Mechanobiology of MicroRNAs in Intervertebral Disk Degeneration
Article Information
Rajiv Supra1, Devendra K. Agrawal2*
1 College of Osteopathic Medicine, Touro University, Henderson, Nevada,
2 Department of Translational Research, College of Osteopathic Medicine of the Pacific,
Pomona, California
*Corresponding Author: Devendra K. Agrawal, MSc, Ph.D. (Biochem), Ph.D. (Med Sci), MBA, MS (ITM), FAAAAI, FAHA, FAPS, FIACS, Professor and Director, Department of Translational Research, Western University of Health Sciences, 309 E. Second Street, Pomona, California 91766-1854, USA.
Received: 10 January 2023; Accepted: 16 January 2023; Published: 17 January 2023
Citation:
Rajiv Supra and Devendra K Agrawal. Mechanobiology of MicroRNAs in Intervertebral Disk Degeneration. Journal of Spine Research and Surgery 5 (2023): 01-09
View / Download Pdf Share at FacebookAbstract
Intervertebral disk degeneration (IDD) is an intricate pathological process contributing to one of the major causes of low back pain. The degradation of the extracellular matrix (ECM), inflammation, and apoptosis have all been investigated as critical factors involved in the pathology of degenerative disk disease. Additionally, the presence of aberrant microRNAs (miRNAs), conserved molecules that regulate the amount protein post-transcriptionally, may play a crucial role in the pathogenesis of IDD. Research regarding the dysfunction of miRNAs in IDD has been well researched over the past five years. Here, we provide a critical overview of the current knowledge of miRNAs, emphasizing the processes involved in the degenerative disk pathology.
Keywords
Apoptosis; Back pain; Degenerative disk disease; Extracellular matrix; Inflammation; low back pain; miRNA; Nucleus pulposus cells
articles about Apoptosis Research articles about Apoptosis review articles about ApoptosisApoptosis PubMed articles Apoptosis PubMed Central articles Apoptosis 2024 articles Apoptosis 2025 articles Apoptosis Scopus articles Apoptosis impact factor journals Apoptosis Scopus journals Apoptosis PubMed journals Apoptosis medical journals Apoptosis free journals Apoptosis best journals Apoptosis top journals Apoptosis free medical journals Apoptosis famous journals Apoptosis Google Scholar indexed journals articles about Back pain Research articles about Back pain review articles about Back painBack pain PubMed articles Back pain PubMed Central articles Back pain 2025 articles Back pain 2026 articles Back pain Scopus articles Back pain impact factor journals Back pain Scopus journals Back pain PubMed journals Back pain medical journals Back pain free journals Back pain best journals Back pain top journals Back pain free medical journals Back pain famous journals Back pain Google Scholar indexed journals articles about Degenerative disk disease Research articles about Degenerative disk disease review articles about Degenerative disk diseaseDegenerative disk disease PubMed articles Degenerative disk disease PubMed Central articles Degenerative disk disease 2026 articles Degenerative disk disease 2027 articles Degenerative disk disease Scopus articles Degenerative disk disease impact factor journals Degenerative disk disease Scopus journals Degenerative disk disease PubMed journals Degenerative disk disease medical journals Degenerative disk disease free journals Degenerative disk disease best journals Degenerative disk disease top journals Degenerative disk disease free medical journals Degenerative disk disease famous journals Degenerative disk disease Google Scholar indexed journals articles about Extracellular matrix Research articles about Extracellular matrix review articles about Extracellular matrixExtracellular matrix PubMed articles Extracellular matrix PubMed Central articles Extracellular matrix 2027 articles Extracellular matrix 2028 articles Extracellular matrix Scopus articles Extracellular matrix impact factor journals Extracellular matrix Scopus journals Extracellular matrix PubMed journals Extracellular matrix medical journals Extracellular matrix free journals Extracellular matrix best journals Extracellular matrix top journals Extracellular matrix free medical journals Extracellular matrix famous journals Extracellular matrix Google Scholar indexed journals articles about low back pain Research articles about low back pain review articles about low back painlow back pain PubMed articles low back pain PubMed Central articles low back pain 2028 articles low back pain 2029 articles low back pain Scopus articles low back pain impact factor journals low back pain Scopus journals low back pain PubMed journals low back pain medical journals low back pain free journals low back pain best journals low back pain top journals low back pain free medical journals low back pain famous journals low back pain Google Scholar indexed journals articles about Inflammation Research articles about Inflammation review articles about InflammationInflammation PubMed articles Inflammation PubMed Central articles Inflammation 2029 articles Inflammation 2030 articles Inflammation Scopus articles Inflammation impact factor journals Inflammation Scopus journals Inflammation PubMed journals Inflammation medical journals Inflammation free journals Inflammation best journals Inflammation top journals Inflammation free medical journals Inflammation famous journals Inflammation Google Scholar indexed journals articles about miRNA Research articles about miRNA review articles about miRNAmiRNA PubMed articles miRNA PubMed Central articles miRNA 2030 articles miRNA 2031 articles miRNA Scopus articles miRNA impact factor journals miRNA Scopus journals miRNA PubMed journals miRNA medical journals miRNA free journals miRNA best journals miRNA top journals miRNA free medical journals miRNA famous journals miRNA Google Scholar indexed journals articles about Nucleus pulposus cells Research articles about Nucleus pulposus cells review articles about Nucleus pulposus cellsNucleus pulposus cells PubMed articles Nucleus pulposus cells PubMed Central articles Nucleus pulposus cells 2031 articles Nucleus pulposus cells 2032 articles Nucleus pulposus cells Scopus articles Nucleus pulposus cells impact factor journals Nucleus pulposus cells Scopus journals Nucleus pulposus cells PubMed journals Nucleus pulposus cells medical journals Nucleus pulposus cells free journals Nucleus pulposus cells best journals Nucleus pulposus cells top journals Nucleus pulposus cells free medical journals Nucleus pulposus cells famous journals Nucleus pulposus cells Google Scholar indexed journals articles about Osteopathic Medicine Research articles about Osteopathic Medicine review articles about Osteopathic MedicineOsteopathic Medicine PubMed articles Osteopathic Medicine PubMed Central articles Osteopathic Medicine 2032 articles Osteopathic Medicine 2033 articles Osteopathic Medicine Scopus articles Osteopathic Medicine impact factor journals Osteopathic Medicine Scopus journals Osteopathic Medicine PubMed journals Osteopathic Medicine medical journals Osteopathic Medicine free journals Osteopathic Medicine best journals Osteopathic Medicine top journals Osteopathic Medicine free medical journals Osteopathic Medicine famous journals Osteopathic Medicine Google Scholar indexed journals
Article Details
Introduction
One of the major causes of low back pain (LBP) is intervertebral disk degeneration (IDD) which has become a global burden greatly affecting the cost of healthcare. LBP affects 80% of adults at some point in their lives, becoming the leading cause of disability worldwide. The economic impact of LBP is vast costing up to 100 billion dollars per year in the United States [1,2]. The pathology of IDD is multifactorial consisting of age, lifestyle, epigenetics, and non-physiologic mechanical loading. The degeneration of connective tissue on the vertebrae occurs on a cellular level leading to gross physiological changes ultimately affecting spinal kinematics and decreased ability to bear compressive loads [2]. The underlying molecular mechanisms are still largely unknown; however, an increasing number of studies support that microRNAs (miRNAs) can influence many facets of cell activity including apoptosis, inflammation, and degradation of the extracellular matrix (ECM).
