Macrocephaly in Children: What to Consider Beyond Ventriculomegaly?
Article Information
Julliet C. Ogu, Laura M. Huisman, Thierry A.G.M. Huisman
Edward B. Singleton Department of Radiology, Texas Children’s Hospital and Baylor College of Medicine, Houston, Texas, USA
*Corresponding Author: Julliet C Ogu. Edward B. Singleton Department of Radiology, Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas, USA
Received: 29 November 2023; Accepted: 07 December 2023; Published: 15 December 2023
Citation: Julliet C Ogu, Laura M. Huisman. Thierry A.G.M. Huisman, Macrocephaly in Children: What to Consider Beyond Ventriculomegaly?. Journal of Radiology and Clinical Imaging 6 (2023): 187-196.
View / Download Pdf Share at FacebookAbstract
Pediatric macrocephaly may result from many benign, transient, acute or chronic entities. Macrocephaly can be secondary to ventriculomegaly, widening of the cerebrospinal fluid filled spaces overlying the brain as well as multiple intrinsic brain pathologies. Early and correct identification of the underlying etiology is essential for patient management and prognosis. The principal goal of this manuscript is to review and discuss the various etiologies of macrocephaly including matching neuroimaging findings beyond the classical well recognized macrocephaly secondary to ventriculomegaly. In addition, the delicate interaction between the ventricular system, the intra- and extracranial arterio-venous system as well as the glymphatic system are briefly reviewed.
Keywords
Macrocephaly; Pediatrics; Neurology; Radiology; Hydrocephalus
Macrocephaly articles; Pediatrics articles; Neurology articles; Radiology articles; Hydrocephalus articles
Macrocephaly articles Macrocephaly Research articles Macrocephaly review articles Macrocephaly PubMed articles Macrocephaly PubMed Central articles Macrocephaly 2023 articles Macrocephaly 2024 articles Macrocephaly Scopus articles Macrocephaly impact factor journals Macrocephaly Scopus journals Macrocephaly PubMed journals Macrocephaly medical journals Macrocephaly free journals Macrocephaly best journals Macrocephaly top journals Macrocephaly free medical journals Macrocephaly famous journals Macrocephaly Google Scholar indexed journals Pediatrics articles Pediatrics Research articles Pediatrics review articles Pediatrics PubMed articles Pediatrics PubMed Central articles Pediatrics 2023 articles Pediatrics 2024 articles Pediatrics Scopus articles Pediatrics impact factor journals Pediatrics Scopus journals Pediatrics PubMed journals Pediatrics medical journals Pediatrics free journals Pediatrics best journals Pediatrics top journals Pediatrics free medical journals Pediatrics famous journals Pediatrics Google Scholar indexed journals Neurology articles Neurology Research articles Neurology review articles Neurology PubMed articles Neurology PubMed Central articles Neurology 2023 articles Neurology 2024 articles Neurology Scopus articles Neurology impact factor journals Neurology Scopus journals Neurology PubMed journals Neurology medical journals Neurology free journals Neurology best journals Neurology top journals Neurology free medical journals Neurology famous journals Neurology Google Scholar indexed journals Radiology articles Radiology Research articles Radiology review articles Radiology PubMed articles Radiology PubMed Central articles Radiology 2023 articles Radiology 2024 articles Radiology Scopus articles Radiology impact factor journals Radiology Scopus journals Radiology PubMed journals Radiology medical journals Radiology free journals Radiology best journals Radiology top journals Radiology free medical journals Radiology famous journals Radiology Google Scholar indexed journals Hydrocephalus articles Hydrocephalus Research articles Hydrocephalus review articles Hydrocephalus PubMed articles Hydrocephalus PubMed Central articles Hydrocephalus 2023 articles Hydrocephalus 2024 articles Hydrocephalus Scopus articles Hydrocephalus impact factor journals Hydrocephalus Scopus journals Hydrocephalus PubMed journals Hydrocephalus medical journals Hydrocephalus free journals Hydrocephalus best journals Hydrocephalus top journals Hydrocephalus free medical journals Hydrocephalus famous journals Hydrocephalus Google Scholar indexed journals cerebrospinal fluid articles cerebrospinal fluid Research articles cerebrospinal fluid review articles cerebrospinal fluid PubMed articles cerebrospinal fluid PubMed Central articles cerebrospinal fluid 2023 articles cerebrospinal fluid 2024 articles cerebrospinal fluid Scopus articles cerebrospinal fluid impact factor journals cerebrospinal fluid Scopus journals cerebrospinal fluid PubMed journals cerebrospinal fluid medical journals cerebrospinal fluid free journals cerebrospinal fluid best journals cerebrospinal fluid top journals cerebrospinal fluid free medical journals cerebrospinal fluid famous journals cerebrospinal fluid Google Scholar indexed journals neuroimaging articles neuroimaging Research articles neuroimaging review articles neuroimaging PubMed articles neuroimaging PubMed Central articles neuroimaging 2023 articles neuroimaging 2024 articles neuroimaging Scopus articles neuroimaging impact factor journals neuroimaging Scopus journals neuroimaging PubMed journals neuroimaging medical journals neuroimaging free journals neuroimaging best journals neuroimaging top journals neuroimaging free medical journals neuroimaging famous journals neuroimaging Google Scholar indexed journals presymptomatic articles presymptomatic Research articles presymptomatic review articles presymptomatic PubMed articles presymptomatic PubMed Central articles presymptomatic 2023 articles presymptomatic 2024 articles presymptomatic Scopus articles presymptomatic impact factor journals presymptomatic