Maternal Serum Soluble Fms- Like Tyrosine Kinase-1 and Placental Growth Factor Ratio as A Short Term Predictor of Pre-Eclampsia
Article Information
Jesika Rizvi Tamanna1*, Mousumi Saha2, khadiza Begum3, Mizanur Rahman4, Nahreen Akthar5, Abeda Sultana6, Tabassum Parveen7
1 Registrar, Department of Gynecology & Obstetrics, Bangabandhu Sheikh Mujib Medical College and Hospital (BSMMCH), Faridpur, Bangladesh
2 Assistant Surgeon, OSD(DGHS), On Deputation, Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
3 Consultant, OSD(DGHS), On Deputation, Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
4 Consultant, Department of Pediatrics, Ibn Sina Medical College. Dhaka, Bangladesh
5 Chairman, Department of Fetomaternal medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
6 Consultant, OSD(DGHS), On Deputation, Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
7 Professor, Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
*Corresponding Author: Dr. Jesika Rizvi Tamanna, Registrar, Department of Gynecology & Obstetrics, Bangabandhu Sheikh Mujib Medical College and Hospital (BSMMCH), Faridpur, Bangladesh.
Received: 15 May 2023; Accepted: 22 May 2023; Published: 04 October 2023
Citation: Dr. Jesika Rizvi Tamanna, Dr. Mousumi Saha, Dr. khadiza Begum, Dr. Md. Mizanur Rahman, Prof. Dr. Nahreen Akthar, Dr. Abeda Sultana, Prof. Dr. Tabassum Parveen. Maternal Serum Soluble Fms- Like Tyrosine Kinase-1 And Placental Growth Factor Ratio as A Short Term Predictor of Pre-Eclampsia. Obstetrics and Gynecology Research. 6 (2023): 251-259.
View / Download Pdf Share at FacebookAbstract
Background:
Preeclampsia (PE) is defined as new onset of HTN (BP≥ 140/90 mm of Hg on two occasions at least 4 hours apart) after 20 weeks of pregnancy and the coexistence of 1 or more of the following new onset conditions: Proteinuria (Protein creatinine ratio of 30 mg/mmol or Albumin creatinine ratio of 8 mg/mmol or at least 1 gm/L[2+] in dip stick) or other maternal organ dysfunction as renal insufficiency, liver involvement, neurological complications, hematological complications and uteroplacental dysfunction. It is an important cause of maternal & perinatal morbidity and mortality globally. It complicates 10-17% of pregnancy particularly in developing countries. Eclampsia causes 20% of maternal death in Bangladesh. Several predictive markers for pre-eclampsia, including placental growth factor, sFlt-1, Plasma protein 13 and Pregnancy-Associated PAPP-A have been evaluated. The imbalance between angiogenic factors like vascular endothelial growth factor or PLG and anti-angiogenic factors like sFlt-1 are known to be related to the disease pathogenesis. In women with PE, sFlt-1 rises approximately 5 weeks prior to disease onset while the level of PLGF decreases before the rising of sFlt-1. Hence, to improve the quality of PE prediction, some studies suggest sFlt-1/PLGF ratio as a better marker compared to measuring sFlt-1 or PLGF separately as predictor of PE. An elevated ratio is highly predictive of PE within 4 weeks, whereas the diagnosis of PE can be ruled out within one week for low ratios.
Objective:
To evaluate sFlt-1/PLGF ratio in maternal serum between 24+0-36+6 gestation weeks as a short term predictor of preeclampsia.
Methodology:
A longitudinal study was conducted in the Outpatient Department of Fetomaternal Medicine and General Obstetrics and Gynecology, BSMMU, Dhaka. Purposive or convenient sampling technique was applied for data collection. According to inclusion criteria 86 pregnant women between 24+0-36+6 weeks' gestation with at least one risk factor for pre-eclampsia were selected from OPD of Fetomaternal Medicine and General Obstetrics and Gynecology department. The purpose and procedure was discussed and after obtaining informed consent, the women were interviewed. After that 5 ml of blood sample was collected by venipuncture from ante cubital vein and taken in a sterile vacuum container and sent to Virology laboratory of BSMMU. Blood was allowed to cool in room temperature, then serum was separated by centrifugation and stored at -200C until analysis. sFlt-1 and PLGF concentrations were measured by ELISA kit. All participants were invited to follow up at 1 week and 4 weeks of enrollment. At each visit they were clinically evaluated by measuring BP and dipstick test for protein if BP raised. Quantification of proteinuria was done by measuring protein creatinine ratio. Patients who developed pre-eclampsia were recorded as pre-eclampsia group (Group 1) & others were labeled as non-pre-eclampsia group (Group 2). Cut off value of sFlt-1/PLGF ratio was determined for developing PE. Data were analyzed using SPSS software. Area Under ROC was used to find the predictive values of sFlt-1 and PLGF ratio.
Result:
Out of total 86 patients 8(9.30 %) patients developed PE. The both groups were almost similar in terms of all baseline demographic and obstetric characteristics except maternal age and BMI which were significantly higher in PE group (Group 1) than that in non PE group (Group 2). The mean age of PE and non PE patients were 27 years and 24.60 years respectively. The mean of sFlt-1 and PLGF ratio were significantly higher in PE group than in non PE group. Multivariate logistic regression analysis demonstrated that a subject with higher sFlt-1/PLGF ratio had OR 1.675(95.0% CI 0.987-1.315) times increase were significantly associated with short term predictor of PE. In ROC the best combination of sensitivity and specificity for prediction of PE which gave 37.8 cut off value of sFlt-1 and PLGF ratio with 87.50% sensitivity and 92.30 % specificity for short term prediction of pre-eclampsia(PE).
Conclusion:
Our findings revealed that patients who developed pre-eclampsia have high serum level of soluble sFlt-1 and PLGF ratio. sFlt-1 and PLGF ratio below 37.8 can rule out preeclampsia for 4 weeks.