MicroRNAs, key mediators of gene expression, are a type of small noncoding RNAs that bind to the 3’-untranslated region inhibiting the translational process of specific mRNA molecules. Dicer is an enzyme that further processes mature miRNA in the cytoplasm after primary miRNA is generated in the nucleus. After incorporation in the RNA-induced silencing complex (RISC), they can suppress the translation of mRNAs ultimately regulating roughly 30% of human genes and cellular processes such as apoptosis and cytokine release [3]. MiRNAs have also been shown to interact with other RNAs such as circular RNAs, noncoding RNAs, and mRNAs, forming an intricate system of gene regulation in vertebral cells [4]. Dysregulation of these miRNA processes have been associated with intervertebral disk degeneration (IDD) [5]. This has motivated a new interest in miRNAs in relation to their role in IDD and potential new therapeutic approaches.
Several pathological mechanisms have been associated with IDD such as degradation of the ECM, cell apoptosis, and inflammation [6]. The ECM plays a critical role in the functionality of the intervertebral disk, consisting of type I and type II collagen providing much of the tensile strength [7]. The nucleus pulposus cells (NPCs), the cells that reside in the central region of the intervertebral disk, synthesize ECM components and dysregulation of this process results in the secretion of ECM degradative proteins such as matrix metalloproteinases (MMPs) [8]. Although this degradation process starts in the nucleus pulposus (NP), the annulus fibrosus (AF), the outer zone of the intervertebral disk, eventually becomes lost contributing to the overall degradation to the intervertebral disk [8,9]. Apoptosis and inflammation further enhance these degradative processes. It is well researched that NPCs release several cytokines such as interleukin (IL)-1b, IL-17, IL-6, and tumor necrosis factor (TNF)-a, which all can contribute to varying degrees of degeneration [10]. These cytokines have been deemed relevant because it is the source of pain associated with degenerative disk disease (DDD) through the infiltration into the nerve fibers and affecting their function [11].
The complex interplay between ECM degradation, inflammation, NP cell proliferation and apoptosis are the hallmarks of the DDD process [1] (Figure 1). Secretion of the inflammatory cytokines upregulates ECM degradative enzymes resulting in downregulation of ECM structure [12]. This degradation leads to a subsequent inflammatory response by NP cells as ECM fragments accumulate extracellularly [13]. Additionally, higher rates of apoptosis have been associated with decreased ECM production in IDD [14]. Based on the current research of the underlying mechanisms involved in IDD, standard therapy has been based on physiotherapy, pharmacological treatments, and arthroplasty [15]. Due to the high costs, limitations, and invasiveness of these treatments, there has been a high demand for novel therapies targeted at reducing pain and the degenerative process. Several therapies are being researched as potential alternatives which involves targeting miRNAs [16] (Table 1).
This review aims to provide a comprehensive overview of the role miRNAs play in the pathogenesis of degenerative disk disease. The pathology of IDD involving apoptosis, ECM degradation, inflammation, and nucleus populous cell function will be emphasized.
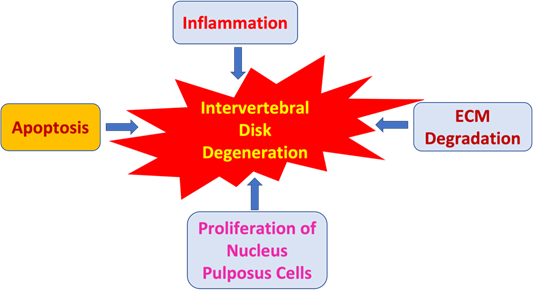
Figure 1: Effect of inflammatory processes, apoptosis, extracellular matrix (ECM) degradation, and proliferation of nucleus pulposus cells in the induction and acceleration of intervertebral disk degeneration.
MicroRNAs in Intervertebral Disk Degeneration
Ribonucleic acids are important in regulating genes. Among these, the miRNAs are a class of single stranded RNAs that function as post-transitional gene regulatory elements [17]. Their role in disk degeneration has been well documented involving the inflammation, NP cell apoptosis, and the ECM degradation processes [18]. NP cell apoptosis can have beneficial effects by offsetting aberrant NP cell proliferation during DDD while also being disadvantageous through the production of interstitial collagen fibers, weakening the tensile strength of the fibrous cap [19,20]. Thus, apoptosis of NP cells plays an integral role in DDD and tensile strength stability.