Scopus journals presymptomatic PubMed journals presymptomatic medical journals presymptomatic free journals presymptomatic best journals presymptomatic top journals presymptomatic free medical journals presymptomatic famous journals presymptomatic Google Scholar indexed journals Brain tumors articles Brain tumors Research articles Brain tumors review articles Brain tumors PubMed articles Brain tumors PubMed Central articles Brain tumors 2023 articles Brain tumors 2024 articles Brain tumors Scopus articles Brain tumors impact factor journals Brain tumors Scopus journals Brain tumors PubMed journals Brain tumors medical journals Brain tumors free journals Brain tumors best journals Brain tumors top journals Brain tumors free medical journals Brain tumors famous journals Brain tumors Google Scholar indexed journals Craniosynostosis articles Craniosynostosis Research articles Craniosynostosis review articles Craniosynostosis PubMed articles Craniosynostosis PubMed Central articles Craniosynostosis 2023 articles Craniosynostosis 2024 articles Craniosynostosis Scopus articles Craniosynostosis impact factor journals Craniosynostosis Scopus journals Craniosynostosis PubMed journals Craniosynostosis medical journals Craniosynostosis free journals Craniosynostosis best journals Craniosynostosis top journals Craniosynostosis free medical journals Craniosynostosis famous journals Craniosynostosis Google Scholar indexed journals
Article Details
1. Introduction
The routine monitoring of head growth in infancy is a fundamental component of pediatric care, as abnormal head growth can serve as a potential indicator of underlying health concerns. Macrocephaly denotes the presence of an abnormally large cranium. It is clinically defined as an occipitofrontal circumference surpassing either 2 standard deviations above the average for age and weight-matched individuals or exceeding the 97th percentile by 0.5 cm [1-4]. Macrocephaly is a common indication for neuroimaging among pediatric patients and affects up to 5% of this population [3], [5]. In infants, macrocephaly can present at birth or as increasing head circumference within the first year of life. During the early months of life, from birth to around 6 months, head circumference can increase by as much as 2 cm per month [5], [6]. This period of rapid growth is crucial for brain development and corresponds to a time when neural connections are rapidly forming. Additionally, the major cranial sutures are still open, allowing for significant expansion of the cranium with any increase in the volume of intracranial contents. The differential diagnosis for macrocephaly is very broad. It encompasses benign causes as well as life threatening genetic syndromes and acquired pathologies requiring prompt intervention. A thorough evaluation, including clinical assessment and appropriate neuro-imaging studies, is essential to accurately diagnose macrocephaly in infants. This paper aims to discuss the key diagnostic considerations, imaging findings, and differential diagnoses for infants presenting with macrocephaly. Radiologists and clinicians should be well versed in the diagnostic features of this prevalent symptom in infants.
2. Imaging Evaluation
Neuroimaging is a powerful tool used to differentiate benign from pathologic etiologies of macrocephaly and guide appropriate treatment. Currently, there are no established guidelines from the American Academy of Pediatrics or the American College of Radiology concerning imaging protocols for macrocephaly in infants [5]–[7]. When deciding whether to conduct imaging on these patients, the potential risks associated with sedation and exposure to ionizing radiation must be carefully weighed, as well as resource availability. In their retrospective review study, Sampson et al concluded that asymptomatic patients with no apparent pathological risk factors may forgo unnecessary imaging [8]. Thomas et al, on the other hand, argued for the use of ultrasound in asymptomatic patients in order to more conclusively rule out life threatening pathologies that may be in a “presymptomatic” stage [7]. Nevertheless, it is advisable to obtain imaging in instances where risk factors are evident. These risk factors include developmental delay, focal neurological symptoms, unexplained irritability, alterations in feeding patterns, or concerns of abusive head trauma. Additionally, complementary neuroimaging should be considered for patients presenting with facial dysmorphism or exhibiting cutaneous or vascular hallmarks, as these may be signs of an underlying genetic pathology [5-8]. Head ultrasound (US) is commonly employed for the initial assessment of cranial abnormalities in infants. US is cost-effective, does not require sedation, and does not expose pediatric patients to potentially harmful radiation. Despite these benefits, US is very user-dependent and provides less structural detail and visualization compared to CT and MRI. However, infants with open fontanels are an ideal population for evaluation using US. The open fontanelles serve as acoustic windows of the cranial vault allowing direct visualization of the underlying brain structures.[4], [5], [7], [9] [10] For patients needing further evaluation, computed tomography (CT) or magnetic resonance imaging (MRI) can be obtained next. MRI is the most diagnostically accurate modality and provides high degree of contrast resolution as well as multiple qualities of information, both anatomical as well as functional. However, MRI is expensive, time consuming, and often requires the use of sedation in infants. Head CT is typically avoided in this patient population due to radiation exposure. However, CT can be advantageous in acute settings and for the assessment of skull abnormalities.