Keywords
Pre-eclampsia; soluble Fms-like tyrosine kinase-1 (sFlt-1); Placental growth factor (PLGF)
Pre-eclampsia articles Pre-eclampsia Research articles Pre-eclampsia review articles Pre-eclampsia PubMed articles Pre-eclampsia PubMed Central articles Pre-eclampsia 2023 articles Pre-eclampsia 2024 articles Pre-eclampsia Scopus articles Pre-eclampsia impact factor journals Pre-eclampsia Scopus journals Pre-eclampsia PubMed journals Pre-eclampsia medical journals Pre-eclampsia free journals Pre-eclampsia best journals Pre-eclampsia top journals Pre-eclampsia free medical journals Pre-eclampsia famous journals Pre-eclampsia Google Scholar indexed journals soluble Fms-like tyrosine kinase-1 (sFlt-1) articles soluble Fms-like tyrosine kinase-1 (sFlt-1) Research articles soluble Fms-like tyrosine kinase-1 (sFlt-1) review articles soluble Fms-like tyrosine kinase-1 (sFlt-1) PubMed articles soluble Fms-like tyrosine kinase-1 (sFlt-1) PubMed Central articles soluble Fms-like tyrosine kinase-1 (sFlt-1) 2023 articles soluble Fms-like tyrosine kinase-1 (sFlt-1) 2024 articles soluble Fms-like tyrosine kinase-1 (sFlt-1) Scopus articles soluble Fms-like tyrosine kinase-1 (sFlt-1) impact factor journals soluble Fms-like tyrosine kinase-1 (sFlt-1) Scopus journals soluble Fms-like tyrosine kinase-1 (sFlt-1) PubMed journals soluble Fms-like tyrosine kinase-1 (sFlt-1) medical journals soluble Fms-like tyrosine kinase-1 (sFlt-1) free journals soluble Fms-like tyrosine kinase-1 (sFlt-1) best journals soluble Fms-like tyrosine kinase-1 (sFlt-1) top journals soluble Fms-like tyrosine kinase-1 (sFlt-1) free medical journals soluble Fms-like tyrosine kinase-1 (sFlt-1) famous journals soluble Fms-like tyrosine kinase-1 (sFlt-1) Google Scholar indexed journals Placental growth factor (PLGF) articles Placental growth factor (PLGF) Research articles Placental growth factor (PLGF) review articles Placental growth factor (PLGF) PubMed articles Placental growth factor (PLGF) PubMed Central articles Placental growth factor (PLGF) 2023 articles Placental growth factor (PLGF) 2024 articles Placental growth factor (PLGF) Scopus articles Placental growth factor (PLGF) impact factor journals Placental growth factor (PLGF) Scopus journals Placental growth factor (PLGF) PubMed journals Placental growth factor (PLGF) medical journals Placental growth factor (PLGF) free journals Placental growth factor (PLGF) best journals Placental growth factor (PLGF) top journals Placental growth factor (PLGF) free medical journals Placental growth factor (PLGF) famous journals Placental growth factor (PLGF) Google Scholar indexed journals Protein creatinine ratio articles Protein creatinine ratio Research articles Protein creatinine ratio review articles Protein creatinine ratio PubMed articles Protein creatinine ratio PubMed Central articles Protein creatinine ratio 2023 articles Protein creatinine ratio 2024 articles Protein creatinine ratio Scopus articles Protein creatinine ratio impact factor journals Protein creatinine ratio Scopus journals Protein creatinine ratio PubMed journals Protein creatinine ratio medical journals Protein creatinine ratio free journals Protein creatinine ratio best journals Protein creatinine ratio top journals Protein creatinine ratio free medical journals Protein creatinine ratio famous journals Protein creatinine ratio Google Scholar indexed journals Albumin creatinine articles Albumin creatinine Research articles Albumin creatinine review articles Albumin creatinine PubMed articles Albumin creatinine PubMed Central articles Albumin creatinine 2023 articles Albumin creatinine 2024 articles Albumin creatinine Scopus articles Albumin creatinine impact factor journals Albumin creatinine Scopus journals Albumin creatinine PubMed journals Albumin creatinine medical journals Albumin creatinine free journals Albumin creatinine best journals Albumin creatinine top journals Albumin creatinine free medical journals Albumin creatinine famous journals Albumin creatinine Google Scholar indexed journals neurological complications articles neurological complications Research articles neurological complications review articles neurological complications PubMed articles neurological complications PubMed Central articles neurological complications 2023 articles neurological complications 2024 articles neurological complications Scopus articles neurological complications impact factor journals neurological complications Scopus journals neurological complications PubMed journals neurological complications medical journals neurological complications free journals neurological complications best journals neurological complications top journals neurological complications free medical journals neurological complications famous journals neurological complications Google Scholar indexed journals hematological complications articles hematological complications Research articles hematological complications review articles hematological complications PubMed articles hematological complications PubMed Central articles hematological complications 2023 articles hematological complications 2024 articles hematological complications Scopus articles hematological complications impact factor journals hematological complications Scopus journals hematological complications PubMed journals hematological complications medical journals hematological complications free journals hematological complications best journals hematological complications top journals hematological complications free medical journals hematological complications famous journals hematological complications Google Scholar indexed journals uteroplacental dysfunction articles uteroplacental dysfunction Research articles uteroplacental dysfunction review articles uteroplacental dysfunction PubMed articles uteroplacental dysfunction PubMed Central articles uteroplacental dysfunction 2023 articles uteroplacental dysfunction 2024 articles uteroplacental dysfunction Scopus articles uteroplacental dysfunction impact factor journals uteroplacental dysfunction Scopus journals uteroplacental dysfunction PubMed journals uteroplacental dysfunction medical journals uteroplacental dysfunction free journals uteroplacental dysfunction best journals uteroplacental dysfunction