Massive Pulmonary Embolism Presenting as Obstructive Shock in a Nigerian Woman: A Case Report
Article Information
Bernard B Akpu1, Chidimma A Ahaneku2*, Agbo J Etim1, Ezoke J Epoke1, David E Elem3, Bassey E Ekeng3, Edikan B Bassey4, Chibueze H Njoku2, Victor O Ansa1, Clement O Odigwe1
1Cardiology Unit, Department of Internal Medicine, University of Calabar Teaching Hospital, Calabar, Cross River State, Nigeria
2Pulmonology Unit, Department of Internal Medicine, University of Calabar Teaching Hospital, Calabar. Cross River State, Nigeria
3Infectious Disease Unit, Department of Internal Medicine, University of Calabar Teaching Hospital, Calabar, Cross River State, Nigeria
4Department of Family Medicine, University of Calabar Teaching Hospital, Calabar, Cross River State, Nigeria
*Corresponding Author: Chidimma A Ahaneku, University of Calabar Teaching Hospital, Calabar, Nigeria.
Received: 18 May 2023; Accepted: 30 May 2023; Published: 19 June 2023
Citation: Bernard B Akpu, Chidimma A Ahaneku, Agbo J Etim, Ezoke J Epoke, David E Elem, Bassey E Ekeng, Edikan B Bassey, Chibueze H Njoku, Victor O Ansa, Clement O Odigwe. Massive Pulmonary Embolism Presenting as Obstructive Shock in a Nigerian Woman: A Case Report. Archives of Clinical and Medical Case Reports. 7 (2023): 280-286.
View / Download Pdf Share at FacebookAbstract
Background: Massive pulmonary embolism is a life-threatening emergency associated with high mortality if prompt diagnosis and urgent intervention is delayed, especially in patients presenting in obstructive shock. It usually requires advanced therapies such as systemic thrombolysis, pharmacomechanical catheter-directed therapy, surgical embolectomy and inferior vena cava filter placement. The dearth in skilled manpower, delay in diagnosis, relative unavailability and high cost of fibrinolytics especially in resource poor environments may account for poor clinical outcomes and eventually death in such patients.
Case Presentation: We present a 50-year-old Nigerian woman of Bahumono ethnicity who was admitted into our hospital with complains of sudden onset breathlessness. On presentation, she was tachypneic, tachycardic and hypotensive. D- Dimer and Troponin I levels were elevated. Computed tomography pulmonary angiography (CTPA) with intravenous contrast confirmed the diagnosis of massive pulmonary embolism. She was initially treated with low molecular weight heparin, then thrombolyzed with intravenous alteplase and later discharged on tablet dabigatran. Her symptoms had resolved at the time of discharge and she has remained stable for over 3 months.
Conclusion: Pulmonary embolism, the most serious clinical presentation of venous thromboembolism can become catastrophic when it presents as the massive type. Prompt diagnosis and urgent appropriate medical intervention ensures good clinical outcomes.
