initial corticosteroid tapering he experienced renewed severe shoulder pain hence we decided to maintain a dose of 40 mg/day with a slower reduction over the following six months. Three months after initiation of treatment, clinical examination showed remission of motor and sensory deficits with persistence of only slight atrophy of left deltoid, supraspinous and interossei muscles. Conduction parameters on EDX were already improving with increased CMAP in the left median and ulnar nerves at 5.8 mV and 4.7 mV respectively. Complete recovery was achieved one year after disease onset.
4. Discussion
Lymphocytic meningoradiculitis (Bannwarth syndrome) is well described in Borrelia infection. CSF analysis usually shows lymphocytic pleocytosis with high protein levels and intrathecal synthesis of Borrelia-specific antibodies [3]. On the other hand, although diffuse myalgia is common in the early stages of infection, franc myositis is quite rare.
In 1993 Reimers [4] published a case series of eight patients with Lyme disease and histologically proven myositis. In this series the pattern of muscle involvement followed the localization of skin lesions, arthritis or neuropathy, leading to the conclusion that muscle damage was caused either by direct local invasion from neighboring lesions or by hematological spreading of spirochetes or toxic factors.
Concerning immune mediated myositis evidence is even more limited. It is hypothesized that infection can trigger an autoimmune response given a genetic predisposition and multiple infectious agents have been implicated in triggering dermatomyositis but only a few case reports document the development of immune mediated myositis in relation to acute LD. Others have described clinical manifestations, and especially cutaneous ones, suggestive of DM associated with or as the initial manifestation of LD [5 - 10]. The activation of autoimmune mechanisms could promote the persistence of the disease[6].
Regarding our case, there are several aspects that provide evidence for both theories of myopathogenesis – direct invasion by the microorganism and activation of endogenous immune mechanisms. First, we found both spirochetal DNA and concomitant HLA type I expression on muscle biopsy specimens. Second, the focal and root distribution of the myopathy indicates a possible axonal transport of spirochetes leading to simultaneous nerve and muscle involvement. The same could be true in the case of hematogenic dissemination with subsequent vascular transmigration. Third, muscle biopsy results, the good response to corticoids and the prolonged tapering period are an argument in favor of an immune mediated process.
In conclusion, we believe that muscle involvement in LD is likely under recognized, but thorough clinical and laboratory examination can improve diagnostic accuracy.
We also believe that peripheral nerve and muscle constitute a single entity. In our opinion muscle involvement should be looked for whenever we are faced with infectious neuropathies.
We hope the future will bring a renewed interest in research aimed at better describing the pathologic mechanisms of infectious dissemination in general and LD in particular. This may in fact have important clinical and therapeutic implications.
5. Conclusion
In conclusion, the patient we described presented with radiculitis and focal myositis of autoimmune appearance secondary to Bg infection. Muscle involvement in Lyme disease is likely underestimated. We believe that focal muscle inflammation should always be considered as one of the clinical manifestations of Lyme disease. It should be explored whenever we are faced with a neuropathy secondary to LD. This may have important clinical and therapeutic implications.
6. Acknowledgements
None
7. Financial Support and Sponsorship
This article has not received funding.
References
- Hatchette TF, Davis I, Johnston BL. Lyme disease: clinical diagnosis and treatment. Can Commun Dis Rep 40 (2014): 194–208.
- Pourel J. Clinical diagnosis of Lyme borreliosis in case of joint and muscular presentations. Med Mal Infect 37 (2007): 523–531.
- Ogrinc K, Lusa L, Lotri?-Furlan S, et al. Course and Outcome of Early European Lyme Neuro borreliosis (Bannwarth Syndrome): Clinical and Laboratory Findings. Clin Infect Dis 63 (2016): 346–353.
- Reimers CD, de Koning J, Neubert U, et al. Borrelia burgdorferi myositis: report of eight patients. J Neurol 240 (1993): 278–283.
- Arniaud D, Mattei JP, Pham T, et al. Coexistent dermatomyositis, relapsing polychondritis, and positive Lyme serology. A case-report. Rev Rhum Engl Ed 64 (1997): 589–590.
- Fraser DD, Kong LI, Miller FW. Molecular detection of persistent Borrelia burgdorferi in a man with dermatomyositis. Clin Exp Rheumatol 10 (1992): 387–390.
- Hoffmann JC, Stichtenoth DO, Zeidler H, et al. Lyme disease in a 74-year-old forest owner with symptoms of dermatomyositis. Arthritis Rheum 38 (1995): 1157–1160.
- Horowitz HW, Sanghera K, Goldberg N, et al. Dermatomyositis associated with Lyme disease: case report and review of Lyme myositis. Clin Infect Dis 18 (1994): 166–171.
- Rodríguez M, Chou S, Fisher DC, De Girolami U, Amato AA, Marty FM. Lyme meningoradiculitis and myositis after allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 41 (2005): e112-114.
- Waton J, Pinault A-L, Pouaha J, Truchetet F. [Lyme disease could mimic dermatomyositis]. Rev Med Interne 28 (2007): 343–345.