top journals uteroplacental dysfunction free medical journals uteroplacental dysfunction famous journals uteroplacental dysfunction Google Scholar indexed journals cerebral hemorrhage articles cerebral hemorrhage Research articles cerebral hemorrhage review articles cerebral hemorrhage PubMed articles cerebral hemorrhage PubMed Central articles cerebral hemorrhage 2023 articles cerebral hemorrhage 2024 articles cerebral hemorrhage Scopus articles cerebral hemorrhage impact factor journals cerebral hemorrhage Scopus journals cerebral hemorrhage PubMed journals cerebral hemorrhage medical journals cerebral hemorrhage free journals cerebral hemorrhage best journals cerebral hemorrhage top journals cerebral hemorrhage free medical journals cerebral hemorrhage famous journals cerebral hemorrhage Google Scholar indexed journals maternal death articles maternal death Research articles maternal death review articles maternal death PubMed articles maternal death PubMed Central articles maternal death 2023 articles maternal death 2024 articles maternal death Scopus articles maternal death impact factor journals maternal death Scopus journals maternal death PubMed journals maternal death medical journals maternal death free journals maternal death best journals maternal death top journals maternal death free medical journals maternal death famous journals maternal death Google Scholar indexed journals
Article Details
INTRODUCTION
Preeclampsia (PE) is defined as new onset of HTN (BP≥ 140/90 mm of Hg on two occasions at least 4 hours apart) after 20 weeks of pregnancy and the coexistence of 1 or more of the following new onset conditions: Proteinuria (Protein creatinine ratio of 30 mg/mmol or Albumin creatinine ratio of 8 mg/mmol or at least 1 gm/L[2+] in dip stick) or other maternal organ dysfunction as renal insufficiency, liver involvement, neurological complications, hematological complications and uteroplacental dysfunction. It is estimated that worldwide prevalence of pre-eclampsia is 5-10% [1]. In the developing world, pre-eclampsia with severe features and eclampsia are more common. Bangladesh is a developing country where higher (about 16.0%) incidence of pre-eclampsia was reported[2]. Documented risk factors of pre-eclampsia are null parity, family history, preeclampsia in previous pregnancy, multiple gestation, and pre gestational diabetes mellitus, chronic hypertension, chronic renal disease and some auto immune diseases[3]. Women under 20 years of age, women with low levels of education, and women in their first pregnancy are all reported to be at higher risk[4]. Pre-eclampsia increases the risk of abruptio-placenta, acute kidney injury, disseminated intravascular coagulation, HELLP syndrome, cerebral hemorrhage and maternal death[5]. Pre-eclampsia also increases the incidence of fetal growth restriction, preterm delivery, low birth weight and neonatal death[6]. If pre-eclampsia is detected earlier these complications can be avoided. Effective management of preeclampsia may be divided into three categories including the prevention of preeclampsia, early detection and treatment[7]. Clinical experience suggests that early detection and monitoring are beneficial[8]. The heterogeneity of its clinical presentation often complicates timely and accurate diagnosis [9]. The pathophysiology is still not completely understood. Normally, during early formation of the placenta, extra villous cytotrophoblasts enter the spiral arteries of the uterus. Angiogenesis is necessary for establishment of adequate placental perfusion. The balance of pro- and anti-angiogenic factors and their receptors, thought to mediate this process. The maternal circulating placental growth factor and soluble fms-like tyrosine kinase1 (sFlt-1) are useful markers for pre-eclampsia [8]. Low ratio of sFlt-1/PLGF <38 in maternal circulation in patients with suspected pre-eclampsia can rule out pre-eclampsia [8]. Because of the high prevalence and seriousness of preeclampsia, evaluation of various angiogenic and antiangiogenic factors in both serum and plasma have been tested as diagnostic markers for preeclampsia along with an assessment for the probability of their use in prediction of pre-eclampsia development[10]. An imbalance of circulating antiangiogenic factors, such as soluble sFlt-1 and angiogenic PLGF, plays a central role in disease pathogenesis[8]. Pregnant women who develop pre-eclampsia have an increased ratio of sFlt-1 to PLGF[8]. Different cut offs of sFlt1/PLGF ratio have been proposed[11]. The pivotal prediction of short term outcome in pregnant women with suspected pre-eclampsia study shed new lights on usefulness of sFlt-1/PLGF ratio for prediction of absence or presence of PE for triage of women with suspected PE. From their study single sFlt-1/PLGF ratio cut off <38 can rule out PE within 1 week with NPV>99% and rule in PE within 4 weeks if the ratio is>38 with PPV 40%. sFlt-1/PLGF ratio >85 under 34 weeks can predict adverse effects and needs delivery within 2 weeks. An increase in the expression of sFlt-1 that binds circulating free VEGF, associated with a decrease in PLGF, plays a key role in the pathogenesis of PE [12]. The increase in circulating sFlt-1 occurs prior to the onset of clinical disease and could be correlated with the disease severity[13]. NICE recommends use of sFlt-1/PLGF along with standard clinical assessment to rule out PE between 20-34+6 weeks. Formerly, many studies assessed sFlt-1 and PLGF for the prediction of preeclampsia development. However, few studies reported the sFlt-1/PLGF ratio in the prediction of preeclampsia. This study is intended to evaluate the prognostic value of the sFlt-1/PLGF ratio for prediction of pre-eclampsia.
OBJECTIVE
General Objective:
To evaluate sFlt-1/PLGF ratio in maternal serum between 24+0-36+6gestation weeks as short term predictor of preeclampsia.
Specific Objectives:
- To estimate the serum sFlt-1/PLGF ratio in pregnant women at 24+0-36+6 weeks of gestation with at least one risk factors of PE.
- To follow up at 1 week and 4 weeks after base line visit to detect development of PE.
- To assess whether a low sFlt-1/PLGF ratio below cut off 38 can predict that, pre-eclampsia will not develop within four weeks of baseline visit.