Keywords
Alteplase; Massive Pulmonary Embolism; Obstructive Shock; Troponin I
Alteplase articles; Massive Pulmonary Embolism articles; Obstructive Shock articles; Troponin I articles
COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Alteplase articles Alteplase Research articles Alteplase review articles Alteplase PubMed articles Alteplase PubMed Central articles Alteplase 2023 articles Alteplase 2024 articles Alteplase Scopus articles Alteplase impact factor journals Alteplase Scopus journals Alteplase PubMed journals Alteplase medical journals Alteplase free journals Alteplase best journals Alteplase top journals Alteplase free medical journals Alteplase famous journals Alteplase Google Scholar indexed journals Massive Pulmonary Embolism articles Massive Pulmonary Embolism Research articles Massive Pulmonary Embolism review articles Massive Pulmonary Embolism PubMed articles Massive Pulmonary Embolism PubMed Central articles Massive Pulmonary Embolism 2023 articles Massive Pulmonary Embolism 2024 articles Massive Pulmonary Embolism Scopus articles Massive Pulmonary Embolism impact factor journals Massive Pulmonary Embolism Scopus journals Massive Pulmonary Embolism PubMed journals Massive Pulmonary Embolism medical journals Massive Pulmonary Embolism free journals Massive Pulmonary Embolism best journals Massive Pulmonary Embolism top journals Massive Pulmonary Embolism free medical journals Massive Pulmonary Embolism famous journals Massive Pulmonary Embolism Google Scholar indexed journals Pulmonary Embolism articles Pulmonary Embolism Research articles Pulmonary Embolism review articles Pulmonary Embolism PubMed articles Pulmonary Embolism PubMed Central articles Pulmonary Embolism 2023 articles Pulmonary Embolism 2024 articles Pulmonary Embolism Scopus articles Pulmonary Embolism impact factor journals Pulmonary Embolism Scopus journals Pulmonary Embolism PubMed journals Pulmonary Embolism medical journals Pulmonary Embolism free journals Pulmonary Embolism best journals Pulmonary Embolism top journals Pulmonary Embolism free medical journals Pulmonary Embolism famous journals Pulmonary Embolism Google Scholar indexed journals Ultrasound articles Ultrasound Research articles Ultrasound review articles Ultrasound PubMed articles Ultrasound PubMed Central articles Ultrasound 2023 articles Ultrasound 2024 articles Ultrasound Scopus articles Ultrasound impact factor journals Ultrasound Scopus journals Ultrasound PubMed journals Ultrasound medical journals Ultrasound free journals Ultrasound best journals Ultrasound top journals Ultrasound free medical journals Ultrasound famous journals Ultrasound Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Obstructive Shock articles Obstructive Shock Research articles Obstructive Shock review articles Obstructive Shock PubMed articles Obstructive Shock PubMed Central articles Obstructive Shock 2023 articles Obstructive Shock 2024 articles Obstructive Shock Scopus articles Obstructive Shock impact factor journals Obstructive Shock Scopus journals Obstructive Shock PubMed journals Obstructive Shock medical journals Obstructive Shock free journals Obstructive Shock best journals Obstructive Shock top journals Obstructive Shock free medical journals Obstructive Shock famous journals Obstructive Shock Google Scholar indexed journals Troponin articles Troponin Research articles Troponin review articles Troponin PubMed articles Troponin PubMed Central articles Troponin 2023 articles Troponin 2024 articles Troponin Scopus articles Troponin impact factor journals Troponin Scopus journals Troponin PubMed journals Troponin medical journals Troponin free journals Troponin best journals Troponin top journals Troponin free medical journals Troponin famous journals Troponin Google Scholar indexed journals Respiratory Examination articles Respiratory Examination Research articles Respiratory Examination review articles Respiratory Examination PubMed articles Respiratory Examination PubMed Central articles Respiratory Examination 2023 articles Respiratory Examination 2024 articles Respiratory Examination Scopus articles Respiratory Examination impact factor journals Respiratory Examination Scopus journals Respiratory Examination PubMed journals Respiratory Examination medical journals Respiratory Examination free journals Respiratory Examination best journals Respiratory Examination top journals Respiratory Examination free medical journals Respiratory Examination famous journals Respiratory Examination Google Scholar indexed journals
Article Details
Abbreviations:
AHA: American Heart Association CT: Computed Tomography; CTPA: Computed Tomography Pulmonary; Angiography DVT: Deep Vein Thrombosis; 0C: Degree Centigrade; ESC: European Society of Cardiology; HRCT: High Resolution Computed Tomography; INR: International Normalized Ratio; LV: Left Ventricular; PE: Pulmonary embolism; RV: Right Ventricular; tPA Tissue plasminogen activator
1. Background
Pulmonary embolism (PE) is an obstructive disease of the pulmonary arterial system caused by the embolization of thrombus originating from outside the lungs especially the deep veins of the lower extremities [1, 2]. The thrombus can also occur de-novo without any evidence of deep vein thrombosis (DVT) [3]. PE is one of the most common cardiovascular diseases, occurring in 1-2 per 1,000 people annually [2]. Virchow first described the process of thrombosis as a triad of stasis, hypercoagulability, and endothelial injury [1, 3]. Patient with PE may present classically with shortness of breath, pleuritic chest pain, cough, orthopnea, wheezing, hemoptysis or less commonly cardiac arrhythmias, syncopal attack or more devastatingly, circulatory collapse [4] depending on the size, number, site of thrombi/emboli and risk factors present [5].