3. Differential Diagnosis of Macrocephaly
Macrocephaly is a nonspecific clinical finding that encompasses a broad range of etiologies. It is sometimes seen in families without any distinct cause. Most frequently, macrocephaly is seen secondary to ventriculomegaly either by a) congenital or acquired intrinsic or extrinsic obstruction of the ventricular system (e.g. congenital aqueductal stenosis or secondary to intraventricular hemorrhage versus mass lesions adjacent to the ventricular system), b) impaired cerebrospinal fluid (CSF) resorption (e.g. obstruction of the Pacchionian granulations after an intracranial hemorrhage/infection or due to an increased central venous pressure in congenital heart disease or arteriovenous malformations), c) overproduction of the cerebrospinal fluid (e.g. plexus papilloma), and d) as part of malformations of the central nervous system (e.g. Chiari II malformation, Dandy Walker malformation). The remaining less frequent causes of macrocephaly in infants can be broadly classified based on their location and the specific brain components or surrounding tissues they impact. Intra-axial factors contributing to macrocephaly arise from within the brain parenchyma, while extra-axial factors involve structures external to the brain tissue, such as the meninges, blood vessels, skull, and cerebrospinal fluid. Certain genetic syndromes including tuberous sclerosis, neurofibromatosis, Canavan’s syndrome, and others, can also present with macrocephaly.
|
Cause |
Description |
|
Ventriculomegaly |
|
|
- Congenital aqueduct stenosis |
Narrowing or blockage of the cerebral aqueduct present at birth. |
|
- Acquired aqueduct stenosis |
Blockage of the cerebral aqueduct due to conditions like infection or tumor growth. |
|
- Brain tumors |
Tumors in the brain can obstruct the flow of cerebrospinal fluid (CSF). |
|
- Impaired CSF outflow |
Conditions that hinder the normal drainage of CSF, such as Chiari malformation. |
|
- CSF overproduction |
Increased production of CSF, often seen in conditions like choroid plexus papilloma. |
|
- Malformation syndromes |
Genetic syndromes that include hydrocephalus as a feature, like Dandy-Walker syndrome. |
|
Extra-axial Etiologies |
|
|
- Benign enlargement of subarachnoid spaces |
An enlargement of the spaces between the brain and the skull. |
|
- Subdural hemorrhage |
Accumulation of blood between the brain's surface and the dura mater. |
|
Skull Abnormalities |
|
|
- Achondroplasia |
A genetic disorder causing abnormal bone growth, including skull bones. |
|
- Craniosynostosis |
Premature fusion of the cranial sutures, affecting skull development. |
|
- Craniometaphyseal dysplasia |
A rare genetic disorder characterized by cranial bone thickening. |
|
Intra-axial Etiologies |
|
|
- Megalencephaly |
Abnormally large brain size often due to genetic or developmental factors. |
4. Macrocephaly secondary to ventriculomegaly
4.1 Congenital or acquired aqueductal stenosis
Congenital aqueductal stenosis is characterized by a focal stenosis of the Sylvian aqueduct connecting the third with the fourth ventricle. The exact etiology of an isolated, intrinsic congenital aqueductal stenosis remains unknown. A genetic etiology has been proposed also known as X-linked hydrocephalus with stenosis of the Sylvian aqueduct (HSAS) [5]. On sagittal imaging the Sylvian aqueduct appears dilated proximal to the stenosis, the third and lateral ventricle are moderately to severely widened while the fourth ventricle is of normal size (Figure 1).
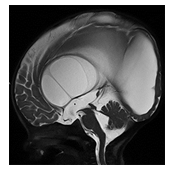
Figure 1: Post-op Sagittal T2-weighted MR image of an infant with high grade aqueductal stenosis with resultant high grade supratentorial ventriculomegaly with thinning of the corpus callosum. A T2-hypointense web/adhesion is noted within the lower third of the Sylvian aqueduct outlined by T2-hyperintense CSF superior and inferior to the stenosis. A T2-hypointense CSF flow related signal void is noted across and superior to the therapeutic, third ventriculostomy.
