Medical Management of Vasovagal Syncope: A Network Meta Analysis
Article Information
Rohit S. Loomba1*, Karan Nijhawan2, Saurabh Aggarwal3, Rohit R. Arora4
1Children’s Hospital of Wisconsin/Medical College of Wisconsin, Milwaukee, WI, USA
2Rush University Medical Center, Chicago, IL, USA
3Creighton University Medical Center, Omaha, NE, USA
4Chicago Medical School, Chicago, IL, USA
*Corresponding Author: Rohit S. Loomba, Children’s Hospital of Wisconsin/Medical College of Wisconsin, Milwaukee, WI, USA
Received: 11 April 2017; Accepted: 06 September 2017; Published: 08 September 2017
View / Download Pdf Share at FacebookAbstract
Introduction: Multiple pharmacologic therapies have been studied to prevent recurrence in patients with vasovagal syncope. Multiple head to head comparisons have been done with varying results. We aimed to perform a network meta-analysis to assess the efficacy of pharmacologic therapies in preventing recurrent syncope in patients with vasovagal syncope.
Methods: A systematic review of the literature was performed to identify randomized controlled trials describing recurrence of vasovagal syncope after initiation of pharmacologic treatment in patients with syncope who had a baseline positive tilt table test. A Bayesian network meta-analysis was conducted utilizing the geMTC package available for R: A Language and Environment for Statistical Computing.
Results: None of the included pharmacological therapies were found to significantly reduce the recurrence of vasovagal syncope when compared to placebo. When compared to one another atenolol did offer significant reduction in syncopal episodes when compared to cafedrine. Dihydroergotamine ranked the highest while cafedrine ranked the lowest.
Conclusions: Pharmacologic therapies do not offer significant reduction in recurrence of vasovagal syncope. First line therapy for these patients should include lifestyle changes such as increased fluid intake, and when appropriate, increased salt intake.
Keywords
Syncope; Vasovagal; Neurocardiogenic; Simple Fainting; Orthostatic
Article Details
1. Introduction
Syncope is the cause of approximately 3% of all emergency department visits and 6% of hospital admissions [1,2] . After exclusion of various other causes, vasovagal syncope is a common diagnosis with which these patients are discharged [3,4] . In the setting of vasovagal syncope, there is sudden loss off sympathetic activity with a concomitant increase in parasympathetic activity which subsequently leads to vasodilation and bradycardia [4,5] . Various triggers of vasovagal syncope have been identified which include changes in body position, changes in temperature, and emotional stressors among others. These may be mediated by abnormal mechanoreceptor activity [4].
While not fatal, vasovagal syncope can cause at least moderate levels of injury in approximately 5% of cases an also leads to decreased quality of life [6-8] . Several pharmacologic therapies have been utilized in the past to help reduce recurrence of vasovagal syncope with studies demonstrating variable efficacy. The purpose of this study was to conduct a network meta-analysis of published data to determine the efficacy of various pharmacological therapies in reducing the recurrence of vasovagal syncope.
2. Methods
2.1 Endpoints
A systematic review of the literature was performed to identify manuscripts describing recurrence of vasovagal syncope after initiation of pharmacologic treatment in patients with syncope who had a baseline positive tilt table test. Recurrence may have been assessed by questionnaire, patient interview, or by repeat tilt table test.
2.2 Manuscript search and identification strategy
Manuscripts were identified using electronic databases including PubMed, EMBASE, and OVID which were queried using one of the following terms: “syncope” or “vasovagal syncope” plus one of the following terms: “pharmacologic therapy”, “treatment”, “beta blocker”, “alpha-adrenergic”, “selective serotonin reuptake inhibitor”, “corticosteroid”. No specific restriction on year of publication was used. Manuscripts were initially screened by title and abstract with full text being retrieved for only select manuscripts.
These full text manuscripts were then reviewed by two of the authors and assessed for quality and bias (RL and SA). Any disparities in scoring of manuscripts were then independently reviewed by another author (RA). The Cochrane Handbook for Systematic Review was used for quality evaluation. Criteria for inclusion consisted of studies published in English comparing pharmacologic therapy for vasovagal syncope. Studies must have had at least two separate arms for inclusion and must have included data for the endpoint of recurrent syncope. Studies not including specific data for each different therapy were excluded as were studies investigating non-pharmacological interventions.
2.3 Data extraction
Data regarding baseline study-level patient characteristics and identified outcomes were extracted from the manuscripts identified for inclusion. Trial level data was extracted independently with use of a data collection form by two authors (RL and KN).