MiR-155 has been well-researched as one the miRNAs involved in regulating apoptotic pathways. Wang et al. revealed various miRNAs exhibiting differential expression in degenerative NP cells, specifically miR-155, which was the most downregulated [21]. When overexpressed, miR-155 inhibits NP cell apoptosis by suppressing Fas-associated protein with death domain (FADD) and caspase-3 expression. Additionally, when miR-155 is expressed in the cytoplasm of NP cells, there is an inverse relationship with FADD and caspase-3 [22,23]. MiR-27a has been another well-researched miRNA and its expression is high in NP cells. Its expression inhibits phosphoinositide-3 kinase (PI3K) by targeting its 3’-UTR and this inhibition was terminated by mutating miR-27a binding site [24]. In essence, when miR-27a is upregulated, apoptosis of NP cells occurs by targeting the PI3K pathway. Other miRNAs involved in the proliferation and degradation pathways of NP cells include miR-10b and miR-21. Previous studies have demonstrated the upregulation of miR-10b in degenerative NP tissues and overexpression of miR-10b increased NP cell proliferation through targeting homeobox D10 (HOXD10) [25]. MiR-21 has led to increased phosphorylation of Akt by targeting phosphatase and tensin homolog protein (PTEN), resulting in NP cell proliferation [26].
In addition to NP cell proliferation and degradation, miRNAs have a major impact on the ECM. The ECM is continuously degraded and synthesized by disk cells which are in a state of equilibrium [27]. This equilibrium becomes shifted towards degeneration in DDD through the alteration in the collagen type and proteoglycan content [28]. Chen et al. revealed the higher expression of miR-155 in lumbar spinal stenosis patients than those with lumbar disk herniation. Increased expression of miR-155 had a positive correlation with ligamentum flavum thickness and levels of type I and III collagen. miR-155 increased protein expression and mRNA of collagens type I and III in the fibroblasts of ligamentum flavum, while downregulation of miR-155 has the opposite effect [29]. Additionally, miR-377 has also been implicated in the pathologic remodeling of ECM. Protein kinase C (PKC) pathway has been shown to be a major regulator of chondrocyte differentiation. MiR-377 upregulation induced PKC signaling coupled with reductions in ADAMTS5, a metalloproteinase with thrombospondin motifs, and cleaved aggrecans [30]. Furthermore, it has been confirmed that miR-93 targets and regulates MMP-3, another collagen degrading enzyme. When downregulating miR-93, NP cells isolated from patients with vertebral disease led to increased levels of MMP-3 subsequently resulting in the degeneration of type II collagen [31]. Another study revealed MMP-13 overexpression in DDD [32]. MiR-27b takes part in this overexpression through downregulating NP cells isolated from degenerated disks [33]. Moreover, miR-133a downregulation observed in spinal tuberculosis and degenerative NP cells led to reduced levels of type II collagen expression [34,35].
Emphasizing miRNAs targeting against signaling pathways involved in ECM degradation, miR-98 was found to be involved in the IL-6/STAT pathway in DDD. When downregulating miR-98, IL-6 levels increased in NP tissue. Additionally, decreased levels of miR-98 initiated the STAT3 signaling cascade by increasing levels of MMP-2, pSTAT3, and STAT3, ultimately contributing to intervertebral disk degeneration [36]. MiRNA-132 and miR-7 have been shown to regulate the expression of growth differentiation factor 5 (GDF5). GDF5 is involved in ECM anabolism and polymorphisms of GDF5 resulted in degenerative diseases such as osteoarthritis [37-39]. Mi-R132 enhanced ECM degradation by targeting GDF5 leading to increased ADAMTS4 and MMP-13 expressions through the mitogen-activated protein kinase/extracellular signal-regulated kinases (MAPK/ERK) pathway. The miR-7 has also been studied enhancing ECM degradation by targeting GDF5, making miR-7 inhibition a promising therapeutic strategy [39].
Thus, findings from several investigations support the critical role of miRNAs in the metabolic pathogenesis of intervertebral disk degeneration. Variations in the miRNA expression of degenerated NP cells lead to dysregulation of metabolic enzymes leading to the compositional change of the ECM. This opens new avenues for potential therapeutic strategies which involves targeting the miRNAs involved in disk degeneration. For example, a preclinical study observed the in vivo changes of injecting an inhibitor of miR-141, a mRNA involved in disk degeneration, and studied its affects. This anti-miR-141 resulted in protective effects against IDD [40]. Further studies need to be conducted to evaluate the role of inflammation in the dysregulation of miRNAs and develop novel therapeutic strategies directed at the inflammation cascade.
MiRNAs and Apoptosis
Normally, apoptosis maintains the stability of the internal environment of the cell through autonomous programmed cell death controlled by genes. There are exogenous and endogenous pathways that play a critical role in human NPC apoptosis. The density of NPCs in the intervertebral disk tissue is reduced through the FasL-Fas signaling pathway. This exogenous signaling pathway involves the Fas-related death domain-containing protein (FADD) and caspase-3 pathway [41]. A major endogenous apoptotic pathway consists of the anti-apoptotic protein B-cell lymphoma leukemia-2 (Bcl-2) family which originates in the mitochondria [42]. Furthermore, miRNAs have been shown to be implicated in cellular senescence changes in the expression profile of various miRNAs have been involved in the regulatory pathways leading to apoptosis in DDD [43,44].
MiR-185 and miR-143-5p are two well researched miRNAs involved in DDD. Studies were conducted using murine models with DDD [45]. The b-galactosidase binding protein involved in apoptosis, galectin 3, was targeted by miR-185 [46]. Rats with DDD had significantly higher levels of galectin 3 than the control group. When miR-185 was inhibited, these expression levels further increased. MiR-143-5p was upregulated in NP tissues of murine models. This was accomplished by eukaryotic elongation factor 2 (eEF2), one of the targets of miR-143-5p. Once targeted, the dysregulation of miR-143-5p led to decreased levels of eEF2 and activation of the adenosine monophosphate activated protein kinase (AMPK) pathway [47,48]. Activating the AMPK pathway subsequently reduced type II collagen and aggrecan levels and when miR-143-5p was inhibited, it resulted in lower levels of senescence through inactivating AMPK (48). Interestingly, NP cells recovered from patients suffering from disk disease exhibited higher levels of miR-143 and its upregulation was shown to increase apoptosis by targeting Bcl2 [49].