PE is grouped into prognostic categories by the European Society of Cardiology (ESC) and American Heart Association (AHA) into high-risk (massive), intermediate-risk (sub-massive) and low-risk (non-massive) at frequencies of 20%, 32% and 48% respectively [6]. Massive PE, an uncommon and life-threatening presentation of PE is defined as obstruction of the pulmonary arterial tree that exceeds 50% of the cross-sectional area, causing acute and severe cardiopulmonary failure from right ventricular overload [7]. Shock refers to a circulatory failure those results in inadequate cellular oxygen supply and utilization [8]. According to Cox and Hinshaw [9], shock is classified into cardiogenic, hypovolemic, distributive and obstructive shock based on the cause. The most frequent causes of obstructive shock include PE, pneumothorax and cardiac tamponade [8]. The average mortality rate from PE is 11%, but may be significantly higher in patients in shock (40-50%) [9].The overall mortality rates for massive, sub-massive and non- massive PE are 71.4%, 44.5% and 28.1% respectively [10]. In patients with massive PE, 50% die within 30 minutes, 70% die within 1 hour, and more than 85% die within 6 hours of the onset of symptoms [11].
Consequently, a high index of suspicion, urgent diagnosis and treatment are of critical importance. Thrombolytic therapy is currently recommended for the treatment of massive PE and Alteplase (tPA) is the most common thrombolytic agent used [12]. Massive PE is rare [13] and to the best of our knowledge, massive PE presenting as obstructive shock has not been reported in Nigeria. We report this case of massive pulmonary embolism presenting as obstructive shock in a 50-year-old Nigerian woman.
2. Case Presentation
A 50-year old female, obese, newly diagnosed diabetic but not a known hypertensive was admitted into the hospital with a one week history of sudden onset of breathlessness. There was associated history of palpitations but no chest pain, orthopnea, paroxysmal nocturnal dyspnea or leg swelling. She had a past history of left leg DVT about a year prior to presentation. There was no history of tobacco or alcohol use. On examination, she was afebrile with a temperature of 37ºC. She was not pale. She was anicteric, acyanotic and had no peripheral edema. She had tachycardia (pulse rate - 135beats /min) and hypotension (blood pressure- 80/ 50mmHg). Heart sounds were S4, S1 and S2. She was also tachypneic (respiratory rate- 36 cycles per minute) with SPO2 of 96%. Abdominal and Neurological examinations were not remarkable. Wells score was 6 (likely PE). D dimer was markedly elevated- 11,580ng/ ml (Normal <500ng/ml). Troponin I was also elevated-12.4ng/l (Normal<6ng/l). White blood cell count, platelet count, serum electrolyte urea and creatinine were essentially within reference limits (Table 1). Arterial blood gas analysis was not done as facilities were not functional. Electrocardiography showed sinus tachycardia, T wave inversion in anteroseptal leads and Mc Ginne white sign- S1Q3T3 pattern (Figure 1), while echocardiography suggested concentric left ventricular remodeling secondary to hypertensive heart disease, preserved biventricular systolic function, grade 1 left ventricular diastolic impairment and mild pulmonary hypertension (Figure 2). The echocardiographic derived values are as shown in Table 2. Chest X-Ray showed cardiomegaly and aortic unfolding suggestive of hypertensive cardiovascular changes (Figure 3). Doppler Ultrasound of both lower limbs showed no obvious intra luminal thrombus, no loss of compressibility of the deep veins of both lower limbs and no calcifications in the vessel walls (Figure 4).
