Management of Traumatic Lumbar Meningo-Radicular Injury caused by Foreign Body Penetration using Sling Technique
Article Information
Ying-Yun Chen1, Chieh-Tsai Wu1, Ting-Wei Chang1, Ko-Ting Chen1, 2*
1Department of Neurosurgery, Chang-Gung Memorial Hospital,Taoyuan, Taiwan
2Ph.D. Program in Biomedical Engineering, Chang Gung University, Taoyuan, Taiwan
*Corresponding Author: Ko-Ting Chen, Department of Surgery, Division of Neurosurgery, Chang Gung Memorial Hospital at Linkou, 5, Fu Xing St., Guishan Township, Taoyuan, 333, Taiwan
Received: 25 May 2022; Accepted: 10 June 2022; Published: 19 July 2022
Citation: Ying-Yun Chen, Chieh-Tsai Wu, Ting-Wei Chang, Ko-Ting Chen. Management of Traumatic Lumbar Meningo-Radicular Injury caused by Foreign Body Penetration using Sling Technique. Journal of Spine Research and Surgery 4 (2022): 087-091.
View / Download Pdf Share at FacebookAbstract
Introduction: Non-missile penetrating spinal injuries (NMPSI) can cause delayed neural injury, including cerebrospinal fluid (CSF) leakage and spinal cord herniation, related to dural defects. To repair ventral dural defect (VDD) is particularly challenging in trauma patients and, in a meanwhile, there has been a well-established method: sling technique, used for patients with idiopathic spinal cord herniation (ISCH) in whom VDD being the primary pathology to deal with.
Case presentation: This 51-year-old man fell down from an altitude of six meters and landed on a plier. The neurological examination revealed decreased muscle strength (grade 3/3) in bilateral lower extremities. Computed tomography showed a pair of pliers penetrating L4 lamina, spinal canal through vertebral body, psoas muscle into retroperitoneal cavity with inferior vena cava (IVC) indentation. Emergent laparotomy revealed intact IVC with no major organ damage, and the plier was removed from back under direct visualization of IVC. Immediate posterior approach showed a through-and-through VDD. Sling technique with COOK® dura substitute was applied to cover the VDD and fixed with 7-0 prolene after neurolysis. There was no CSF leakage or nerve tissue herniation afterward. He regained working ability with full muscle strength except for a minor sequal of paresthesia of right toe.
Conclusions: In patients with NMPSI with VDD, indirect duraplasty using sling technique originally developed for treating patients with ISCH is suitable and effective in preventing CSF leakage and delayed neural injury. We further propose an algorithm emphasizing key decision makings for repairing dural defect while preventing delayed neural injury.
Keywords
Duraplasty, Sling Procedure, Traumatic Spinal Dural Defect, Ventral Dural Defect
Duraplasty articles Duraplasty Research articles Duraplasty review articles Duraplasty PubMed articles Duraplasty PubMed Central articles Duraplasty 2023 articles Duraplasty 2024 articles Duraplasty Scopus articles Duraplasty impact factor journals Duraplasty Scopus journals Duraplasty PubMed journals Duraplasty medical journals Duraplasty free journals Duraplasty best journals Duraplasty top journals Duraplasty free medical journals Duraplasty famous journals Duraplasty Google Scholar indexed journals Sling Procedure articles Sling Procedure Research articles Sling Procedure review articles Sling Procedure PubMed articles Sling Procedure PubMed Central articles Sling Procedure 2023 articles Sling Procedure 2024 articles Sling Procedure Scopus articles Sling Procedure impact factor journals Sling Procedure Scopus journals Sling Procedure PubMed journals Sling Procedure medical journals Sling Procedure free journals Sling Procedure best journals Sling Procedure top journals Sling Procedure free medical journals Sling Procedure famous journals Sling Procedure Google Scholar indexed journals Traumatic Spinal Dural Defect articles Traumatic Spinal Dural Defect Research articles Traumatic Spinal Dural Defect review articles Traumatic Spinal Dural Defect PubMed articles Traumatic Spinal Dural Defect PubMed Central articles Traumatic Spinal Dural Defect 2023 articles Traumatic Spinal Dural Defect 2024 articles Traumatic Spinal Dural Defect Scopus articles Traumatic Spinal Dural Defect impact factor journals Traumatic Spinal Dural Defect Scopus journals Traumatic Spinal Dural Defect PubMed journals Traumatic Spinal Dural Defect medical journals Traumatic Spinal Dural Defect free journals Traumatic Spinal Dural Defect best journals Traumatic Spinal Dural Defect top journals Traumatic Spinal Dural Defect free medical journals Traumatic Spinal Dural Defect famous journals Traumatic Spinal Dural Defect Google Scholar indexed journals Ventral Dural Defect articles Ventral Dural Defect Research articles Ventral Dural Defect review articles Ventral Dural Defect PubMed articles Ventral Dural Defect PubMed Central articles Ventral Dural Defect 2023 articles Ventral Dural Defect 2024 articles Ventral Dural Defect Scopus articles Ventral Dural Defect impact factor journals