Another major miRNA involved in the apoptosis of NPCs is miR-138-5p. Wang et al. discovered that miR-138-5p was significantly upregulated in degenerated disks and its inhibition profoundly reduced apoptosis [50]. NPCs can be protected from excessive apoptosis by knocking out miR-138-5p. Protection from apoptosis was mediated through sirtuin 1 (SIRT 1) upregulation, which is induced by PTEN/PI3K/Akt pathways [51]. Activation of the AMPK signal pathway inhibited the differentiation of NPCs and promoted apoptosis. Additionally, miR-141 further progressed the process of DDD through targeting the SIRT1/NF-kB pathway, leading to apoptosis [52]. In vitro studies revealed knocking out miR-141 attenuated DDD by delivering the down-regulated miR-141 through nanoparticles in the DDD murine model. MiR-222 was also shown to be upregulated in degenerated NPCs and the overexpressed miR-222 activated Bax and caspase -3 but inhibited Bcl-2. Of note, activation of BCL-2 can inhibit apoptosis while activation of Bax and caspase-3 promotes apoptosis [53].
A recent study also revealed that miR-494 upregulation led to lower levels of SRY-Box Transcription Factor (SOX)-9, a gene that has been shown to protect against IL-1b-induced apoptosis [54]. MiR-494 downregulating SOX9 is seen in DDD, shedding light on the process of apoptosis in disk degeneration [55]. Additionally, targeting JunD and cytochrome c can lead to inhibition of miR-494 and may protect NPCs from TNF-a induced apoptosis [56]. Researchers have also identified increased levels of miR-494 in DDD murine models, whereas inhibitors of miR-494 led to increased Bcl-2 and neuro-oncological ventral antigen 1 (NOVA1) levels and reduced expression of caspase-3 and Bax [57]. MiR-494 is heavily involved in the apoptosis of NPCs and plays an integral role in DDD. The abnormal expression of miR-129 5p may also serve as a role in DDD. Studies have shown reduced levels of miR-129 5p in humans with DDD, whereas NPCs treated with miR-129-5-p and bone morphogenic protein 2 (BMP-2) silencing RNAs displayed improved survival and decreased apoptotic activity [58]. Similarly, miR-499a-5p also exhibited significant downregulation in human degenerated NP cells. Knocking out miR-499a-5p enhanced NPC apoptosis, MMP-13, and MMP-3 expression while decreasing aggrecan and type II collagen levels. Furthermore, overexpressing miR-499a-5p reduced apoptosis in TNF-a treated NPCs. The abnormal expression of SOX4, however, weakened the negative of miR-499a-5p on apoptosis of NP cells [59]. This suggests that miR-499a-5p may be influenced by targeting SOX4.
A deeper understanding of the apoptotic processes involved in DDD is needed and may provide new therapeutic approaches in delaying or possibly reversing DDD. Another component that is heavily involved in DDD is inflammation and further research can lead to the development of novel therapies in reducing DDD.
Inflammation
Numerous studies have revealed inflammation as a key factor in the process of DDD. Inflammatory mediators such as ILs (interleukins), tumor necrosis factor (TNF)-a, nitric oxide, and prostaglandin E2 (PGE2) are the main regulators of the inflammatory response within the intervertebral disk [60]. Studies reveal that miRNAs can accelerate or delay the process of DDD through regulating inflammatory cytokines such as IL-1b and TNF-a [61].
MiR-146a was reported to inhibit mRNA expression of IL-1 b-mediated catabolic proteinases and MMPs [62]. However, Lv et al. revealed in peripheral mononuclear cells of patients with degenerative disk disease, miR-146 was significantly downregulated [63]. Additionally, they discovered that overexpression of miR-146a downregulated levels of TNF- a, IL-6, and IL-1b in lipopolysaccharide-stimulated NPCs. MiR-194-5p was also found to be downregulated in patients with intervertebral disk degeneration through miRNA microarray analysis [64]. Overexpression of miR-194-5p resulted in inhibiting and accelerating the expression of the cullin family genes 4A (CUL4A) and CUL4B. The inflammatory cytokines TNF-a and IL-6 also reduced levels of CUL4A and CUL4B in NPCs and AF cells. Similarly, miR-149 levels were significantly decreased in LPS-induced NPCs [64]. MiR-149 when overexpressed reduced the expression of collagen II and aggrecan and mitigated effects of MMP-3, ADAMTS4, and inflammatory mediators through targeting myeloid differentiation factor 88 [65].
MiR-27a was upregulated in the inflammatory DDD model using LPS stimulation. When miR-27a was inhibited, however, the p-p38/NF-kB expression and IL-6, IL-1, and TNF-a were decreased [66]. MiR-203-3p was upregulated in degenerated NP tissue and negatively correlated with estrogen receptor alpha (ERa) expression[67]. In the DDD murine model, miR-203-3p was shown to inhibit the inflammatory response and disk degeneration through targeting ERa. Activating TLR4/NF-kB pathways increased levels of inflammatory factors and expression of miR-625-5p while decreasing COL1A1 [68]. The rates of NPC apoptosis and TNF-a, IL-6, and IL-1 were also increased through the upregulation of miR-589-3p while levels of COL II and aggrecan were reduced through inhibiting Smad4 [69]. Interestingly, peripheral blood mononuclear cells from DDD patients revealed reduced expression of miR-146a while in the DDD murine model, miR-146a suppressed protein levels of TRAF6/NF-kB, leading to reduced levels of inflammatory cytokines in NPCs [70]. Collectively, the miRNAs that have been studied have a multitude affects contributing to inflammation, apoptosis, and ECM degradation seen in DDD (Table 1).