|
Test |
Value |
Normal Range |
|
Hemoglobin (g/dl) |
12.7 |
11.5-15.5 |
|
White-cell count (per mm3) |
9,400 |
4,000-11,000 |
|
Platelet count (per mm3) |
199,000 |
150000-400000 |
|
Urea (mmol/l) |
3.57 |
2.2- 7.2 |
|
Creatinine (umol/l) |
97.24 |
44.2 -106 |
|
Sodium (mmol/l) |
141.3 |
135-145 |
|
Potassium (mmol/l) |
3.7 |
3.5-5.1 |
|
PT(s) |
11 |
11- 14 |
|
PTTK(s) |
44 |
20- 40 |
|
INR (International Normalized Ratio) |
1.3 |
<1.1 |
|
D- Dimer (ng/mL) |
11,580 |
<500 |
|
Troponin I (ng/l) |
12.4 |
< 6 |
|
FBS (mmol/l) |
13.1 |
3.3 – 5.5 |
|
HbA1c (%) |
8.3 |
4.0 – 6.0 |
|
Total Cholesterol (mmol/l) |
4.5 |
< 5.2 |
|
HDL- C (mmol/l) |
1.04 |
>1.0 |
|
LDL- C (mmol/l) |
2.9 |
<3.0 |
|
VLDL- C (mmol/l) |
21.6 |
14- 24 |
|
Triglyceride (mmol/l) |
1.19 |
<2.0 |
Table 1: Laboratory finding.
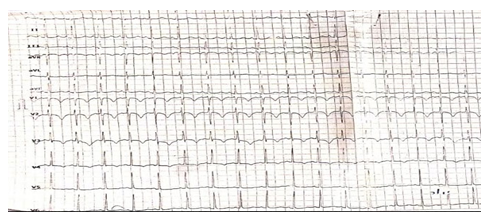
Figure 1: Electrocardiogram showing Sinus tachycardia, S1Q3T3 sign and T wave inversion in leads V1 to V4.
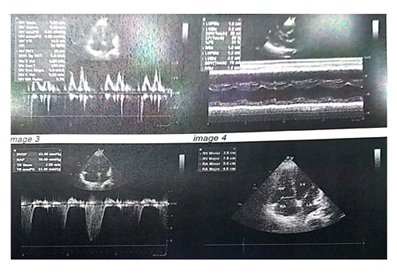
Figure 2: Two-dimensional Echocardiography showing features suggestive of hypertensive heart disease with diastolic dysfunction.
|
Parameter |
Value |
Normal Range |
|
EF (%) |
59 |
52- 74 |
|
FS (%) |
31 |
25- 45 |
|
EPSS (mm) |
9 |
<6 |
|
E/ A |
0.78 |
0.9- 1.5 |
|
E/e |
7. 99 |
<8 |
|
DT (ms) |
136 |
140- 240 |
|
IVRT (ms) |
83 |
70- 90 |
|
IVSd (mm) |
12 |
6- 9 |
|
LVPWd (mm) |
12 |
6- 9 |
|
LVIDd (mm) |
42 |
37. 8- 52. 2 |
|
RWT |
0.57 |
0. 42 |
|
LVM (g) |
178. 16 |
67- 162 |
|
LVMI (g/m2) |
84.43 |
43- 95 |
|
PASP (mmHg) |
43. 46 |
<30 |
|
TAPSE (mm) |
20 |
>17 |
|
RVS’ (cm/s) |
11 |
>9.5 |
|
RA Major (cm) |
4. 6 |
<5. 3 |
|
RA Minor (cm) |
5. 0 |
<4.4 |
|
RV Major (cm) |
7. 9 |
<7. 4 |
|
RV Minor (cm) |
3. 8 |
<3. 8 |
Table 2: Echocardiographic parameters.
A diagnosis of massive pulmonary embolism presenting with obstructive shock was made and the patient was commenced on anticoagulant therapy with subcutaneous enoxaparin at 1mg/kg 12hrly, inotropic support with dopamine, oxygen therapy, beta blockers (tablet bisoprolol 5mg dly), antidiabetic therapy (insulin and metformin) and artovastatins. However, she remained hypotensive despite inotropic support.
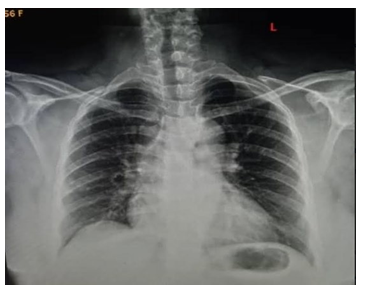
Figure 3: Chest X-Ray showing cardiomegaly and aortic unfolding suggestive of Hypertensive cardiovascular changes.