Ventral Dural Defect Scopus journals Ventral Dural Defect PubMed journals Ventral Dural Defect medical journals Ventral Dural Defect free journals Ventral Dural Defect best journals Ventral Dural Defect top journals Ventral Dural Defect free medical journals Ventral Dural Defect famous journals Ventral Dural Defect Google Scholar indexed journals spinal injuries articles spinal injuries Research articles spinal injuries review articles spinal injuries PubMed articles spinal injuries PubMed Central articles spinal injuries 2023 articles spinal injuries 2024 articles spinal injuries Scopus articles spinal injuries impact factor journals spinal injuries Scopus journals spinal injuries PubMed journals spinal injuries medical journals spinal injuries free journals spinal injuries best journals spinal injuries top journals spinal injuries free medical journals spinal injuries famous journals spinal injuries Google Scholar indexed journals lower cervical articles lower cervical Research articles lower cervical review articles lower cervical PubMed articles lower cervical PubMed Central articles lower cervical 2023 articles lower cervical 2024 articles lower cervical Scopus articles lower cervical impact factor journals lower cervical Scopus journals lower cervical PubMed journals lower cervical medical journals lower cervical free journals lower cervical best journals lower cervical top journals lower cervical free medical journals lower cervical famous journals lower cervical Google Scholar indexed journals upper thoracic articles upper thoracic Research articles upper thoracic review articles upper thoracic PubMed articles upper thoracic PubMed Central articles upper thoracic 2023 articles upper thoracic 2024 articles upper thoracic Scopus articles upper thoracic impact factor journals upper thoracic Scopus journals upper thoracic PubMed journals upper thoracic medical journals upper thoracic free journals upper thoracic best journals upper thoracic top journals upper thoracic free medical journals upper thoracic famous journals upper thoracic Google Scholar indexed journals vascular injury articles vascular injury Research articles vascular injury review articles vascular injury PubMed articles vascular injury PubMed Central articles vascular injury 2023 articles vascular injury 2024 articles vascular injury Scopus articles vascular injury impact factor journals vascular injury Scopus journals vascular injury PubMed journals vascular injury medical journals vascular injury free journals vascular injury best journals vascular injury top journals vascular injury free medical journals vascular injury famous journals vascular injury Google Scholar indexed journals hemicolectomy articles hemicolectomy Research articles hemicolectomy review articles hemicolectomy PubMed articles hemicolectomy PubMed Central articles hemicolectomy 2023 articles hemicolectomy 2024 articles hemicolectomy Scopus articles hemicolectomy impact factor journals hemicolectomy Scopus journals hemicolectomy PubMed journals hemicolectomy medical journals hemicolectomy free journals hemicolectomy best journals hemicolectomy top journals hemicolectomy free medical journals hemicolectomy famous journals hemicolectomy Google Scholar indexed journals spinal canal articles spinal canal Research articles spinal canal review articles spinal canal PubMed articles spinal canal PubMed Central articles spinal canal 2023 articles spinal canal 2024 articles spinal canal Scopus articles spinal canal impact factor journals spinal canal Scopus journals spinal canal PubMed journals spinal canal medical journals spinal canal free journals spinal canal best journals spinal canal top journals spinal canal free medical journals spinal canal famous journals spinal canal Google Scholar indexed journals
Article Details
1. Introduction
The majority of nonmissile penetrating spinal injuries (NMPSIs) is attributed to assaults by knives involving lower cervical and upper thoracic [1]. Despite a controversial role of surgical intervention in treating patients with NPMSI with non-retained foreign body [2, 3], an invasive treatment comprising safe removal of foreign body and repair of injured structure is oftentimes recommended in those with retained foreign body [4]. NMPSI can be categorized into immediate and delayed types. Immediate spinal injury is caused by direct penetrating injury and vascular injury [5]. Delayed neurological deficit may be caused by CSF leakage, spinal cord herniation, retained foreign body, infection and edema [6, 7]. Since immediate injuries occurred before reaching medical cares, efforts should be paid to prevent delayed neurological deficits. Currently, there is no guideline on treating patients with NMPSI, particularly addressing issues on preventing delayed injuries mainly CSF leakage and spinal cord herniation, in other words, repairing dural defect. By presenting this case, we discuss and propose an algorithm for dural defect repair (Figure 4).