The data extraction was then independently reviewed by another author (SA) to ensure integrity of the extracted data. Baseline data was collected for each separate therapy included in the respective study. If therapy level data was not available then study level characteristics were extracted for baseline variables. Authors of included studies were not contacted for additional data.
2.4 Bias analysis
Bias was assessed using the Cochrane Risk of Bias assessment tool. Specifically, patient eligibility, randomization and concealment of allocation, blinding, completeness of outcome data, and statistical integrity were assessed using this scale.
2.5 Network meta-analysis
A Bayesian network meta-analysis was conducted utilizing the geMTC package available for the R: A Language and Environment for Statistical Computing (Version 3.2.2, Vienna, Austria). Markov chain Monte Carlo methods were utilized for this analysis. First a network plot was created to visually demonstrate the evidence base. Nodes represented specific pharmacologic therapies while the edges represented comparisons with direct evidence. Inconsistency was then assessed using the node splitting functionality. In this visual inspection of forest plots demonstrating the odds ratios for direct, indirect, and overall comparisons are plotted for each comparison.
Convergence was then assessed qualitatively by visual assessment of trace plots and visual assessment of density plots. Convergence was quantitatively assessed using via the Gelman-Rubin diagnostic and a resulting potential scale reduction factor. This was done multiple times with a different number of iterations until convergence was deemed acceptable. A potential scale reduction factor of 1 was targeted. The number of iterations corresponding to the most ideal convergence was then used for the remainder of the analysis.
Heterogeneity was assessed by utilizing the I2 statistic at the level of direct, pairwise comparisons. An I2 value of greater than 50% was considered to represent significant heterogeneity. A random-effects model was then used to determine a pooled odds-ratio comparing all included pharmacological therapies utilizing a number of iterations determined from the aforementioned convergence analysis. Rank probabilities were then calculated with all pharmacological therapies included in the model. These data were then used to create subsequent visual rankograms.
Reporting for this manuscript was done in concordance with the Preferred Reporting Items for Systematic Review and Meta-analyses extension statement for reporting of systematic review incorporating network meta-analyses [9] .
3. Results
3.1 Manuscript identification and characteristics
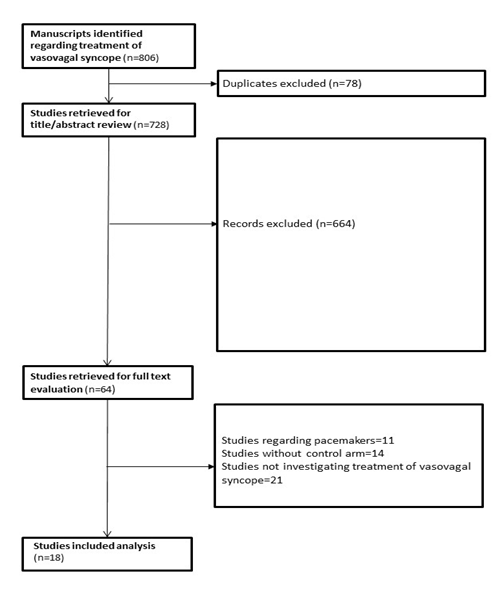
A total of 806 manuscripts were initially identified with 728 remaining after duplicated were excluded. After title and abstract review 664 manuscripts were excluded, leaving 64 manuscripts for which full text was obtained and reviewed. Studies were then excluded if they were based on evaluation of pacemaker implantation for vasovagal syncope, did not include a comparison arm, or did not investigate therapies for vasovagal syncope. A total of 18 studies were included in the final analysis [10-27] (Figure 1). All studies were randomized with 4 studies also having a crossover component (Table 1).
A total of 1,109 patients were included in this analysis with 15 different pharmacological therapies included. Table 1 demonstrates what specific therapies were included in each study and how many patients were present in each study for each respective therapy. Most studies included patients in their late 30s to early 40s, although 3 studies included patients in their teenage years. We elected to include these studies as they did not impact the direction of the pooled effect. Syncopal episodes had occurred for 0.5 to 6 years prior to study enrollment with 0.1 to 1.1 syncopal episodes occurring a month (Table 1).
Table 1: Characteristics of included studies
3.2 Bias analysis
A majority of studies demonstrated a low risk for bias per the Cochrane Risk of Bias assessment tool. A study by Zhang and colleagues as well as a study by Nakagawa and colleagues was found to have higher bias than the other included studies (Table 2). We did elect to include these in the overall analysis as the overall bias was still felt to be acceptable.