The corpus callosum may be thinned secondary to high grade hydrocephalus. The overlying hemispheric white and gray matter may be thinned. Congenital aqueductal stenosis should be suspected on prenatal ultrasound or MRI if the supratentorial ventricles are disproportionally widened relative to a normal appearing fourth ventricle.
Acquired aqueductal stenosis (Figure 2) is typically seen as a complication of an intraventricular hemorrhage or infection with resultant webs typically occurring in the region of the Sylvian aqueduct [5]. The corpus callosum may be thinned secondary to high grade hydrocephalus. The overlying hemispheric white and gray matter may be thinned. Congenital aqueductal stenosis should be suspected on prenatal ultrasound or MRI if the supratentorial ventricles are disproportionally widened relative to a normal appearing fourth ventricle. Acquired aqueductal stenosis (Figure 2) is typically seen as a complication of an intraventricular hemorrhage or infection with resultant webs typically occurring in the region of the Sylvian aqueduct [5].
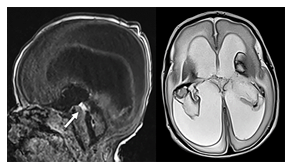
Figure 2: Sagittal T1 and axial T2-weighted MR image of a neonate with a bilateral germinal matrix and intraventricular hemorrhage. T2-hypointense blood products are seen covering the choroid plexus bilaterally as well as T2-hypointense lining of the ventricles. In addition, T1-hyperintense blood is noted within the Sylvian aqueduct (arrow) obstructing the aqueduct with resultant high grade supratentorial ventriculomegaly
Intraventricular blood products in neonates are often secondary to germinal matrix hemorrhages with intraventricular extension. On MRI webs may be seen on T2-weighted imaging as a T2-hypointense structure outlined by T2-hyperintense CSF. Occasionally, the blood products can be directly visualized as T1-hyperintense and T2-hypointense focal signal alterations.
4.2 Brain tumors
Posterior fossa tumors that obstruct CSF outflow may cause hydrocephalus which can manifest with macrocephaly in infants. Tumors in pediatric patients in the posterior fossa can obstruct the cerebral aqueduct and/or 4th ventricle. Medulloblastoma and pilocytic astrocytoma are the most prevalent pediatric posterior fossa tumors (Figure 3).
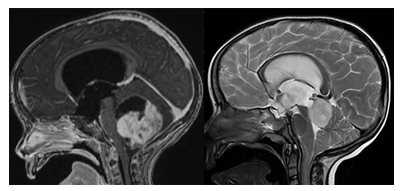
Figure 3: Sagittal contrast enhanced T1-weighted and T2-weighted MR images of two children with posterior fossa tumors. The first child has a large partially cystic, partially solid and strongly contrast enhancing pilocytic astrocytoma located within the cerebellar hemisphere compressing the fourth ventricle. The second child shows a well circumscribed T2-hyperintense mass lesion centered within the tectal plate compatible with a low grade tectal glioma. This focal lesion is compressing/narrowing the adjacent Sylvian aqueduct. Both tumors obstruct the outflow of the third ventricle with resultant high grade supratentorial ventriculomegaly involving the third and lateral ventricles.
Medulloblastoma frequently presents as a hypercellular mass characterized by diminished diffusion and variable enhancement [4], [11], [12]. Pilocytic astrocytomas present as large cystic masses with an enhancing nodular component. Ependymomas also typically arise within the fourth ventricle and can extend into the foramina of Luschka and Magendie. These tumors are avidly enhancing and are more often associated with calcifications and hemorrhages compared to medulloblastomas [4], [11], [12]. Tumors in the tectal plate (Figure 3) or pineal region can cause marked supratentorial hydrocephalus via obstruction of the cerebral aqueduct. Tectal plate neoplasms are typically low-grade gliomas that expand the tectal plate with resultant narrowing of the Sylvian aqueduct. Pineal germinomas are the most common type of tumor in the pineal gland region. They are characterized on imaging as heterogeneous, avidly enhancing masses that are hyperattenuating on CT and show restricted diffusion and T2 hypointensity on MRI. Pineal teratomas may present as fetal intracranial neoplasms and can cause congenital hydrocephalus. Neuroimaging classically reveals a heterogenous mass with fat, calcifications, and cystic components. Lastly, pineoblastomas are malignant tumors typically manifesting in pediatric patients as large, necrotic, hypercellular masses with peripheral calcifications. These tumors can be differentiated from pineal germinomas by their larger degree of diffusion restriction and markedly low apparent diffusion coefficient (ADC) values [4], [5], [11]
4.3 Impaired cerebrospinal fluid (CSF) resorption
For many decades, the dynamics of cerebrospinal fluid (CSF) have been regarded as a delicate equilibrium between its production and resorption, commonly referred to as bulk CSF flow. Furthermore, until recently, it was widely believed that the primary route for CSF resorption occurred at the Pacchionian granulations. This presumption has received indirect validation through observations in various medical conditions, such as those resulting from subarachnoid hemorrhaging or infectious exudates in cases of meningitis (as illustrated in Figure 4). Several pathophysiological factors contribute to the impaired functionality of the Pacchionian granulations, including subarachnoid fibrosis, ciliary dysfunction, adhesions, and mechanical obstruction by blood clots [13], [14],[15]. However multiple studies have shown that the process of CSF resorption is much more complex than commonly assumed. It is intricately linked to both intracranial and extracranial arterio-venous hemodynamics as well as the complex interaction between CSF dynamics and propagated arterial pulsations at the outlets of the cranial vault, particularly at the level of the foramen of magnum. This was recognized as early as 1997 by the Greitz brothers [16]. Multiple articles and study groups followed discussing the hydrodynamic hypotheses versus the bulk flow hypothesis [17]. The importance of arteriovenous dynamics becomes evident when we acknowledge that CSF resorption can be restricted in situations of elevated intracranial venous pressure, such as in cases of congenital heart disease or as a result of arteriovenous malformations such as vein of Galen aneurysmal malformation (VGAM).