2. Case Presentation
This is a 51–year-old male construction worker with the past history of colon perforation status post right hemicolectomy. While working, he fell down from an altitude of six meters and landed on a plier placed on the floor vertically (Figure 1A, B). At the emergency department, he was alert and oriented, with stable vital signs: T: 36.1°C, P:81/min, R:20/min, BP:149/99 mmHg. He complained of low back pain, bilateral extremities numbness and weakness. Physical examination showed smooth breathing sound, no distant heart sound, no Cullen’s sign, no abdominal tenderness, and no deformity of extremities. Neurological examination revealed decreased muscle strength (grade 3/3) in bilateral lower extremities, paresthesia below knee and decrease deep tendon reflex of ankle jerk. There was normal anal sphincter tone. Lab data confirmed no anemia (Hb=15.4 g/dL), no renal or hepatic function impairment (BUN=9.8 mg/dL, creatinine=0.86 mg/dL and ALT=33 U/L). A computed tomography showed a pair of pliers penetrating L4 lamina, spinal canal, through vertebral body and psoas muscle into retroperitoneal cavity with IVC indentation (Figure 2). There was no contrast extravasation or bloody ascites in the peritoneal cavity.
Under the diagnosis of NMPSI with retained foreign body at lumbar level, the patient then underwent emergent combined surgery of trauma surgeon and neurosurgeon for the prevention of IVC bleeding upon foreign body removal. The patient was placed in three-quarter supine position (Figure 1D). A paramedian laparotomy with transverse extension was done first to expose right retroperitoneal space after elevation of liver. The plier tip was palpated, confirming that the medial arm located at the lateral aspect of supra-renal IVC and the lateral arm located closely to the upper and lateral pole of right kidney (Figure 1E). The IVC was intact, accompanying with no injury to renal pedicle artery and mild kidney abrasion. After affirmation that there was no active bleeding or major organ damage, the foreign body was removed cautiously from the back of the patient by neurosurgeon (Figure 1D). There was CSF leakage and a simple stitch closure was made. After closure of abdominal wound, the patient was then placed in prone position. A midline incision was made and muscle was dissected to expose L3-L4 lamina. After laminectomy and bone chip removal, a through-and-through dural defect was found (Figure 3A) and several adherent rootlets related to penetrating friction were seen at the margin of VDD. The VDD was tunneled to L3/4 disc space and packed with a piece of antibiotic-soaked gelfoam (Figure 3B). We applied sling technique with COOK® artificial dura to cover the VDD after neurolysis and fixed it with 7-0 prolene (Figure 3C). The dorsal dura defect (DDD) was closed primarily with watertight manner (Figure 3D). The patient’s lower extremities muscle strength improved to grade 4 and his paresthesia of calf and toes gradually faded away. One week after the operation, he could urinate and ambulate without assistance. There was no positional headache, nausea, vomiting, neck pain or stiffness post-operatively, indicating no CSF leakage. The patient returned to normal life with mild sequelae of right toe numbness. A follow-up MRI showed patent CSF flow without pseudomeningocele (Figure 4).