Table 2: Bias analysis of included studies
3.3 Network meta-analysis
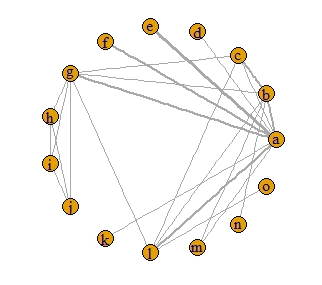
A network plot was constructed and demonstrated that the most frequent direct comparisons were between midodrine and placebo as well as metoprolol and placebo which were present in 3 studies. Several other direct comparisons were found in 2 studies (Figure 2).
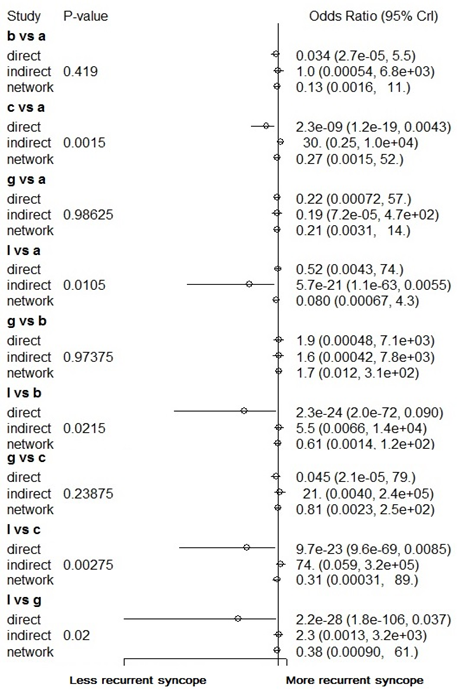
Nodesplitting analysis demonstrated inconsistency in the following comparisons: nadolol versus placebo, metoprolol versus propranolol, metoprolol versus nadolol, and metoprolol versus atenolol (Figure 3). Significant heterogeneity was present in the direct, pair-wise analysis with an I2 of 62%, thus a random effects model was used for the overall network meta-analysis with a total of 5,000 iterations. This was based on a potential scale reduction factor of 1.35 being achieved with 5,000 iterations.
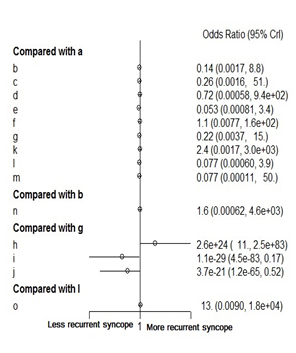
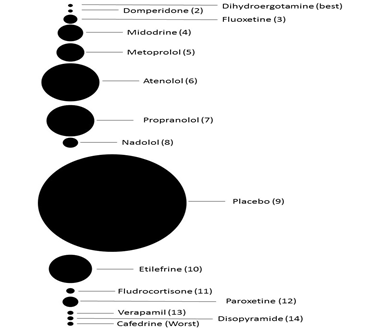
None of the included pharmacological therapies were found to significantly reduce the recurrence of vasovagal syncope when compared to placebo. When compared to one another atenolol did offer significant reduction in syncopal episodes when compared to cafedrine (Figure 4). Figure 5 demonstrates the rank probabilities of all the included pharmacological therapies and the number of patients for each therapy. Dihydroergotamine ranked as the most effective therapy while cafedrine ranked as the least effective therapy. Of note, midodrine was the highest ranked therapy with a significant number of patients. Placebo ranked 9th out of 15.
4. Discussion
The current study demonstrates a lack of significant difference between pharmacological therapies and placebo. This held true for all included therapies which included a variety of beta-blockers, alpha-adrenergic agents, corticosteroids, and calcium channel blockers. To our knowledge, this is the first network meta-analysis regarding the efficacy of these interventions for recurrent syncope.