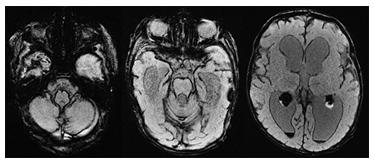
Figure 4: Axial susceptibility weighted (SWI) MR images of a neonate with extensive superficial SWI-hypointense hemosiderosis after an intraventricular hemorrhage. The hemosiderosis obstructs the CSF resorption with resultant ventriculomegaly.
VGAM (Figure 5) are rare arteriovenous malformations characterized by one or more arteries of the Circle of Willis draining directly into the deep venous system, causing subsequent “aneurysmal” dilation of the vein of Galen [11].
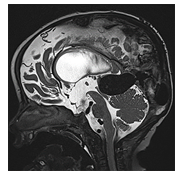
Figure 5: Sagittal T2-weighted MR image of an infant with a large vein of Galen aneurysmal malformation. The dilated vein of Galen compresses the tectal plate with narrowing of the Sylvian aqueduct with resultant supratentorial ventriculomegaly.
Ventriculomegaly in VGAM is a complex condition arising from a combination of factors. One of these factors involves arteriovenous shunting, which can lead to ongoing damage to the developing brain. This damage occurs due to chronic venous hypertension, parenchymal venous stasis, and arterial steal phenomena. This cumulative damage has been colloquially referred to as "melting brain" with e vacuo dilatation of the ventricular system due to global volume loss." Additionally, ventriculomegaly may develop as a result of impaired cerebrospinal fluid (CSF) resorption. Elevated venous pressure and compression of the Sylvian aqueduct by the dilated Vein of Galen can hinder CSF resorption. Moreover, the systemic consequences of intracranial arteriovenous shunting can contribute to progressive heart failure, affecting both cerebral perfusion and venous drainage. A VGAM typically manifests between weeks 6-11 of gestation. It can be detected during routine prenatal ultrasound screenings either by directly identifying the dilated vein or by observing systemic hemodynamic effects such as polyhydramnios, cardiomegaly, fetal ascites, pleural effusion, or fetal hydrops. Fetal and postnatal MRI imaging confirm the ultrasound findings, revealing a T1/T2-hypointense aneurysmal dilation of the vein of Galen, ventriculomegaly, and chronic injury and volume loss in the hemispheric white and gray matter. Additional fetal MRI observations may include a swollen umbilical cord, dilated umbilical vessels, an enlarged heart, and T2-hyperintense fetal pericardial and pleural effusion. The overall prognosis hinges on the hemodynamic significance of the arteriovenous shunting and its subsequent impact on both the brain and cardiovascular system. When left untreated, the condition leads to high morbidity and mortality rates in affected children, who often present with symptoms such as congestive heart failure, respiratory distress, cyanosis, macrocephaly, prominent scalp veins, and an audible bruit over the scalp [11-12], [41]. More recently, it was discovered that interstitial fluid within the brain and CSF may also interact with the lymphatic system of the brain known as glymphatic system [18]. It was shown that the CSF partially drains via the cribriform plate and nasal mucosa to the cervical lymph nodes in rats and sheep and to a lesser extent in humans [19]. Multiple subsequent studies have confirmed and discussed the existence of such a glymphatic system [20], [21]. Recently a manuscript reviewed the interaction between the cerebral venous and lymphatic system, describing the interplay between the brain parenchyma, arteries, veins, CSF and glymphatic system [22]. The exact significance and impact of the glymphatic system on the development of ventriculomegaly has not yet been proven. A discussion of this research would however go beyond the scope of this manuscript.