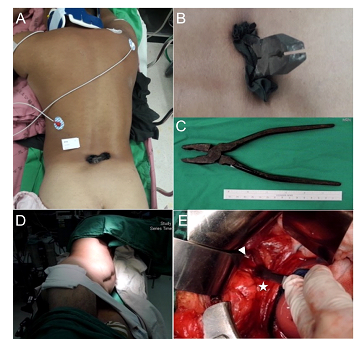
Figure 1: Pre- and intra-operative pictures. (A) A pair of pliers penetrated into the patient from behind at lower lumbar area in the midline. (B) A closer look of the pilers and the dragged fragment of his clothes. (C) A total 14cm long handle was inside the patient’s body. (D) Intraoperatively before laparotomy, a left decubitus position was prepared. (E) Direct vision of the handle tip of pliers (arrowhead) after we opened the retroperitonium cavity and retracted inferior vena cava (asterisk) away.
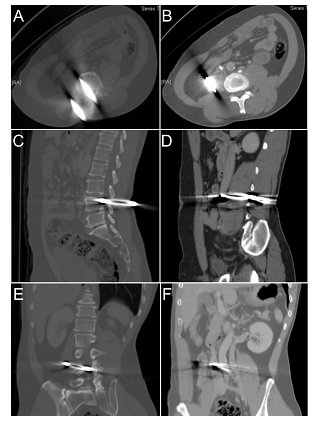
Figure 2: Preoperative abdominal CT. A pair of pliers penetrated L4 lamina, spinal canal and vertebral body through right psoas muscle into retroperitoneal cavity was shown in axial (A,B), sagittal (C,D) and coronal (E,F) view. Note the inferior vena cava was pushed aside by the tip of pliers (B,F).
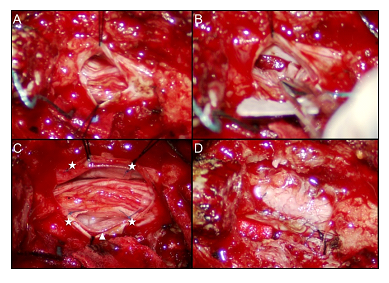
Figure 3: Intraoperative microscopic pictures of dura repair using sling technique. (A) After partial L3 and L4 laminectomy, a traumatic durotomy was found and it was extended rostrally and caudally for better inspection and management of intrathecal damage. (B) A ventral dural defect was packed with gelfoam for hemostasis and all injured rootlets were gently reduced into intradural cavity. (C) Sling technique was selected to repair the ventral dural defect by using artificial dural graft (arrowhead), which was sutured on the ventral (not shown) and the dorsal side (asterisks). (D) A final look of the fully-expanded thecal sac after primary repair of traumatic dural defect.
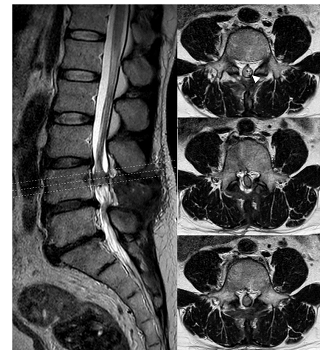
Figure 4: Postoperative 6 month MRI of Lumbar spine showed good coverage of artificial dural graft (arrowhead) circumferentially. There was no pseudomeningoceal.