MATERIALS AND METHODS
This was a Cohort study. Selected by purposive sampling method. Conducted during November 2020 to October 2021. The study sample size was 86. The patient was pregnant women of 24 - 36 weeks of gestation with risk factors of pre-eclampsia who will come at outpatient Department of Fetomaternal Medicine and General Obstetrics and Gynecology, Bangabondhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh within the study period.
Inclusion Criteria:
Pregnant women 18 years of age or older between 24+0-36+6 week’s gestation at the first visit with at least one risk factors for preeclampsia. Gestational hypertension [BP 140/90], Chronic HTN, DM, SLE/Thrombophilia, Chronic kidney disease, First pregnancy, Family history of pre-eclampsia, Previous history of pre-eclampsia
Study procedure
A longitudinal study was conducted in the Outpatient Department of Fetomaternal Medicine and General Obstetrics and Gynecology, BSMMU, Dhaka. Purposive or convenient sampling technique was applied for data collection. According to inclusion criteria 86 pregnant women between 24+0-36+6 weeks' gestation with at least one risk factor for pre-eclampsia were selected from OPD of Feto-maternal Medicine and General Obstetrics and Gynecology department. Information of demographic profile, family histories were recorded. Obstetrics history documented gravidity, parity, gestational weeks, past history of any other medical disorders. Then a complete physical examination was carried out including height, weight, BMI, BP, anemia, edema, etc. Urinary protein was examined by dipstick test to exclude pre-eclampsia. FGR, multiple pregnancy and fetus with congenital anomaly were excluded by ultrasonography report. After that 5 ml of blood sample was collected by venipuncture from ante cubital vein and taken in a sterile vacuum container and sent to Virology laboratory of BSMMU. Blood was allowed to cool in room temperature, and then serum was separated by centrifugation at 2000-3000 r.p.m for 20 minutes and stored at -20°C until analysis. sFlt-1 was quantified by enzyme immunoassay using Bio Vendor ELISA kit and PLGF concentrations was measured by DRG ELISA Kit according to manufacturer’s instructions at the end of sample collection in the virology lab of BSMMU. For sFlt-1 the lower detection limit was 0.16 ng/ml or 320 pg /ml. The intra-assay coefficient of variation was 5.5%, inter-assay coefficient of variation was 5.1%, absorbance was quantified at a wave length of 450 nm in an automatic ELISA plate reader. The concentrations of sFlt-1 were determined by interpolation from the standard curve and the results were expressed as pg /ml. The lowest detection limit for PLGF was 25 pg/ml, intra-assay coefficient of variation was (1.7-2.8) %, inter-assay coefficient of variation was (4.1-7) %. The concentrations of PLGF were determined by interpolation from the standard curve and the results were expressed as pg/ml (pico gram/ml). Finally, the sFlt-1/PLGF ratios were calculated for each sample.
Procedures of data analysis and interpretation
Collected data was processed and analyzed by using SPSS version 22.0. Categorical variables were presented in the form of frequency and percentage and quantitative data was presented in the form of mean and standard deviation. Data presented on categorical scale were compared between groups using Chi square test, while the data presented on continuous scale were compared between groups using unpaired t-test. The level of significance was set 5% and p value <0.05 will be considered as statistically significant. For this study purpose the cut off value of sFlt-1/PLGF ratio was taken 38 from previous study. Association between the level of sFlt-1/PLGF ratio and development of pre-eclampsia was done by Chi-square test. The accuracy of sFlt-1/PLGF ratio and other maternal factors in predicting the development of PE were calculated using multiple logistic regressions. ROC was used to decide the best cut off point of sFlt-1/PLGF ratio.
Ethical implication
Ethical issues were address as follows: the study was done in accordance with Helsinki declaration for research involving human subject, 1964. Last amended in 2013. prior to the commencement of this study was approved by the institutional review board in BSMMU.
RESULTS
|
Characteristics |
Pre-eclampsia group (n=8) |
Non Pre-eclampsia group (n=78) |
P Value |
||
|
Age in years |
Frequency (n) |
Percentage (%) |
Frequency (n) |
Percentage (%) |
|
|
18-24 yrs. |
2 |
25 |
52 |
66.66 |
0.0015 |
|
25-30 yrs. |
4 |
50 |
19 |
24.35 |
|
|
31-40 yrs. |
2 |
25 |
7 |
8.97 |
|
|
Mean ± SD |
27.00±3.81 |
24.6±3.21 |
|||
|
Range(min - max) |
18 - 34 |
17 - 17 |
|||
Table 1: Age of the study population (N=86)
Table 1 showed 50% patients were of pre-eclampsia group from 25 to 30 years of age and24.35 % patient were of non-pre-eclampsia group. The Mean age of the preeclampsia patients were 27.00±3.81 years and non-pre-eclampsia patients were, 24.60±3.21. The differences were statistically significant (p<0.05) between two groups.