This network meta-analysis had results that did differ from a recent pairwise, meta-analysis by Vyas and colleagues. Vyas and colleagues did not compare individual therapies but instead grouped them by mechanisms of action such as beta-blockers and alpha-adrenergic agents. Using a random-effects model, they found Beta-blockers to be associated with a 64% odds reduction in recurrent syncope when compared to control while alpha-adrenergic agents were associated with a 79% odds reduction in recurrent syncope when compared to control. It is important to note than when midodrine and etilefrine were analyzed separately only midodrine was associated with reduction in recurrent syncope [28]. The difference in results between our analysis and that of Vyas and colleagues is likely the result of slightly different study selection as well as the addition of data from indirect comparisons due to the network nature of our analysis. Additionally, the pairwise meta-analysis by Vyas and colleagues also commented on how the effect of beta-blockers was lost when considering studies comparing to placebo only [28]. Again, different beta-blockers may have different effects and hence combining them may give the analysis adequate power to detect subtle differences but individual therapy choice cannot be guided by such analyses.
Beta-blockers have been proposed for use in the setting of vasovagal syncope based on beta-receptor involvement in baroreceptor mediated reflexes [3,29,30] . Additionally, the observation that isoproterenol, a beta-agonist was able to facilitate hypotension, bradycardia, and subsequent syncope during tilt table testing also lead to the use of beta-blockers to help reduce the recurrence of vasovagal syncope [3]. Perhaps the largest trial to look at the beta-blockers was the Prevention of Syncope Trial. With 108 patients randomized to metoprolol and 100 patients randomized to placebo, this trial demonstrated no significant difference between the two groups in respect to recurrent vasovagal syncope [20] . Thus, overall it appears that beta-blockers do not offer a significant reduction in recurrence of vasovagal syncope.
Alpha-adrenergic agonists such as midodrine and etilefrine have also been used for treatment of vasovagal syncope. These agents act on the alpha receptors present on both venous and arterial vasculature, causing both veno- and vaso-constriction. There has been varying success with midodrine noted in previous studies. The Prevention of Syncope Trial IV is currently ongoing and is randomizing patients with vasovagal syncope to either midodrine or placebo with hopes to have a study with enough power to draw firm conclusions regarding the efficacy of midodrine in prevention of recurrent vasovagal syncope [31] . As of yet it appears that alpha-adrenergic agonists also do not lead to a significant reduction in recurrence of vasovagal syncope.
Corticosteroids, particularly fludrocortisone, have also been used in the setting of vasovagal syncope. Corticosteroids are intended to increase circulating volume by retaining sodium and, subsequently water. Pediatric studies have demonstrated improvement in symptoms with use of fludrocortisone although neither the pairwise meta-analysis by Vyas and colleagues nor the current network meta-analysis demonstrated significant reduction in recurrence of vasovagal syncope. Hence, corticosteroids also appear ineffective. The Prevention of Syncope Trial II was conducted with randomization of patients to either fludrocortisone or placebo. Although data from this study has yet to be published in full, Raj and colleagues obtained data from the authors of this trial, learning that the study demonstrated a 26% relative risk reduction associated with fludrocortisone when compared to placebo. This was not statistically significant with a p-value of 0.066 [32,33] .
Another group of pharmacological therapies that has been utilized to reduce recurrence of vasovagal syncope is the selective serotonin reuptake inhibitors. As their name implies these increase the serotonin levels in the brain, with serotonin being known to help facilitate regulation of blood pressure and heart rate via the central nervous system. Individual studies have demonstrated mixed results in regards to reduction in recurrent vasovagal syncope. With that, in mind, our analysis found no statistically significant reduction.
It is possible that pharmacological therapies may be helpful for a select subset of patients. Prospective identification of this subset, however, is not possible at this time and for the overall group of patients with vasovagal syncope it appears that no pharmacological therapies offer an advantage over placebo with respect to eliminating recurrence of episodes. Patients should receive counseling about what vasovagal syncope is and the mechanism by which it causes syncope. Patients should be encouraged to increase their fluid intake and, if appropriate, to increase their salt intake [34,35] . Patients should be instructed to sit down or lie down when they fell that a syncopal episode is about to occur in order to avoid any injury associated with the fall during a syncopal episode. For those with recurrence that is still troublesome to the patient despite counseling, then this analysis suggests that midodrine and metoprolol are the most likely to reduce recurrence of vasovagal syncope, although in the overall vasovagal syncope cohort neither treatment offered benefit over placebo.
This study offers a concise overview of the current literature regarding pharmacologic therapies for vasovagal syncope. The network nature of this meta-analysis allows for data from both direct and indirect comparisons to be pooled and also allows for rank probability analysis which cannot be done with conventional, pairwise meta-analysis. Additionally, this analysis only included randomized studies with acceptable levels of bias. The most important strength of this analysis lies in the fact that multiple therapies to be compared simultaneously for guidance to a physician.