4.4 Overproduction of CSF
Choroid plexus papillomas are benign, slow growing tumors that arise from the choroid plexus within the ventricles and produce CSF (Figure 6).
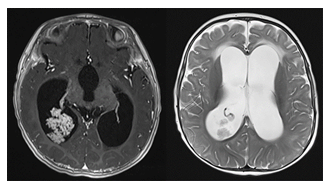
Figure 6: Axial contrast-enhanced T1- and axial T2-weighted MR images of an infant with a confirmed choroid plexus papilloma within the atrium of the right occipital horn. The overproduction of CSF results in a high grade ventriculomegaly.
In pediatric patients, these tumors are typically located in the lateral ventricles. The overproduction of CSF by these tumors as well as the potential for ventricular obstruction may lead to macrocephaly in infants with open sutures. Imaging will typically reveal solid, avidly enhancing intraventricular masses, sometimes with speckled calcifications. If the mass is fast growing with heterogeneous signal intensity, central necrosis and hemorrhagic components, choroid plexus carcinoma should be suspected. Choroid plexus carcinoma is a very rare type of brain cancer with high recurrence rates [4], [23].
4.5 Malformations
Ventriculomegaly is seen in a wide range of brain malformations (e.g., Chiari 2, Dandy Walker, Rhombencephalosynapsis, Joubert, Aicardi syndrome). Imaging is essential to identify the exact kind and degree of malformation. It would go beyond the scope of this manuscript to describe all malformations that may present with ventriculomegaly and subsequent macrocephaly, but one should be aware that ventriculomegaly/macrocephaly may be just the tip of the iceberg of the malformation.
5. Extra-axial Etiologies
Benign enlargement of subarachnoid spaces (BESS), also known as benign external hydrocephalus, is a common cause of pediatric macrocephaly (Figures 7,8). These patients typically present with a rapidly increasing head circumference in the first few months of life. The growth gradually tapers off over time, leading to the eventual normalization of head circumference around the ages of 2 to 3 years [1], [4], [6], [23-25].
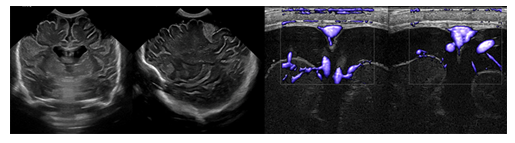
Figure 7: Coronal and sagittal ultrasound and color-coded Doppler sonography show the classical findings of enlarged subarachnoid spaces with draining superficial veins crossing the widened subarachnoid spaces overlying the cerebral hemispheres.
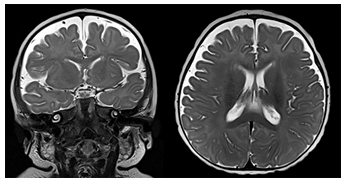
Figure 8: Coronal and axial T2-weighted MR images of an infant with BESS. The subarachnoid spaces overlying both cerebral hemispheres are widened with veins crossing the widened space.
BESS is thought to be caused by the delayed maturation of the arachnoid villi. Most patients remain asymptomatic and go on to develop normally. Imaging will typically reveal enlargement of the fronto-parietal subarachnoid spaces without associated mass effect. Some mild lateral ventriculomegaly may also be observed. Bridging and superficial veins may be seen traversing the enlarged subarachnoid spaces, appearing as flow voids on T2 weighted MR images. This is called the cortical vein sign and can be used to distinguish BESS from subdural fluid collections [24], [25]. BESS is associated with an increased risk for subdural hematoma, as the bridging veins crossing through the enlarged subarachnoid spaces are prone to mechanical trauma and bleeding. Because BESS is self-limiting, treatment is typically conservative. A subdural hemorrhage (SDH) is a form of extra-axial intracranial bleeding that takes place between the dura mater and the arachnoid mater enveloping the brain [13], [16], [17]. SDH arises from the bridging veins which are delicate vessels prone to mechanical shearing and rupture. Subdural hemorrhages are frequently linked with head trauma involving rapid deceleration or rotational force. Infants are at a higher risk of SDH due to their large head to body ratio and weak neck muscles that are less able to support their heads. In instances of abusive shaking, the violent back-and-forth motion can cause the bridging veins to rupture in the subdural space, leading to bleeding and potentially severe brain damage. The presence of subdural hemorrhage, retinal hemorrhages, and neurological symptoms is a hallmark of shaken baby syndrome (Figure 9). In infants with open sutures and fontanelles, the accumulating blood can cause a sudden increase in cranial size. SDH presents on imaging as an area of crescentic hyperdensity commonly found along the cortical convexity [23, [24], [26] Neurosurgical intervention is frequently necessary to mitigate the risk of lifelong disability and potential fatality.