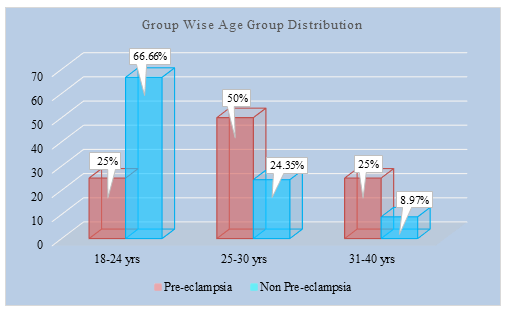
Figure 1: Group wise Age distribution of study population (N=86)
|
BMI (Kg/m2) |
Pre-eclampsia group (n=8) |
Non Pre-eclampsia group (n=78) |
P Value |
||
|
|
Frequency (n) |
Percentage (%) |
Frequency (n) |
Percentage(%) |
|
|
<18.5 |
0 |
0 |
0 |
0 |
0.002 |
|
18.5-24.9 |
2 |
25 |
20 |
25.64 |
|
|
25-29.9 |
4 |
50 |
48 |
61.82 |
|
|
>30 |
2 |
25 |
10 |
12.82 |
|
|
Mean ± SD |
27.87±5.62 |
|
23.81±3.01 |
|
|
|
Range (min-max) |
20.67 - 36.69 |
|
15.36 - 36.69 |
|
|
Table 2: Baseline anthropometrics of study population (N=86)
Table 2 showed the mean BMI was 27.87±5.62 (kg/m2) in pre-eclampsia group and 23.81±3.01 in non-Pre-eclampsia group (kg/m2). The difference of BMI was statistically significant (p<0.05) between two groups
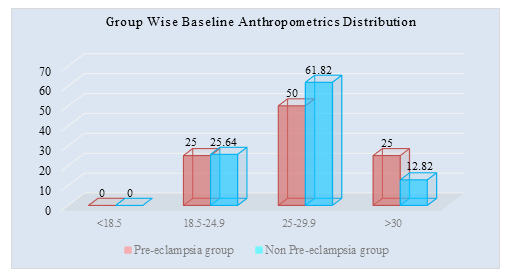
Figure II: Group wise Baseline Anthropometrics Distribution of study population (N=86)
|
Gestational age |
BP (mm of Hg) |
Frequency (%) |
|
<34 weeks(early onset PE) |
160/110 |
5(62.50%) |
|
>34 weeks(late onset PE) |
160/110 |
3 (37.50%) |
Table 3: Gestational age and blood pressure of preeclampsia patients (N=8)
Table 3 showed among the 86 patients, seven developed clinical PE: 5(62.50 %) before 34 gestation weeks, and 3(37.50%) after 34 weeks
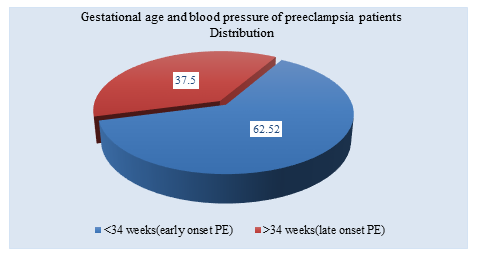
Figure III: Distribution of Gestational age and blood pressure of preeclampsia patients (n=8)
|
Variables |
Primary Enrollment |
Pre-eclampsia group (n=8) |
Non Pre-eclampsia group (n=78) |
P value |
|
sFlt-1 (pg/mL) |
4.434±499 |
7,044±509 |
4,079±1,652 |
0.001 |
|
sFlt-1 >95th percentile |
24(27.9%) |
6(46.15%) |
18(24.66%) |
|
|
PLGF (pg/mL) |
405±41 |
153±45 |
440±30 |
|
|
PLGF <5th percentile |
13(15.11%) |
2(15.38%) |
11(15.06%) |
|
|
sFlt-1/PLGF ratio |
36.5±12 |
69±13 |
32±25 |
Table 4: Distribution of sFlt-1 and PLGF concentrations and sFlt-1/PLGF ratio
Table 4 showed SFLT-1 and PLGF concentrations and sFlt-1/PLGF ratio assessments. 24(27.9%) patients had a sFlt-1 concentration higher than the 95th percentile, with 6(46.15%) in the pre-eclampsia group. For PLGF, 13(15.11%) patients had a concentration lower than the 5th percentile, with two in the PE group (15.06%.) The mean sFlt-1/PLGF ratio was 36.5±12 for the entire cohort, which was significantly higher (69±13) for women of pre-eclampsia group than for women non pre-eclampsia group (32±25).
|
Variables |
Primary Enrollment |
Pre-eclampsia group (n=8) |
Non Pre-eclampsia group (n=78) |
|
At 1 week Follow up |
|
|
|
|
s-Flt1/PLGF ratio <38 |
73 |
0 (FN) |
73 (TN) |
|
s-Flt1/PLGF ratio >38 |
13 |
2 (TP) |
11 (FP) |
|
Negative predictive value |
100% |
||
|
Positive predictive value |
15.38% |
||
|
Sensitivity |
100% |
||
|
Specificity |
86.90% |
||
Table 5: Predictive performance of s-Flt1/PLGF ratio<38 (1st week follow up)
Table 5 showed sFlt-1/PLGF ratio threshold of 38, 13 women exhibited a high sFlt-1/PLGF ratio. Among them, two developed PE within less than one week. The negative predictive value of the sFlt-1/PLGF ratio at one week was 100%; the positive predictive value was 15.38%. Sensitivity and specificity were 100% and 86.90%, respectively.
|
Variables |
Pre-eclampsia group (n=8) |
Non Pre-eclampsia group (n=78) |
|
At 1 week Follow up |
|
|
|
s-Flt1/PLGF ratio <38 |
6 (FN) |
67 (TN) |
|
s-Flt1/PLGF ratio >38 |
2 (TP) |
11 (FP) |
|
Negative predictive value |
91.78% |
|
|
Positive predictive value |
15.38% |
|
|
Sensitivity |
25% |
|
|
Specificity |
85.89% |
|
Table 6: Predictive performance of s-Flt1/PLGF ratio> 38 (1st week follow up)
Table 6 showed, sFlt-1/PLGF ratio>38, 2 women exhibited a high sFlt-1/PLGF ratio and developed PE. The negative predictive value of the sFlt-1/PLGF ratio>38 at one week was 91.78%; the positive predictive value was 15.38%. Sensitivity and specificity were 25% and 85.89% respectively.
|
Variables |
Primary Enrollment |
Pre-eclampsia group (n=8) |
Non pre-eclampsia group (n=78) |
|
At 4th weeks follow up |
|
|
|
|
s-Flt1/PLGF ratio<38 |
73 |
1 (FN) |
72 (TN) |
|
s-Flt1/PLGF ratio>38 |
11 |
5 (TP) |
6 (FP) |
|
Negative predictive value |
98.63% |
||
|
Positive predictive value |
45.45% |
||
|
Sensitivity |
83.33% |
||
|
Specificity |
92.30% |
||
Table 7: Predictive performance of s-Flt1/PLGF ratio<38 (4th week Follow up)
Table 7 showed, concerning the risk of PE occurring within four weeks at the same threshold and after excluding the two patients who presented with PE before one week, five of the remaining patients developed PE. The negative predictive value sFlt-1/PLGF ratio for PE occurring at four weeks was 98.63%; positive predictive value was 45.45%. Sensitivity was 83.33%, and specificity was 92.30%.