|
miRNA |
Expression |
Function |
Target |
Reference |
|
miR-155 |
Decrease |
Apoptosis |
FADD |
21 |
|
miR-27a |
Increase |
Apoptosis |
PI3K |
24 |
|
miR-21 |
Increase |
NP Proliferation |
PTEN |
26 |
|
miR-377 |
Increase |
ECM remodel |
ADAMTS5 |
30 |
|
miR-93 |
Decrease |
Collagen Degrade |
MPP3 |
31 |
|
miR-27b |
Increase |
NP Proliferation |
MMP-13 |
33 |
|
miR-133a |
Decrease |
Decrease Collagen |
Type II Collagen |
34, 35 |
|
miR-98 |
Decrease |
ECM Degradation |
IL-6/STAT |
36 |
|
miR-132 |
Increase |
ECM Degradation |
GDF5 |
37, 38 |
|
miR-7 |
Increase |
EMC Degradation |
GDF5 |
39 |
|
miR-185 |
Increase |
Apoptosis |
Galectin 3 |
46 |
|
miR-143-5p |
Increase |
Reduce Type II Collagen |
eEF2/AMPK |
47, 48 |
|
miR-138-5p |
Increase |
Apoptosis |
PTEN/PI3K/Akt |
51 |
|
miR-141 |
Increase |
Apoptosis |
SIRT1/NF-kB |
52 |
|
miR-222 |
Increase |
Apoptosis |
BAX/Caspase 3 |
53 |
|
miR-494 |
Increase |
Apoptosis |
SOX9 |
54 |
|
miR-129-5p |
Increase |
Apoptosis |
- |
58 |
|
miR-499a-5p |
Decrease |
Apoptosis |
MMP-13 |
59 |
|
miR-146a |
Increase |
Inflammation |
IL-1/MMP |
62 |
|
miR-194-5p |
Decrease |
Inflammation |
CUL4A/CUL4B |
64 |
|
miR-149 |
Increase |
Decrease Collagen II/Aggrecan |
MyD88 |
65 |
|
miR-203-3p |
Increase |
Inflammation |
ERa |
67 |
|
miR-625-5p |
Increase |
Inflammation |
TLR4/NF-kB |
68 |
|
miR-589-3p |
Increase |
Decrease COL II/Aggrecan |
SMAD4 |
69 |
|
miR-146a |
Increase |
Reduced Inflammation |
TRAF6/NF-kB |
70 |
Table 1: MiRNAs involved in degenerative disk disease
Therapeutic Approaches in Degenerative Disk Disease (DDD)
Currently, there are several areas of growing research in novel strategies for DDD therapy (Figure 2). Genetic modification, stem cells, and growth factors all have shown beneficial effects in in vivo and in vitro studies [71].
Injecting growth factors into degenerated disks can stimulate the ECM and delay the degeneration process [72]. Growth factors such as bone morphogenic proteins (BMPs), insulin like growth factor-1 (IGF-1), platelet derived growth factor (PDGF), and transforming growth factor beta (TGF-b) are peptides that cause proliferation and differentiation of cells. They can stimulate anabolic function while reducing inflammatory cytokines such as IL-1, IL-6, and TNF-a. Growth factors are used for the restoration of DDD but are limited due to their biological half-lives [73,74]. The growth factors within platelet-rich plasma have been shown to be a promising therapeutic strategy for DDD [75]. In the rabbit DDD model, administering platelet-rich plasma with gelatin-based microspheres in the degenerated NPCs suppressed the degeneration process significantly. Administering platelet rich plasma in a similar rabbit model also revealed significant restoration of chondrocyte cells and disk height [76,77].
Gene therapy has been another growing field in the therapeutic strategies of DDD. This method involves introducing genes to target cells using viral and non-viral vectors. Target cells are then removed and put into culture medium where the cells can be altered and eventually re-implanted into target organs. The cells with genetic alterations can go on to produce proteins
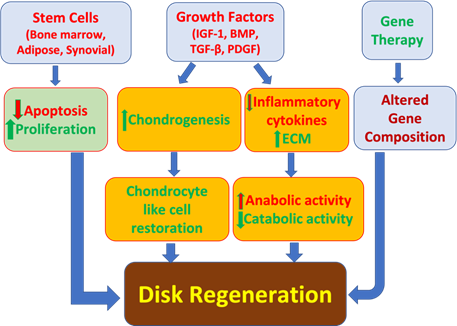
Figure 2: Current approaches in the treatment of degenerative disk disease. BMP, bone morphogenic protein; ECM, extracellular matrix; IGF-1, insulin like growth factor-1; PDGF, platelet derived growth factor.
that support intervertebral disk regeneration [78]. Additionally, stem cells from adipose tissue, synovial tissue, and bone marrow can mediate changes in fibrocartilage like tissues. By transplanting cells, they can induce paracrine signaling to affect endemic cells to produce regenerative substances in intervertebral disks. De novo cells can also influence homeostasis and production of ECM proteins. These cells are obtained from embryonic human NPCs; mesenchymal stem cells (MSCs), and chondrocytes through autologous adipose tissue and bone marrow [79,80]. Adipose mesenchymal stem cells (ASCs) used on NPCs was shown to reduce expressions of TNF-a and IL-1b and decrease apoptosis. ASCs on AF cells increased proliferation and had anabolic effects while decreasing catabolic factors and inflammatory cytokines [81]. Collectively, gene therapy, stem cells, and growth factors have shown promising results in the regeneration of intervertebral disks, however further studies are needed to fully understand the limitations and applications of these approaches (Figure 2).
Conclusion
MiRNAs are a critical component in regulating genes and are involved in the pathogenesis of degenerative disk disease. There is no optimal treatment for DDD, but substantial progress has been made in studying miRNAs and there association DDD. The studies currently available highlight miRNAs as a major factor in disk degeneration. Further studies are needed however, to research the impact miRNAs have on DDD and to develop new promising therapeutic strategies.
Author Contributions:
Concept and design: RS, DKA; Literature Search: RS, DKA; Critical review and interpretation of the findings: RS, DKA; Drafting the article: RS; Revising and editing the manuscript: RS, DKA; Final approval of the article: RS, DKA.