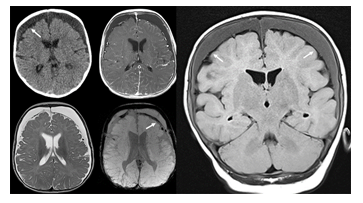
Figure 9:Axial CT and contrast enhanced T1-weighted (upper row), axial T2- and SWI-weighted (lower row), coronal fluid attenuated inversion recovery MR images of an infant who was exposed to non-accidental injury. Large bilateral subdural chronic hematomas are seen overlying both cerebral hemispheres. The subdural effusions appear mildly hyperdense on CT and FLAIR-hyperintense compared to the mildly compressed subarachnoid space (arrows). On T2- and SWI-weighted MR images subarachnoid hypointense blood products are noted. The subdural effusions are mildly hyperdense/FLAIR-hyperintense due to the increased protein content which is typical for chronic subdural hematomas. No vessels appear to cross the subdural effusions assisting to differentiate this entity from BESS.
6. Skull Abnormalities
Achondroplasia (Figure 10) is the most common cause of primary skeletal dysplasia in humans and is caused by an autosomal dominant mutation in the fibroblast growth factor receptor 3 (FGFR3) gene [26]. In newborns affected by this condition, there is often a notable presence of macrocephaly that tends to progress throughout infancy. Among the distinctive characteristics are rhizomelic shortening of the limbs, frontal bossing, midface retrusion, and prominent genu varum. Foramen magnum stenosis and jugular foramen hypoplasia are also prevalent in these patients. These conditions can lead to significant hydrocephalus and intracranial venous hypertension which may contribute to the development of macrocephaly [23], [28], [29].
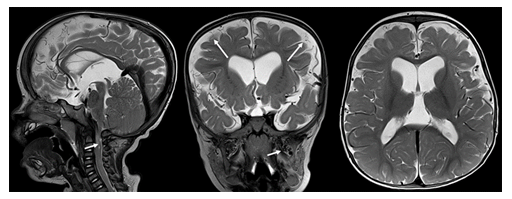
Figure 10: Sagittal, coronal and axial T2-weighted MR images in a 6-year-old child with achondroplasia. Moderate ventriculomegaly, high-grade narrowing of the foramen magnum with spinal cord compression (short arrow), frontal bossing, prominent superficial veins (long arrows) and mildly dilated pterygoid plexus (short arrow on coronal image) are noted.
Craniosynostosis (Figure 11) refers to the premature closure of cranial sutures, resulting in craniofacial deformities. Craniosynostosis can be isolated without any additional anomalies or syndromic, often caused by a genetic mutation. Neuroimaging will depict prematurely fused sutures with associated brain and skull abnormalities. Progressive hydrocephalus and intracranial hypertension can also occur, most commonly as a result of hypoplastic posterior fossa and venous outlet obstruction.
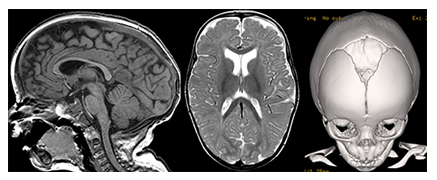
Figure 11: Sagittal T1- and axial T2-weighetd MR images and a 3D surface shaded CT reconstruction of the skull show a mild macrocephaly with elongated skull shape due to a premature closure of the sagittal suture while the coronal and metopic sutures are open compatible with sagittal suture craniosynostosis.
Craniometaphyseal dysplasia (CMD) is a rare genetic disease (Figure 12) characterized by progressive thickening of the craniofacial bones and abnormal development of the metaphyses in long bones. CMD is caused by pathogenic variants of the ANKH gene, which is involved in the regulation of matrix bone formation. Craniofacial abnormalities commonly associated with this condition include frontonasal bossing, hypertelorism, an enlarged skull, a broad nasal root, and dental abnormalities. Additionally, hyperostosis of the skull base can lead to cranial nerve compression. Characteristic imaging findings include diffuse cortical thickening, marked sclerosis, and diploic space expansion of the craniofacial bones [23], [30]. As a result of diffuse hyperostosis of the skull base, neurological symptoms associated with cranial nerve compression such as reduced vision, cranial nerve palsy, and deafness can occur.
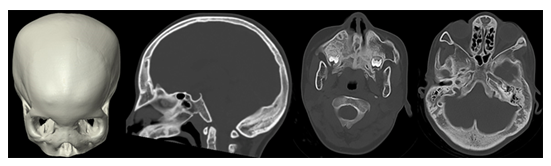
Figure 12: 3D surface shaded CT reconstruction and sagittal and axial CT images of an infant with a Craniometaphyseal dysplasia with a widening of the diploic space, hyperostosis of the skull base and a wide nasal root.