|
Variables |
Pre-eclampsia group (n=8) |
Non Pre-eclampsia group (n=78) |
|
At 1 week Follow up |
|
|
|
s-Flt1/PLGF ratio <38 |
3(FN) |
72 (TN) |
|
s-Flt1/PLGF ratio >38 |
5 (TP) |
6 (FP) |
|
Negative predictive value |
96% |
|
|
Positive predictive value |
45.45% |
|
|
Sensitivity |
62.50% |
|
|
Specificity |
92.30% |
|
Table 8: Predictive performance of s-Flt1/PLGF ratio> 38 (4th week follow up)
Table 8 showed, for a sFlt-1/PLGF ratio >38 at 4thweek, 11 women exhibited a high sFlt-1/PLGF ratio and 5 women developed PE. The negative predictive value of the sFlt-1/PLGF ratio>38 at 4th week was 96%; the positive predictive value was 45.45%. Sensitivity and specificity were 62.50% and 92.30% respectively.
|
Predictor |
Pre-eclampsia (n=8) |
Non pre-eclampsia (n=78) |
Total |
|
Positive (sFlt-1/PLGF)>38 |
7(TP) |
6 (FP) |
13 |
|
Negative (sFlt-1/PLGF)<38 |
1(FN) |
72(TN) |
73 |
TP=true positive, FP=False positive, FN=false negative, TN=true negative
Table 9: Diagnostic validity test
|
Validity test |
Percentage (%) |
|
Sensitivity |
87.50% |
|
Specificity |
92.30% |
|
Positive predictive value(PPV) |
53.84% |
|
Negative predictive value(NPV) |
98.63% |
|
Diagnostic accuracy |
91.26% |
Table 10: Sensitivity, Specificity, accuracy, positive and negative predictive value of sFlt-1/PLGF ratio for prediction of PE (N=86)
Table 10 showed, sensitivity, Specificity, accuracy, positive and negative predictive value of sFlt-1/PLGF ratio for prediction of PE 87.50%, 92.30%, 53.84%, 98.63% and Diagnostic accuracy 91.26% respectively.
|
Variables |
P value |
OR |
95% C.1 |
|
|
Lower |
Upper |
|||
|
Maternal age |
0.001 |
1.005 |
0.791 |
1.278 |
|
BMI |
0.016 |
1.711 |
0.539 |
0.938 |
|
Gestational Weeks |
0.731 |
0.86 |
0.74 |
0.99 |
|
Parity |
0.389 |
0.451 |
0.088 |
2.306 |
|
sFlt-1/PLGF ratio |
0.043 |
1.675 |
0.987 |
1.315 |
Table 11: Factor associated with predictor of pre-eclampsia in multivariate logistic regression analysis (N=86)
Table 11 showed, a subject with sFlt-1/PLGF ratio had 1.675 (95.0% CI 0.987-1.315) times increase in odds were significantly associated with predictor of preeclampsia. A subject with BMI had 1.711 (95.0% CI 0.539 to 0.938) times increase in odds were significantly associated with predictor of preeclampsia.
|
Variables |
Cut of value |
Sensitivity |
Specificity |
Area under the ROC curve |
95% Confidence interval (CI) |
|
|
Lower bound |
Upper Bound |
|||||
|
Serum sFIt-1/PLGF ratio |
37.8 |
87.50% |
92.30% |
0.9 |
0.83 |
0.98 |
Table 12: Receiver-operator characteristic (ROC) curve of maternal serum sFIt-1/PLGF ratio for prediction of preeclampsia
Table 12 showed based on receiver-operator characteristic(ROC) curve sFlt-1/PLGF ratio had the best area under the curve. The results showed AUC of 0.90 (95% CI=0.83–0.98). The best cut-off value of sFlt-1/PLGF ratio was 37.8 with sensitivity of 87.50% (95% CI =68.7-94) and specificity 92.30% (95% CI = 62.1-96.8) as predictor of pre-eclampsia.