Funding:
This work was supported by the research grants R01 HL144125 and R01HL147662 to DKA from the National Heart, Lung, and Blood Institute, National Institutes of Health, USA. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement:
Not applicable.
Informed Consent Statement:
Not applicable
Data Availability Statement:
Not applicable since the information is gathered from published articles.
Acknowledgments:
None
Conflicts of Interest:
The authors declare no conflict of interest.
References
- Urban J P G, Roberts S. Degeneration of the intervertebral disk. Arthritis Res Ther. 2003; 5(3): 120-130.
- Adams M A, Roughley P J. What is intervertebral disk degeneration, and what causes it? Spine (Phila Pa 1976) [Internet]. 2006 Aug [cited 2022 Dec 10]; 31(18): 2151-2161.
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function, and decay. Nat Rev Genet. 2010 Sep; 11(9): 597-610.
- Zhu J, Zhang X, Gao W, et al., lncRNA/circRNA miRNA mRNA ceRNA network in lumbar intervertebral disk degeneration. Mol Med Rep. 2019 Oct; 20(4): 3160-3174.
- Ohrt-Nissen S, Døssing KB v., Rossing M, et al., Characterization of miRNA Expression in Human Degenerative Lumbar Disks. Connect Tissue Res. 2013 Jun 15; 54(3): 197-203.
- Clouet J, Vinatier C, Merceron C, et al. The intervertebral disk: from pathophysiology to tissue engineering. Joint Bone Spine. 2009 Dec; 76(6): 614-618.
- Eyre D R, Muir H. Quantitative analysis of types I and II collagens in human intervertebral disks at various ages. Biochimica et Biophysica Acta (BBA) - Protein Structure. 1977 May; 492(1): 29-42.
- Roberts S, Caterson B, Menage J, et al., Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disk. Spine (Phila Pa 1976). 2000 Dec; 25(23): 3005-3013.
- Dowdell J, Erwin M, Choma T, et al., Intervertebral Disk Degeneration and Repair. Neurosurgery. 2017 Mar 1; 80(3S): S46-54.
- Johnson Z, Schoepflin Z, Choi H, Shapiro I, Risbud M. Disk in flames: Roles of TNF-α and IL-1β in intervertebral disk degeneration. Eur Cell Mater. 2015 Sep; 30: 104-117.
- Risbud M V, Shapiro I M. Role of cytokines in intervertebral disk degeneration: pain and disk content. Nat Rev Rheumatol. 2014 Jan; 10(1): 44-56.
- Wuertz K, Haglund L. Inflammatory Mediators in Intervertebral Disk Degeneration and Diskogenic Pain. Global Spine J. 2013 Jun 21; 3(3): 175-184.
- Quero L, Klawitter M, Schmaus A, et al., Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disk cells through modulation of toll-like receptor 2 signalling pathways. Arthritis Res Ther. 2013 Aug; 15(4): R94.
- Liebscher T, Haefeli M, Wuertz K, et al., Age-related variation in cell density of human lumbar intervertebral disk. Spine (Phila Pa 1976). 2011 Jan 15; 36(2): 153-159.
- Fernandez-Moure J, Moore C A, Kim K, et al., Novel therapeutic strategies for degenerative disk disease: Review of cell biology and intervertebral disk cell therapy. 2018 Jan;6:205031211876167.
- Henry N, Clouet J, le Bideau J, et al., Innovative strategies for intervertebral disk regenerative medicine: From cell therapies to multiscale delivery systems. Biotechnol Adv. 2018 Jan; 36(1): 281-294.
- Fabbri M, Calin G A. Epigenetics and miRNAs in human cancer. Adv Genet. 2010; 70: 87-99.
- Wei A, Brisby H, Chung S A, Diwan A D. Bone morphogenetic protein-7 protects human intervertebral disk cells in vitro from apoptosis. Spine J. 2008; 8(3): 466-474.
- Murata Y, Nannmark U, Rydevik B, et al., The role of tumor necrosis factor-alpha in apoptosis of dorsal root ganglion cells induced by herniated nucleus pulposus in rats. Spine (Phila Pa 1976). 2008 Jan 15; 33(2): 155-162.
- Ha K Y, Kim B G, Kim K W, et al., Apoptosis in the sequestrated nucleus pulposus compared to the remaining nucleus pulposus in the same patient. Spine (Phila Pa 1976). 2011 Apr 20; 36(9): 683-689.
- Wang H Q, Yu X D, Liu Z H, et al., Deregulated miR-155 promotes Fas-mediated apoptosis in human intervertebral disk degeneration by targeting FADD and caspase-3. J Pathol. 2011 Oct; 225(2): 232-242.
- Drayton R M, Dudziec E, Peter S, et al., Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 2014 Apr; 20(7): 1990-2000.
- Zhao X, Yang L, Hu J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. Journal of Experimental & Clinical Cancer Research. 2011 Dec; 30(1): 55.
- Liu G, Cao P, Chen H, et al., MiR-27a Regulates Apoptosis in Nucleus Pulposus Cells by Targeting PI3K. PLoS One. 2013 Sep; 8(9): e75251.
- Yu X, Li Z, Shen J, Wu WKK, Liang J, Weng X, et al. MicroRNA-10b Promotes Nucleus Pulposus Cell Proliferation through RhoC-Akt Pathway by Targeting HOXD10 in Intervetebral Disk Degeneration. 2013 Dec 20; 8(12): e83080.
- Liu H, Huang X, Liu X, et al., miR-21 Promotes Human Nucleus Pulposus Cell Proliferation through PTEN/AKT Signaling. Int J Mol Sci. 2014 Mar 5; 15(3): 4007-4018.
- Clouet J, Pot-Vaucel M, Grimandi G, et al., Characterization of the age-dependent intervertebral disk changes in rabbit by correlation between MRI, histology and gene expression. BMC Musculoskelet Disord. 2011 Dec; 12(1): 147.
- Chen W H, Lo W C, Lee J J, et al., Tissue-engineered intervertebral disk and chondrogenesis using human nucleus pulposus regulated through TGF-β1 in platelet-rich plasma. J Cell Physiol. 2006 Dec; 209(3): 744-754.