3. Discussion
Dorsal dura defects (DDD) can often be assessed and approached directly without further manipulation of intrathecal neural structures, therefore a primary repair for linear defect and an augmented duraplasty using various autograft or allograft dural substitutes are suitable (Figure 5). In a series of 51 patients with spinal stab injury, 78% of whom underwent dorsal dura primary repair with only 2 patients (4%) developed CSF leakage related to wound infection [5]. While dorsal approach provides access to dorsal and lateral dura sac, it does not provide adequate access ventrally unless retracting or mobilizing of the neural structures which is often dangerous especially in cervical and thoracic level [8]. To prevent secondary injury to the spinal cord, two techniques of indirect repair have been reported in literature of ISCH, which are duraplasty and widening of dural defect, respectively [9]. A comparison made by Saito et al showed more patients who underwent primary ventral dura closure had worsened neurological outcome compared to those underwent duraplasty or widening of the defect, suggesting an indirect repair is recommended than direct one in VDD [10]. A study of duraplasty after acute cervical laceration spinal cord injury in a rat model concluded that duraplasty was able to improve CSF flow by limiting meningeal fibrosis, reduce connective tissue formation, attenuate macrophage accumulation and progressive secondary injury, further supporting the use of duraplasty over widening of dural defect regarding choosing between indirect repair techniques [11]. An indirect duraplasty can further be classified into patch attachment and sling procedure [12, 13]. Patch attachment requires manipulation of the neural structures for direct visualization of the defect edge to apply stitches or fibrin glue [12]. Meanwhile in sling procedure, dura graft is glided into the ventral space and fixed to bilateral dura wall without retraction of spinal cord [14].
Based on above evidences, for repair of NMPSI with ventral dura defect, the risk of spinal cord mobilization should be of primary concern for choosing appropriate technique (Figure 5). One thing makes the patients with NMPSI comparable to patients with ISCH is that a blunt injury with dural tissue loss in patients with NMPSI mimicking a spontaneous dura defect in ISCH. With sling procedure, even a larger defect can be repaired indirectly simply by dividing dentate ligament and designing sleeves for segmental roots to pass through. The use of sling procedure in NMPSI has never been reported to our knowledge, and a good outcome in our case demonstrates that sling procedure should be considered in managing patients with ventral dura defect. For the choice of dural substitute, a great variety of grafts including autograft (ex: muscle fascia, fat), allograft (ex: cadaveric dural graft), xenograft (ex: bovine pericardium) and synthetic graft (ex: ePTFE dural substitute, Teflon, Gore-Tex) [12, 15]. Autograft was the first applied graft owning to its easy availability, but was limited by insufficient soft tissue and additional incisions [16, 17]. Allograft and xenograft are more flexible choices regarding to the customized sizes, but they still obtain the concern of immune reactivity and transmissible disease which includes the risk of prion disease [17, 18]. To avoid spinal cord damage during the insertion of dural graft, the material should be as thin and soft as possible, which is achievable in synthetic graft that can be less than 1mm. Synthetic graft is also associated with lower rates of wound infections, adhesions, CSF leaks and reoperation comparing to autograft in literature [19]. In the era of biodesign, various innovative grafts aiming biocompatibility, stronger sealing and better manipulation have been presented. The COOK® dural graft we used is an example of using decellularized extracellular matrix to prevent immune response while still providing natural scaffold for cell growth [20]. Additional sealant to the duraplasty edge may be beneficial to preventing further CSF leakage.
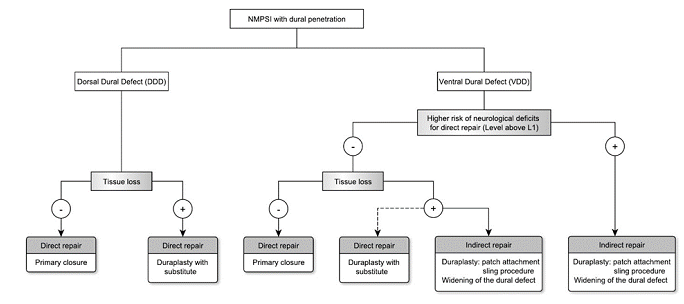
Figure 5: Dural defect in NMPSI can be categorized into VDD(ventral dural defect) and DDD(dorsal dural defect). In VDD with higher risk of neurological deficits or tissue loss, indirect repair is recommended to minimize secondary injury. In DDD and VDD without tissue loss, direct repair is indicated. Direct repair includes primary closure and duraplasty with substitute; indirect repair includes sling procedure, patch attachment and widening of dural defect.
4. Conclusions
In conclusion, managing patients of NMPSI with dura penetration, safe repair of dural defect is mandatory to prevent delayed neural injury. The location of defect (DDD vs. VDD), level of injury, and whether tissue loss or not, are practical determinants for choosing an appropriate method of dura repair. An indirect repair with sling technique is an easily adapted and effective surgical technique for traumatic VDD.