This analysis isn’t without its limitations, however. Some comparisons were limited in the number of patents included. This is particularly true in respect to dihydroergotamine, domperidone, cafedrine, disopyramide, and fluoxetine. It is also important that not only there be a good number of patients but an adequate number of studies present in a network meta-analysis and some direct comparisons lacked more than 1 study with data from a direct comparison. We were also unable to produce relative risk estimates and only were able to generate odds ratios due to the methodology used in this network meta-analysis. While the odds ratios and relative risks do converge when events are frequent enough, we are unable to comment on the relationship between odds ratios and relative risk in this current study. The underlying conventional, pairwise meta-analysis demonstrated heterogeneity. We did not look at this as a limitation of our study as it seems unnecessary to perform any pooled analysis if there is no heterogeneity and all studies have demonstrated similar effects direction and magnitude. We did utilize a random effects model due to the heterogeneity.
5. Conclusion
Pharmacological therapies do not offer significant reduction in recurrence of vasovagal syncope. First line therapy for these patients should include lifestyle changes such as increased fluid intake, and when appropriate, increased salt intake.
References
- Day SC, Cook EF, Funkenstein H, Goldman L. Evaluation and outcome of emergency room patients with transient loss of consciousness. The American journal of medicine 73 (1982): 15-23.
- Gendelman HE, Linzer M, Gabelman M, Smoller S, Scheuer J. Syncope in a general hospital patient population. Usefulness of the radionuclide brain scan, electroencephalogram, and 24-hour Holter monitor. New York state journal of medicine 83 (1983): 1161-1165.
- Waxman MB, Yao L, Cameron DA, Wald RW, Roseman J. Isoproterenol induction of vasodepressor-type reaction in vasodepressor-prone persons. The American journal of cardiology 63 (1989): 58-65.
- Benditt DG, Remole S, Bailin S, Dunnigan A, Asso A, Milstein S. Tilt table testing for evaluation of neurally-mediated (cardioneurogenic) syncope: rationale and proposed protocols. Pacing and clinical electrophysiology : PACE 14 (1991): 1528-1537.
- Benditt DG, Ferguson DW, Grubb BP, Kapoor WN, Kugler J, et al. Tilt table testing for assessing syncope. American College of Cardiology. Journal of the American College of Cardiology 28 (1996): 263-275.
- Bartoletti A, Fabiani P, Bagnoli L, Cappelletti C, Cappellini M, et al. Physical injuries caused by a transient loss of consciousness: main clinical characteristics of patients and diagnostic contribution of carotid sinus massage. European heart journal 29 (2008): 618-624.
- Giada F, Silvestri I, Rossillo A, Nicotera PG, Manzillo GF, et al. Psychiatric profile, quality of life and risk of syncopal recurrence in patients with tilt-induced vasovagal syncope. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 7 (2005): 465-471.
- Baron-Esquivias G, Gomez S, Aguilera A, Campos A, Romero N, et al. Short-term evolution of vasovagal syncope: influence on the quality of life. International journal of cardiology 102 (2005): 315-319.
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Annals of internal medicine 162 (2015): 777-784.
- Flevari P, Livanis EG, Theodorakis GN, Zarvalis E, Mesiskli T, et al. Vasovagal syncope: a prospective, randomized, crossover evaluation of the effect of propranolol, nadolol and placebo on syncope recurrence and patients' well-being. Journal of the American College of Cardiology 40 (2002): 499-504.
- Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, et al. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. Journal of the American College of Cardiology 33(1999): 1227-1230.
- Kaufmann H, Saadia D, Voustianiouk A. Midodrine in neurally mediated syncope: a double-blind, randomized, crossover study. Annals of neurology 52 (2002): 342-345.
- Moya A, Permanyer-Miralda G, Sagrista-Sauleda J, Carne X, Rius T, et al. Limitations of head-up tilt test for evaluating the efficacy of therapeutic interventions in patients with vasovagal syncope: results of a controlled study of etilefrine versus placebo. Journal of the American College of Cardiology 25 (1995): 65-69.
- Madrid AH, Ortega J, Rebollo JG, Manzano JG, Segovia JG, et al. Lack of efficacy of atenolol for the prevention of neurally mediated syncope in a highly symptomatic population: a prospective, double-blind, randomized and placebo-controlled study. Journal of the American College of Cardiology 37 (2001): 554-559.