DISCUSSION
Among total 86 participants, 7 patients had developed pre-eclampsia during the follow up period and 79 patients did not develop pre-eclampsia. The present study findings were discussed and compared with previous relevant published studies. In this current study, out of total 86 patients 8(9.3%) patients developed PE. [14] in their studies showed that the incidence of pre-eclampsia in developing countries ranges from 1-17%. Thus our pre-eclampsia developed group falls within the range of incidence. Our study shows 50% patients were of pre-eclampsia group from 25 to 30 years of age and 24.35 % patient were of non-pre-eclampsia group. The mean age of the pre-eclampsia patients was 27.00±3.81 years and non-pre-eclampsia patients were 24.60±3.21. The differences were statistically significant between two groups. Kumari et al., (2014) [15] in their studies showed that the mean age of the pre-eclampsia patients was 28.00±3.91 years and non-pre-eclampsia patients were 24.0±3.1. In both studies the mean age of pre-eclampsia patients were found more than the patients without pre-eclampsia. In our study most of the patients were housewife 83.33% in non-pre-eclampsia group and 62.50% pregnant patient with pre-eclampsia group. 35(44.87%) patients complete their primary education in non-pre-eclampsia group in compare to 2(25%) pregnant patient with pre-eclampsia group. Opitasari, C. and Andayasari,( 2014) [16] in their studies showed that women with low education level had 86% greater risk of pre-eclampsia (RRa=1.86, P=0.005) which is similar to our study. Ren QW, Yang FF, et al (2021) [17] showed in their studies that mean BMI was 22.71±3.37 kg/m2 in non-pre-eclampsia group and 34.25±3.59 kg/m2 in pre-eclampsia group. In our study the mean BMI was 27.87±5.62 (kg/m2) in pre-eclampsia group and 23.81±3.01 in non-Pre-eclampsia group (kg/m2) . In both study it was found that mean BMI is more in pre-eclampsia group. In our study 24(27.9%) patients had a sFlt-1 concentration higher than the 95th percentile, with six (46.15%) in the pre-eclamptic group. For PLGF, 13(15.11%) patients had a concentration lower than the 5th percentile; with two in the PE group (15.06%). The mean sFlt-1/PLGF was significantly higher (69±13) for women who developed PE than for women without PE (32±25). Park et al., (2014) [18] In their study showed that the mean sFlt-1/PLGF ratio was significantly higher 76.3(15.1-352.7) for women who developed PE than for women without PE 8(0.8-156). These results demonstrated that sFlt-1/PLGF ratio can be used as a predictor of pre-eclampsia. the National Institute for Health and Care Excellence guidance recommends sFlt-1/PLGF ratio testing to rule out PE in women presenting with suspected PE between 20 and 34+6 gestation weeks. Among the 86 patients, eight developed clinical PE: 5(62.50%) patients developed severe PE before 34 gestation weeks and 3(37.50%) after 34 weeks. Our study showed dipstick proteinuria was positive with 13(15.11%) in pre-eclampsia group and 7(8.1%) in non-pre-eclampsia group. Sensitivity and specificity were 100% and 86.90%, respectively. Concerning the risk of PE occurring within four weeks at the same threshold and after excluding the two patients who presented with PE before one week, six of the remaining patients developed PE. The negative predictive value of the ratio for the risk of PE occurring at four weeks was 98.63%; positive predictive value was 45.45%. Sensitivity was 83.33%, and specificity was 92.30%. The best cut-off value was 37.8% with sensitivity of 87.50% (95% CI=68.7-94) and specificity 92.30% (95% CI=62.1-96.8). (Zeisler et al ., 2016.) [8] identified an sFlt-1: PLGF ratio cutoff of 38 as having important predictive value. In a subsequent validation study among an additional 550 women, an sFlt-1:PLGF ratio of 38 or lower had a negative predictive value (i.e., no pre-eclampsia in the subsequent week) of 99.3% (95% confidence interval [CI], 97.9 to 99.9), with 80.0% sensitivity (95% CI, 51.9 to 95.7) and 78.3% specificity (95% CI, 74.6 to 81.7). The positive predictive value of an sFlt-1: PLGF ratio above 38 for a diagnosis of preeclampsia within 4 weeks was 36.7% (95% CI, 28.4 to 45.7), with 66.2% sensitivity (95% CI, 54.0 to 77.0) and 83.1% specificity (95% CI, 79.4 to 86.3).Caillon, H et al., 2018) [19] in their study showed that among the 67 patients , 53 had a sFlt-1/PLGF ratio lower than 38; none developed subsequent PE leading to a negative predictive value of 100%. Eight patients developed clinical PE. The positive predictive value was 21% at one week and 78% at four weeks. The above studies including our study reveals that sFlt-1/PLGF ratio below the cut off value can rule out pre-eclampsia in one week, and above the cut off value can predict pre-eclampsia within 4 weeks. (Perales et al., 2017) [20] in their study showed receiver-operating characteristics curves of 0.86 (95% CI, 0.77-0.95) which is almost similar to our study of 0.90 (95% CI=0.83-0.98). In this current study, it was observed in multivariate logistic regression analysis that a subject with sFlt-1/PLGF ratio had 1.675 (95.0% CI 0.987-1.315) times increase in odds were significantly associated with predictor of preeclampsia. Broader use of the sFlt-1/PLGF ratio in maternity care may support targeted clinical care by helping to identify women who are at high risk of developing PE and require close monitoring and management from women who are at a low risk of developing PE and can simply be reassured, thus avoiding unnecessary hospitalization (Klein E, Schlembach D, 2016) [21]. The utility of these markers as an aid in prognostication and diagnosis of pre-eclampsia was especially useful when a ratio was calculated. The present study showed that the sFlt-1/PLGF ratio improved the sensitivity to predict preeclampsia risk than any of the individual factors. Women with levels of sFlt-1/PLGF ratio greater than 37.8 were at a higher risk of consequent development of pre-eclampsia compared with women with values lower than that cutoff value. These data were constant with previous studies which declared that the sFlt-1/PLGF ratio was the best predictor of pre-eclampsia when compared with individual measure. Our study demonstrated that a cut-off of 37.8 for the sFlt-1/PLGF ratio can rule out PE in high-risk patients for 4 weeks. Use of the sFlt-1/PLGF ratio in clinical practice will improve management of the disease and reduce health expenditures, while ensuring safety.
LIMITATIONS
The study population was selected from one selected hospital in Dhaka city, so that the results of the study may not reflect the exact picture of our country. Present study was conducted at a very short period of time. Small sample size was also a limitation of present study. Therefore, in future further study may be under taken with large sample size.
CONCLUSIONS
Among 86 patients 8(9.3%) patients developed pre-eclampsia. In these PE group 7 patients had sFlt-1/PLGF ratio >38.Among 86 patients 78 patients did not develop PE. In these non PE group 72 patients had sFlt-1/PLGF ratio<38. Sensitivity, Specificity, accuracy, positive and negative predictive value of sFlt-1/PLGF ratio for prediction of PE 87.50%, 92.30%, 53.84%, 98.63% and Diagnostic accuracy 99.30% respectively. The serum sFlt-1/PLGF ratio showed highly predictive performances for ruling out PE. Using these biomarkers in routine management of PE may improve clinical care and avoid over testing and over treating, which has a significant economic impact. Beside this assessment of sFlt-1/PLGF ratio helps to avoid unnecessary stress and anxiety for the patients. This study was undertaken to evaluate sFlt-1/PLGF ratio among the pregnant patients from 24+0 - 36+6 weeks of gestation with at least one risk factors for pre-eclampsia as a short term predictor of pre-eclampsia. The study revealed sFlt-1/PLGF ratio below cut off value 37.8% can rule out pre-eclampsia for 4 weeks. Therefore sFlt-1/PLGF ratio can be used as a short term predictor of pre-eclampsia.