References
- Wallace DJ, Sy C, Peitz G, et al. Management of non-missile penetrating spinal injury. Neurosurg Rev 42 (2019): 791-798.
- McCunniff PT, Ramey JS, Scott ML, et al. Operative Versus Nonoperative Management of Civilian Gunshot Wounds to the Spinal Cord: Novel Use of the Functional Independence Measure for Validated Outcomes. World Neurosurg 106 (2017): 240-246.
- Klimo P, Ragel BT, Rosner M, et al. Can surgery improve neurological function in penetrating spinal injury? A review of the military and civilian literature and treatment recommendations for military neurosurgeons. Neurosurg Focus 28 (2010): E4.
- Simsek O, Kilincer C, Sunar H, et al. Surgical management of combined stab injury of the spinal cord and the aorta--case report. Neurol Med Chir (Tokyo) 44 (2004): 263-265.
- Enicker B, Gonya S, Hardcastle TC. Spinal stab injury with retained knife blades: 51 Consecutive patients managed at a regional referral unit. Injury 46 (2015): 1726-1733.
- Eltoukhy M, Gkolemis C. Late-Onset Post-Traumatic Spinal Cord Herniation as a Rare and Overlooked Cause of Late Neurologic Deterioration After Penetrating Injury to the Thoracic Spine: A Case Report and Review of the Literature. World Neurosurg 142 (2020): 408-412.
- Peacock WJ, Shrosbree RD, Key AG. A review of 450 stabwounds of the spinal cord. S Afr Med J 51 (1977): 961-964.
- Sasani M, Ozer AF, Vural M, et al. Idiopathic spinal cord herniation: case report and review of the literature. J Spinal Cord Med 32 (2009): 86-94.
- Randhawa PS, Roark C, Case D, et al. Idiopathic Spinal Cord Herniation Associated With a Thoracic Disc Herniation: Case Report, Surgical Video, and Literature Review. Clin Spine Surg 33 (2020): 222-229.
- Saito T, Anamizu Y, Nakamura K, et al. Case of idiopathic thoracic spinal cord herniation with a chronic history: a case report and review of the literature. J Orthop Sci 9 (2004): 94-98.
- Iannotti C, Zhang YP, Shields LB, et al. Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J Neurotrauma 23 (2006): 853-865.
- Akutsu H, Takada T, Nakai K, et al. Surgical technique for idiopathic spinal cord herniation: the Hammock method. Technical note. Neurol Med Chir (Tokyo) 52 (2012): 238-242.
- Herring EZ, Shin JH, Nagel SJ, et al. Novel Strategy of Ventral Dural Repair for Idiopathic Thoracic Spinal Cord Herniation: Report of Outcomes and Review of Techniques. Oper Neurosurg (Hagerstown) 17 (2019): 21-31.
- Batzdorf U, Holly LT. Idiopathic thoracic spinal cord herniation: report of 10 patients and description of surgical approach. J Spinal Disord Tech 25 (2012): 157-162.
- Payer M, Zumsteg D, De Tribolet N, et al. Surgical management of thoracic idiopathic spinal cord herniation. Technical case report and review. Acta Neurochir (Wien) 158 (2016): 1579-1582.
- Bi X, Liu B, Mao Z, et al. Applications of materials for dural reconstruction in pre-clinical and clinical studies: Advantages and drawbacks, efficacy, and selections. Materials Science and Engineering: C (2020).
- Warren WL, Medary MB, Dureza CD, et al. Dural repair using acellular human dermis: experience with 200 cases: technique assessment. Neurosurgery 46 (2000): 1391-1396.
- Alleyne CH, Barrow DL. Immune response in hosts with cadaveric dural grafts. J Neurosurg 81 (1994): 610.
- Abla AA, Link T, Fusco D, et al. Comparison of dural grafts in Chiari decompression surgery: Review of the literature. J Craniovertebr Junction Spine 1 (2010): 29-37.
- Hodde J, Janis A, Ernst D, et al. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med 18 (2007): 537-543.