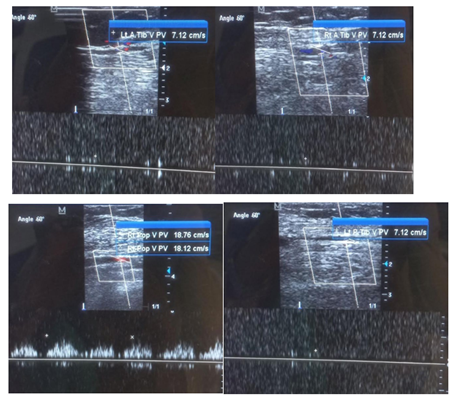
Figure 4: No obvious intra luminal thrombus in both lower limbs. No loss of compressibility of the deep veins of both lower limbs. No calcifications seen in vessel walls.
Computed Tomography Pulmonary Angiography (CTPA) was then requested and it showed bilateral pulmonary emboli with more than 75% luminal occlusion of both main bronchial, interlobar and segmental pulmonary arteries confirming the diagnosis of massive PE (Figure 5).
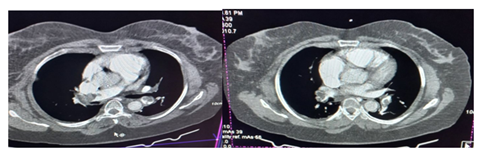
Figure 5: CT pulmonary angiography showing multiple, large hypo dense filling defects involving both main bronchial -right >Left (with greater than 75% luminal occlusion), interlobar and segmental pulmonary arteries.
The patient was then commenced on systemic thrombolysis with IV alteplase 100mg over 2 hours. Samples for repeat clotting profile was taken and anticoagulation with SC enoxaparin 100iu 12 hourly was continued for 7 days. Repeat D dimer done after systemic thrombolysis showed a marked reduction from the initial value of 11,580ng/ml to 2,510ng/ml. Tablet Dabigatran 150mg twice daily was commenced after enoxaparin administration to last for 3 months with a target INR of 1.2 to 1.8.
The patient showed marked clinical improvement and has remained stable since after discharge.
3. Discussion
Patients with massive PE present with profound dyspnea at rest, often accompanied by anxiety, syncope, or lightheadedness; and medical history may include recent surgery or trauma, congestive heart failure, chronic lung disease, prior venous thromboembolism, or cancer [14]. Thirty percent of patients with PE have no detectable provoking factors [2]. In this index patient, the cardiovascular risk factors were diabetes mellitus, past history of deep vein thrombosis, obesity, sedentary lifestyle, anabolic steroid use and dyslipidemia.
The recently published 2019 ESC guidelines provides a revised definition of high-risk (massive) PE encompassing the following clinical presentations: (i) cardiac arrest; (ii) obstructive shock (defined as systolic blood pressure <90mmHg or vasopressors required to achieve a blood pressure ≥90mmHg despite adequate filling status, in combination with end-organ hypoperfusion); or (iii) persistent hypotension (defined as systolic blood pressure <90mmHg or a systolic pressure drop by ≥40mmHg for longer than 15min, if not caused by new-onset arrhythmia, hypovolemia or sepsis) [15]. Our patient had dyspnea, features of obstructive shock and hypotension confirming the diagnosis of massive (high risk) PE.
Aniteye et al [16] observed that the commonest clinical presentations of the patients admitted with the diagnosis of massive PE were dyspnoea (100.0%) and hypotension (70.3%), which were both present in this index case. Similarly, Ebner et al [15] reported that among their patients classified as having massive (high-risk) PE, 43.0% presented with cardiac arrest, 44.2% with obstructive shock, and 12.8% with persistent hypotension only. Pivetti et al [17] found obstructive shock in 10.2% of their PE cases.