- Chen J, Liu Z, Zhong G, et al., Hypertrophy of Ligamentum Flavum in Lumbar Spine Stenosis Is Associated with Increased miR-155 Level. Dis Markers. 2014; 20(14): 1-8.
- Tsirimonaki E, Fedonidis C, Pneumaticos S G, et al., PKCε Signalling Activates ERK1/2, and Regulates Aggrecan, ADAMTS5, and miR377 Gene Expression in Human Nucleus Pulposus Cells. 2013 Nov; 8(11): e82045.
- Jing W, Jiang W. MicroRNA-93 regulates collagen loss by targeting MMP3 in human nucleus pulposus cells. Cell Prolif. 2015 Jun; 48(3): 284-292.
- Vo N v., Hartman R A, Yurube T, et al., Expression and regulation of metalloproteinases and their inhibitors in intervertebral disk aging and degeneration. The Spine Journal. 2013 Mar; 13(3): 331-341.
- Li H ran, Cui Q, Dong Z yin, et al., Downregulation of miR-27b is Involved in Loss of Type II Collagen by Directly Targeting Matrix Metalloproteinase 13 (MMP13) in Human Intervertebral Disk Degeneration. Spine (Phila Pa 1976). 2016 Feb; 41(3): E116-123.
- Wang X W, Liu J J, Wu Q N, et al., RETRACTED: The in vitro and in vivo effects of microRNA-133a on intervertebral disk destruction by targeting MMP9 in spinal tuberculosis. Life Sci. 2017 Nov; 188: 198-205.
- Xu Y qiang, Zhang Z hui, Zheng Y fa, Feng S qing. Dysregulated miR-133a Mediates Loss of Type II Collagen by Directly Targeting Matrix Metalloproteinase 9 (MMP9) in Human Intervertebral Disk Degeneration. Spine (Phila Pa 1976). 2016 Jun; 41(12): E717-724.
- Ji M liang, Lu J, Shi P liang, et al., Dysregulated miR-98 Contributes to Extracellular Matrix Degradation by Targeting IL-6/STAT3 Signaling Pathway in Human Intervertebral Disk Degeneration. Journal of Bone and Mineral Research. 2016 Apr; 31(4): 900-909.
- Ikegawa S. The Genetics of Common Degenerative Skeletal Disorders: Osteoarthritis and Degenerative Disk Disease. Annu Rev Genomics Hum Genet. 2013 Aug; 14(1): 245-256.
- Chujo T, An H S, Akeda K, et al., Effects of Growth Differentiation Factor-5 on the Intervertebral Disk−In Vitro Bovine Study and In Vivo Rabbit Disk Degeneration Model Study. Spine (Phila Pa 1976). 2006 Dec; 31(25): 2909-2917.
- Liu W, Zhang Y, Xia P, et al., MicroRNA-7 regulates IL-1β-induced extracellular matrix degeneration by targeting GDF5 in human nucleus pulposus cells. Biomedicine & Pharmacotherapy. 2016 Oct; 83: 1414-1421.
- Ji M liang, Jiang H, Zhang X jun, et al., Preclinical development of a microRNA-based therapy for intervertebral disk degeneration. Nat Commun. 2018 Dec; 9(1): 50-51.
- Seyrek K, Ivanisenko N v., Richter M, et al., Controlling Cell Death through Post-translational Modifications of DED Proteins. Trends Cell Biol. 2020 May; 30(5): 354-369.
- Chong S J F, Marchi S, Petroni G, et al., Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020 Jul; 30(7): 537-555.
- Panda A C, Abdelmohsen K, Gorospe M. SASP regulation by noncoding RNA. Mech Ageing Dev. 2017 Dec;168: 37-43.
- Ding Y, Wang L, Zhao Q, et al., MicroRNA 93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF κB signaling pathway. Int J Mol Med. Dec(2018).
- Yun Z, Wang Y, Feng W, et al., Overexpression of microRNA-185 alleviates intervertebral disk degeneration through inactivation of the Wnt/ β -catenin signaling pathway and downregulation of Galectin-3. Mol Pain. 2020 Jan; 16: 174480692090255.
- Hisamatsu K, Niwa M, Kobayashi K, et al., Galectin-3 expression in hippocampal CA2 following transient forebrain ischemia and its inhibition by hypothermia or antiapoptotic agents. Neuroreport. 2016 Mar; 27(5): 311-317.
- Kameshima S, Okada M, Ikeda S, et al., Coordination of changes in expression and phosphorylation of eukaryotic elongation factor 2 (eEF2) and eEF2 kinase in hypertrophied cardiomyocytes. Biochem Biophys Rep. 2016 Sep: 218-224.
- Yang Q, Guo X P, Cheng Y L, Wang Y. MicroRNA-143-5p targeting eEF2 gene mediates intervertebral disk degeneration through the AMPK signaling pathway. Arthritis Res Ther. 2019 Dec; 21(1): 97.
- Zhao K, Zhang Y, Kang L, et al., Epigenetic silencing of miRNA-143 regulates apoptosis by targeting BCL2 in human intervertebral disk degeneration. Gene. 2017 Sep; 628: 259-266.
- Wang B, Wang D, Yan T, Yuan H. MiR-138-5p promotes TNF-α-induced apoptosis in human intervertebral disk degeneration by targeting SIRT1 through PTEN/PI3K/Akt signaling. Exp Cell Res. 2016 Jul; 345(2): 199-205.
- Yang Q, Guo X P, Cheng Y L, Wang Y. MicroRNA-143-5p targeting eEF2 gene mediates intervertebral disk degeneration through the AMPK signaling pathway. Arthritis Res Ther. 2019 Dec; 21(1): 97.
- Ji M liang, Jiang H, Zhang X jun, et al., Preclinical development of a microRNA-based therapy for intervertebral disk degeneration. Nat Commun. 2018 Dec; 9(1): 5051.
- Wang W, Wang J, Zhang J, Taq W, Zhang Z. miR 222 induces apoptosis in human intervertebral disk nucleus pulposus cells by targeting Bcl 2. Mol Med Rep. Oct(2019).