REFERENCES
- Mou A D, Barman Z, Hasan M, Miah R, Hafsa J M, Das Trisha A, Ali N. Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci Rep. 2021 Oct 29; 11(1): 21339.
- Rudra P, Basak S, Patil D. and Latoo M Y, 2011. Recent advances in management of pre-eclampsia. BJMP, 4(3), pp.433-441.
- Vousden N, Lawley E, Seed P T, Gidiri M F, Goudar S, Sandall J, Chappell L C, Shennan A H. and CRADLE Trial Collaborative Group, 2019. Incidence of eclampsia and related complications across 10 low-and middle-resource geographical regions: secondary analysis of a cluster randomised controlled trial. PLoS medicine, 16(3), p.e1002775.
- Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel J P, Souza J P. and WHO Multicountry Survey on Maternal and Newborn Health Research Network, 2014. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the W orld H ealth O rganization Multicountry S urvey on M aternal and N ewborn H ealth. BJOG: An International Journal of Obstetrics & Gynaecology, 121, pp.14-24.
- Biradar, A M, Yaliwal R G, Kori S S, Gamini B S and Pujeri S U, 2020. A 5-Year Clinical Experience of Haemolysis, Elevated Liver Enzymes and Low Platelet Count (HELLP) Syndrome at a Tertiary Care Teaching Hospital in North Karnataka--A Retrospective Analysis. Journal of Evolution of Medical and Dental Sciences, 9(40), pp.2938-2942.
- Suhag A. and Berghella V, 2013. Intrauterine growth restriction (IUGR): etiology and diagnosis. Current Obstetrics and Gynecology Reports, 2(2), pp.102-111.
- English F A, Kenny L C, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control. 2015 Mar 3; 8: 7-12.
- Zeisler H, Llurba E, Chantraine F, Vatish M, Staff A C, Sennström M, Olovsson M, Brennecke S P, Stepan H, Allegranza, D. and Dilba P, 2016. Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. N Engl J Med, 374, pp.13-22.
- Stepan H, Hund M, Gencay M, Denk B, Dinkel C, Kaminski W E, Wieloch P, Semus B, Meloth T, Dröge L A and Verlohren, S., 2016. A comparison of the diagnostic utility of the sFlt-1/PlGF ratio versus PlGF alone for the detection of preeclampsia/HELLP syndrome. Hypertension in pregnancy, 35(3), pp.295-305.
- Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F, Klein E, Lapaire O, Llurba E, Ramoni A and Vatish M, 2015. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound in Obstetrics & Gynecology, 45(3), p.241.
- Graupner O, Enzensberger C. Prediction of Adverse Pregnancy Outcome Related to Placental Dysfunction Using the sFlt-1/PlGF Ratio: A Narrative Review. Geburtshilfe Frauenheilkd. 2021 Aug; 81(8): 948-954.
- Kojovic D, V Workewych N, Piquette-Miller M. Role of Elevated SFLT-1 on the Regulation of Placental Transporters in Women With Pre-Eclampsia. Clin Transl Sci. 2020 May; 13(3): 580-588.
- Wang C C, Li K, Gaylord S. Wang et al. Respond. Am J Public Health. 2019 Sep; 109(9): e5-e6.
- Osungbade K O. and Ige O K, 2011. Public health perspectives of preeclampsia in developing countries: implication for health system strengthening. Journal of pregnancy, 2011.
- Kumari A, Chakrawarty A, Singh A. and Singh R, 2014. Maternofoetal complications and their association with proteinuria in a tertiary care hospital of a developing country. Journal of pregnancy, 2014.
- Opitasari C and Andayasari L, 2014. Parity, education level and risk for (pre-) eclampsia in selected hospitals in Jakarta. Health Science Journal of Indonesia, 5(1), pp.35-39.
- Ren Q W, Yang F F, Han T B, Guo M Z, Zhao N, Feng Y L, Yang H L, Wang S P, Zhang Y W, Wu W W. [Relationship between the pre-pregnancy BMI, gestational weight gain, and risk of preeclampsia and its subtypes]. Zhonghua Liu Xing Bing Xue Za Zhi. 2021 Nov 10; 42(11): 2037-2043.
- Park H J, Kim S H, Jung Y W, Shim S S, Kim J Y, Cho Y K, Farina A, Zanello M, Lee K J and Cha D H, 2014. Screening models using multiple markers for early detection of late-onset preeclampsia in low-risk pregnancy. BMC pregnancy and childbirth, 14(1), pp.1-11.
- Caillon H, Tardif C, Dumontet E, Winer N and Masson D, 2018. Evaluation of sFlt-1/PlGF ratio for predicting and improving clinical management of pre-eclampsia: experience in a specialized perinatal care center. Annals of laboratory medicine, 38(2), pp.95-101.
- Perales A, Delgado J L, De La Calle M, García-Hernández J A, Escudero A I, Campillos J M, Sarabia M D, Laíz B, Duque M, Navarro M and Calmarza P, 2017. sFlt-1/PlGF for prediction of early-onset pre-eclampsia: STEPS (Study of Early Pre-eclampsia in Spain). Ultrasound in Obstetrics & Gynecology, 50(3), pp.373-382.
- Klein, E., Schlembach, D., Ramoni, A., Langer, E., Bahlmann, F., Grill, S., Schaffenrath, H., van der Does, R., Messinger, D., Verhagen-Kamerbeek, W.D. and Reim, M., 2016. Influence of the sFlt-1/PlGF ratio on clinical decision-making in women with suspected preeclampsia. 11(5), p.e0156013.