The combination of clinical features and predisposing risk factors has been incorporated into clinical scoring systems that are used to predict the likelihood of PE and determining which investigations to perform [18]. These clinical scoring systems include the Wells score, simplified Geneva score and Pulmonary Embolism Severity Index (PESI). The most extensively validated and widely used is the Wells score where PE is unlikely at values of 0-4 and likely at > 4 [6]. Wells score in our patient was 6 (likely PE). The initial diagnostic tests for PE include the D-Dimer assay which is usually elevated in PE. It has good sensitivity, but poor specificity [19]. The D-Dimer level in our patient was 11,580ng/ml (Normal <500ng/ml) and this further heightened our clinical suspicion.
Patients with massive PE present with obstructive shock as abrupt elevation in afterload impairs right ventricular (RV) contractility and causes RV dilatation with bowing of the interventricular septum, impeding left ventricular (LV) diastolic filling and ultimately reducing cardiac output [9, 20]. Worsening tricuspid regurgitation and RV ischemia secondary to RV wall stress and reduced right coronary artery perfusion also exacerbate this process [20]. Biomarkers, including serum troponin I or T may be useful in detecting evidence of this RV dysfunction as their levels increase with subsequent leakage from the RV myocytes into the bloodstream [6]. Troponin I was elevated-12.4ng/l (Normal<6ng/l) in our patient. Computed Tomography Pulmonary Angiography (CTPA) has over 90% specificity and sensitivity in diagnosing PE in the main, lobar, and segmental pulmonary arteries [21] and will show filling defect, upon contrast enhancement, in these arteries [22]. CTPA was done for our patient whose image showed multiple, large hypo dense filling defects involving both main, interlobar and segmental pulmonary arteries.
Ventilation-Perfusion (V/Q) scanning can be used for diagnosis of PE when CTPA is contraindicated. Echocardiography in PE shows a nonspecific right ventricle (RV) strain pattern, enlarged RV, tricuspid regurgitation and sometimes RV thrombi while electrocardiography shows sinus tachycardia and deep S wave in lead 1, Q wave in lead 3, T wave inversion in lead 3 -S1Q3T3 (these were seen in our patient). Tall P wave (P pulmonale), right bundle branch block, right ventricular hypertrophy and right axis deviation are other features [23]. Chest X-Ray sometimes shows segmental atelectasis, pleural effusion, cardiomegaly, regional oligemia, enlarged pulmonary artery, and parenchymal opacities with unilateral diaphragmatic elevation [23].
Patients with PE are treated according to their hemodynamic status and their risk profile [24]. Thrombolytic therapy is a useful adjunct to heparin in patients who have PE and are hemodynamically unstable [25]. Rapid improvement of RV function and pulmonary perfusion, accomplished with thrombolytic therapy followed by heparin, may lead to a lower rate of death and recurrent PE [25] as the main cause of mortality in massive PE is obstructive shock and associated right ventricular (RV) failure [6]. Alteplase, a tissue plasminogen activator (tPA) is the most common thrombolytic used for the treatment of PE, currently approved by the US Food and Drug Administration (FDA) at a dose of 100mg infused over 2 hours [12] was used for our patient with subsequent resolution of signs and symptoms of PE.
Pivetti et al [17] reported that of the 24 PE patients who suffered obstructive shock, 13 patients given thrombolysis were all alive and showed stable hemodynamic parameters at discharge, whereas 11 patients who were not thrombolysed died during hospital stay. Poor et al [26] reported a case series of two patients in the intensive care unit with massive PE and obstructive shock who had resolution of shock after repeated administration of alteplase.
5. Conclusion
Early recognition, correct diagnosis and treatment of patients with massive PE and obstructive shock using thrombolytic agents is recommended to increase their chances of full recovery and survival. We therefore recommend that practitioners should have a high index of suspicion as mortality is high within minutes to few hours of onset of symptoms of massive PE.
Declarations
Ethics Approval and Consent to Participate
Not Applicable.
Consent for Publication
Written informed consent was obtained from the patient to publish this case report. A copy of the written consent is available for review by the editor of this journal.
Availability of Data and Materials
Not applicable.
Competing Interests
The authors declare no potential conflicts of interest with respect to the case report, authorship, and/or publication of this article.
Funding
The authors received no financial support for the case report.