- Lu H, Zeng C, Chen M, et al., Lentiviral vector-mediated over-expression of Sox9 protected chondrocytes from IL-1β induced degeneration and apoptosis. Int J Clin Exp Pathol. 2015; 8(9): 10038-10049.
- Kang L, Yang C, Song Y, et al., MicroRNA-494 promotes apoptosis and extracellular matrix degradation in degenerative human nucleus pulposus cells. Oncotarget. 2017 Apr; 8(17): 27868-27881.
- Wang T, Li P, Ma X, et al., MicroRNA-494 inhibition protects nucleus pulposus cells from TNF-α-induced apoptosis by targeting JunD. Biochimie. 2015 Aug; 115: 1-7.
- Li L, Zhang L, Zhang Y. Roles of miR-494 in Intervertebral Disk Degeneration and the Related Mechanism. World Neurosurg. 2019 Apr; 124: e365-372.
- Yang W, Sun P. Downregulation of microRNA-129-5p increases the risk of intervertebral disk degeneration by promoting the apoptosis of nucleus pulposus cells via targeting BMP2. J Cell Biochem. 2019 Dec; 120(12): 19684-19690.
- Sun J chuan, Zheng B, Sun R xin, et al., MiR-499a-5p suppresses apoptosis of human nucleus pulposus cells and degradation of their extracellular matrix by targeting SOX4. Biomedicine & Pharmacotherapy. 2019 May; 113: 108652.
- Wang Y, Che M, Xin J, et al., The role of IL-1β and TNF-α in intervertebral disk degeneration. Biomedicine & Pharmacotherapy. 2020 Nov; 131: 110660.
- Haro H. Translational research of herniated disks: current status of diagnosis and treatment. J Orthop Sci. 2014 Jul; 19(4): 515-520.
- Gu S X, Li X, Hamilton J L, et al., MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disk. Gene. 2015 Jan; 555(2): 80-87.
- Lv F, Huang Y, Lv W, et al. MicroRNA-146a Ameliorates Inflammation via TRAF6/NF-κB Pathway in Intervertebral Disk Cells. Medical Science Monitor. 2017 Feb; 23: 659-664.
- Chen Z, Han Y, Deng C, et al., Inflammation-dependent downregulation of miR-194-5p contributes to human intervertebral disk degeneration by targeting CUL4A and CUL4B. J Cell Physiol. 2019 Nov; 234(11): 19977-19989.
- Qin C, Lv Y, Zhao H, Yang B, Zhang P. MicroRNA-149 Suppresses Inflammation in Nucleus Pulposus Cells of Intervertebral Disks by Regulating MyD88. Med Sci Monit. 2019 Jul; 25: 4892-900.
- Cao Z, Chen L. Inhibition of miR-27a suppresses the inflammatory response via the p38/MAPK pathway in intervertebral disk cells. Exp Ther Med. 2017 Nov; 14(5): 4572-4578.
- Cai Z, Li K, Yang K, et al., Suppression of miR-203-3p inhibits lipopolysaccharide induced human intervertebral disk inflammation and degeneration through upregulating estrogen receptor α. Gene Ther. 2020 Sep; 27(9): 417-426.
- Shen L, Xiao Y, Wu Q, et al., TLR4/NF-κB axis signaling pathway-dependent up-regulation of miR-625-5p contributes to human intervertebral disk degeneration by targeting COL1A1. Am J Transl Res. 2019; 11(3): 1374-1388.
- Lu A, Wang Z, Wang S. Role of miR-589-3p in human lumbar disk degeneration and its potential mechanism. Exp Ther Med. 2018 Feb; 15(2): 1616-1621.
- Xi Y, Jiang T, Wang W, et al., Long non-coding HCG18 promotes intervertebral disk degeneration by sponging miR-146a-5p and regulating TRAF6 expression. Sci Rep. 2017 Oct; 7(1): 13234.
- Iatridis J C, Nicoll S B, Michalek A J, et al., Role of biomechanics in intervertebral disk degeneration and regenerative therapies: what needs repairing in the disk and what are promising biomaterials for its repair? Spine J. 2013 Mar; 13(3): 243-262.
- Dowdell J, Erwin M, Choma T, et al., Intervertebral Disk Degeneration and Repair. Neurosurgery. 2017 Mar; 80(3S): S46-54.
- Wu P H, Kim H S, Jang I T. Intervertebral Disk Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disk Disease. Int J Mol Sci. 2020 Mar; 21(6): 2135.
- Tamama K, Kawasaki H, Wells A. Epidermal Growth Factor (EGF) Treatment on Multipotential Stromal Cells (MSCs). Possible Enhancement of Therapeutic Potential of MSC. J Biomed Biotechnol. 2010; 2010: 1-10.
- Obata S, Akeda K, Imanishi T, et al., Effect of autologous platelet-rich plasma-releasate on intervertebral disk degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res Ther. 2012; 14(6): R241.
- Fernandez-Moure J, Moore C A, Kim K, et al., Novel therapeutic strategies for degenerative disk disease: Review of cell biology and intervertebral disk cell therapy. 2018; 6: 2050312118761674.
- Obata S, Akeda K, Imanishi T, et al., Effect of autologous platelet-rich plasma-releasate on intervertebral disk degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res Ther. 2012; 14(6): R241.
- Sampara P, Banala R R, Vemuri S K, et al., Understanding the molecular biology of intervertebral disk degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 2018 Apr; 25(2): 67-82.
- Tong W, Lu Z, Qin L, Mauck RL, Smith HE, Smith LJ, et al. Cell therapy for the degenerating intervertebral disk. Translational Research. 2017 Mar; 181: 49-58.
- Sakai D, Schol J. Cell therapy for intervertebral disk repair: Clinical perspective. J Orthop Translat. 2017 Apr; 9: 8-18.
- Sharma A. The Role of Adipokines in Intervertebral Disk Degeneration. Medical Sciences. 2018 Apr; 6(2): 34.