- Perez-Lugones A, Schweikert R, Pavia S, Sra J, Akhtar M, Jaeger F, et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. Journal of cardiovascular electrophysiology 12 (2001): 935-938.
- Brignole M, Menozzi C, Gianfranchi L, Lolli G, Bottoni N, Oddone D. A controlled trial of acute and long-term medical therapy in tilt-induced neurally mediated syncope. The American journal of cardiology 70 (1992): 339-342.
- Qingyou Z, Junbao D, Chaoshu T. The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. The Journal of pediatrics 149 (2006): 777-780.
- Raviele A, Brignole M, Sutton R, Alboni P, Giani P, et al. Effect of etilefrine in preventing syncopal recurrence in patients with vasovagal syncope: a double-blind, randomized, placebo-controlled trial. The Vasovagal Syncope International Study. Circulation 99 (1999): 1452-1457.
- Salim MA, Di Sessa TG. Effectiveness of fludrocortisone and salt in preventing syncope recurrence in children: a double-blind, placebo-controlled, randomized trial. Journal of the American College of Cardiology 45 (2005): 484-488.
- Sheldon R, Connolly S, Rose S, Klingenheben T, Krahn A, et al. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 113 (2006): 1164-1170.
- Theodorakis GN, Leftheriotis D, Livanis EG, Flevari P, Karabela G, et al. Fluoxetine vs. propranolol in the treatment of vasovagal syncope: a prospective, randomized, placebo-controlled study. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 8 (2006): 193-198
- Cox MM, Perlman BA, Mayor MR, Silberstein TA, Levin E, et al. Acute and long-term beta-adrenergic blockade for patients with neurocardiogenic syncope. Journal of the American College of Cardiology 26 (1995): 1293-1298.
- Zhang Q, Jin H, Wang L, Chen J, Tang C, et al. Randomized comparison of metoprolol versus conventional treatment in preventing recurrence of vasovagal syncope in children and adolescents. Medical science monitor : international medical journal of experimental and clinical research 14 (2008): CR199-203.
- Nakagawa H, Kobayashi Y, Kikushima S, Shinohara M, Obara C, et al. Long-term effects of pharmacological therapy for vasovagal syncope on the basis of reproducibility during head-up tilt testing. Japanese circulation journal 62 (1998): 727-732.
- Mahanonda N, Bhuripanyo K, Kangkagate C, Wansanit K, Kulchot B, et al. Randomized double-blind, placebo-controlled trial of oral atenolol in patients with unexplained syncope and positive upright tilt table test results. American heart journal 130 (1995): 1250-1253.
- Kluger J, Bazunga M, Goldman R, O'Rangers E, Azar P, et al. Usefulness of intravenous metoprolol to prevent syncope induced by head-up tilt. The American journal of cardiology 82 (1998): 820-823.
- Jhamb DK, Singh B, Sharda B, Kaul U, Goel P, et al. Comparative study of the efficacy of metoprolol and verapamil in patients with syncope and positive head-up tilt test response. American heart journal 132 (1996): 608-611.
- Vyas A, Swaminathan PD, Zimmerman MB, Olshansky B. Are treatments for vasovagal syncope effective? A meta-analysis. International journal of cardiology 167 (2013): 1906-1911.
- Sheldon R, Killam S. Methodology of isoproterenol-tilt table testing in patients with syncope. Journal of the American College of Cardiology 19 (1992): 773-779.
- Waxman MB, Asta JA. Induction of paradoxic bradycardia in rats by inferior vena cava occlusion during the administration of isoproterenol: the essential role of augmented sympathetic tone. Journal of cardiovascular electrophysiology 8 (1997): 405-414.
- Raj SR, Faris PD, McRae M, Sheldon RS. Rationale for the prevention of syncope trial IV: assessment of midodrine. Clinical autonomic research : official journal of the Clinical Autonomic Research Society 22 (2012): 275-280.
- Raj SR, Rose S, Ritchie D, Sheldon RS, Investigators PI. The Second Prevention of Syncope Trial (POST II)--a randomized clinical trial of fludrocortisone for the prevention of neurally mediated syncope: rationale and study design. American heart journal 151 (2006): 1186-1187.
- Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Progress in cardiovascular diseases 55 (2013): 425-433.
- Jordan J, Shannon JR, Black BK, Ali Y, Farley M, et al. The pressor response to water drinking in humans : a sympathetic reflex? Circulation 101 (2000): 504-509
- Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water. Lancet 353 (1999): 723.