Authors’ Contributions
BBA: Conceptualization, writing-original draft, review and editing, CAA: Conceptualization, literature review, writing-original draft, review and editing, AJE, EJE, DEE, BEE and EBB: Participated in writing, CHN, VOA and COO: Constructive review and editing. All authors have agreed to the final version of this manuscript.
Acknowledgement
Not Applicable.
References
- Dalen JE. Pulmonary embolism: What have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest 122 (2002):1440-1456.
- Morrone D, Morrone V. Acute pulmonary embolism: Focus on the clinical picture. Korean Circ J 48 (2018): 365-381.
- Van Gent JM, Zander AL, Olson EJ, et al. Pulmonary embolism without deep venous thrombosis: De novo or missed deep venous thrombosis? J Trauma Acute Care Surg 76 (2014):1270-124.
- Stein PD, Beemath A, Matta F, et al. Clinical Characteristics of Patients with Acute Pulmonary Embolism: Data from PIOPED II. Am J Med 120 (2007): 871-879.
- Saleh JA, Shovlin C, Alasia DD. Acute Pulmonary Embolism: A review. Niger J Med 16 (2007): 11-17.
- Moorjani N, Price S. Massive Pulmonary Embolism. Cardiol Clin 31 (2013): 503-518.
- Riedel M. Acute Pulmonary Embolism 1: Pathophysiology, Clinical presentation and Diagnosis. Heart 85 (2001): 229-240.
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med 369 (2013): 1726-1734.
- Zotzmann V, Rottmann FA, Müller-Pelzer K, et al. Obstructive Shock, from Diagnosis to Treatment. Rev Cardiovasc Med 23 (2022):1-10.
- Gupta R, Ammari Z, Dasa O, et al. Long-term mortality after massive, submassive, and low-risk pulmonary embolism. Vasc Med (United Kingdom) 25 (2020): 141-149.
- Reza AQM, Attawar S, Munwar S, et al. Acute Massive Pulmonary Embolism (AMPE): From Therapeutic to Protective Management - A Case Report. Cardiovasc J 3 (2011): 213-217.
- Brandt K, McGinn K, Quedado J. Low-Dose Systemic Alteplase (tPA) for the Treatment of Pulmonary Embolism. Ann Pharmacother (2015): 1-7.
- Kucher N, Rossi E, Rosa M De, et al. Massive Pulmonary Embolism. Circulation 113 (2006): 577-578.
- Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 112 (2005): e28-e32.
- Ebner M, Sentler C, Harjola V-P, et al. Outcome of patients with different clinical presentations of high-risk pulmonary embolism. Eur Hear Journal Acute Cardiovasc Care 10 (2021): 787-796.
- Aniteye E, Tettey M, Sereboe L, et al. Outcome of thrombolysis for massive pulmonary embolism. Ghana Med J 43 (2009): 19-23.
- Pivetti S, Aluffi E, Bonino L, et al. Obstructive shock in pulmonary embolism: thrombolytic therapy and survival. Crit Care 3 (1999): 120.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 172 (2005): 1041-1046.
- Nikolaou K, Thieme S, Sommer W, et al. Diagnosing pulmonary embolism: New computed tomography applications. J Thorac Imaging 25 (2010):151-160.
- Konstantinides SV. ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 35 (2014): 3145-3146.
- Riedel M. Diagnosing pulmonary embolism. Postgrad Med J 80 (2004): 309-319.
- Remy-Jardin M, Pistolesi M, Goodman LR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: A statement from the Fleischner Society. Radiology 245 (2007): 315-329.
- Ogunkoya J, Oluwole A, Adefuye B, et al. Acute Pulmonary Thromboembolism: A Retrospective Study in a Nigerian Private Tertiary Hospital. Ann Heal Res 7 (2021):107-117.
- Erythropoulou-Kaltsidou A, Alkagiet S, Tziomalos K. New guidelines for the diagnosis and management of pulmonary embolism: Key changes. World J Cardiol 12 (2020): 161-166.
- Goldhaber SZ. Thrombolysis for Pulmonary Embolism. N Engl J Med 34 (2002): 1131-1132.
- Poor AD, Poor HD. Successful treatment of refractory massive pulmonary embolism with repeated administration of systemic thrombolysis. Tanaffos 17 (2018): 127-131.