7. Intra-axial Etiologies
7.1 Megalencephaly
Atypical enlargement of brain tissues characterizes megalencephaly. In affected individuals, the size and weight of the brain are at least 2 standard deviations beyond the age and gender-matched norms [23], [30]–[32]. Megalencephaly is most commonly a result of various genetic syndromes and is classically divided into two subgroups: anatomic/developmental and metabolic. Anatomic megalencephaly is characterized by an increase in brain parenchymal cells caused by disruptions of essential signaling pathways that regulate brain cellular proliferation. Metabolic megalencephaly, however, results from the pathological accumulation of metabolic substances within the brain [31], [32].
7.2 Anatomic Megalencephaly
Most genetic disorders associated with anatomic megalencephaly involve mutations in the rapamycin (mTOR) and RAS-related/mitogen-activated protein kinase 1 (MAPK1) pathways. Among these are neurocutaneous disorders, also known as phakomatoses, which include neurofibromatosis and tuberous sclerosis. Neurofibromatosis type 1 (NF1) is the most common phakomatosis and is caused by mutations in the NF1 gene which encodes the tumor suppressor protein neurofibromin. Up to 40% of patients with NF1 have macrocephaly often associated with diffuse white matter overgrowth, increased total brain volume, and thickening of the corpus callosum [4], [33-35]. Other distinctive intracranial findings include optic and non-optic pathway gliomas, nerve sheath tumors, hamartomas, and vascular malformations [24], [33]. Tuberous sclerosis is an autosomal dominant disorder caused by mutations in the TSC1 or TSC2 tumor suppressor genes. The neuropathological hallmarks of tuberous sclerosis include subependymal nodules, subependymal giant cell astrocytomas, along with various white matter abnormalities. Megalencephaly in these patients is often secondary to asymmetric white matter overgrowth. Obstructive hydrocephalus is also commonly observed and can further contribute to macrocephaly [23], [24], [36]. Other genetic syndromes include the PI3KA related overgrowth Spectrum (PROS). PROS encompasses a spectrum of genetic disorders caused by pathologic variants of the PIK3CKA gene (Figure 13). The clinical manifestations of these conditions are very broad, but key features include early onset of asymmetric overgrowth involving the adipose, skeletal, and/or nervous tissues often alongside vascular malformations and cutaneous lesions. The brain, limbs, trunk, and face are most affected. Brain matter overgrowth may be accompanied by ventriculomegaly, thickening of the corpus callosum, cerebellar ectopia, and crowding of the posterior fossa [5], [10].
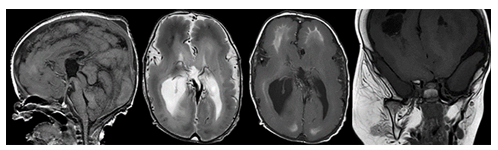
Figure 13: Sagittal T1-, axial T2-, axial T1-weighted MR images of the brain and a coronal T1-weighted MR image of the face show a megalencephaly polymicrogyria overgrowth syndrome with diffusely malformed cortical ribbon, abnormal white matter myelination, ventriculomegaly and a right dominant soft tissue hyperplasia in the maxillofacial region.
7.3 Metabolic Megalencephaly
Canavan syndrome (Figure 14) is a neurometabolic disorder caused by a deficiency of aspartoacylase leading to the accumulation of N-acetyl aspartic acid in the brain’s white matter [11], [36]. Symptom onset begins around 3-6 months of age resulting in rapidly increasing head circumference, seizures, severe hypotonia, and behavioral aberrations. MRI findings include diffuse T2 hyperintensities within the white matter as well as white matter thickening.
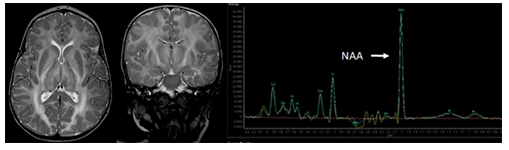
Figure 14: Axial and coronal T2-weighted MR images and a 1H Magnetic resonance spectroscopy spectrum of the white matter in a 6-year-old female patient with Canavan’s disease shows a moderate macrocephaly with diffuse T2-hyperintensity of the white matter and a significantly enlarged NAA peak on MRS.
Lysosomal storage diseases can also cause megalencephaly. Tay-Sachs disease is one of the most common lysosomal storage diseases and is caused by a deficiency of the beta-hexosaminidase enzyme leading to toxic accumulation of fatty acids. Patients present at around 6 months of age with progressive developmental delay, hypotonia, seizures, and visual degeneration. Macrocephaly with megalencephaly is frequently observed due to the accumulation of fatty acids within the brain. Neuroimaging typically reveals diffuse, cystic white matter and cerebellar degeneration [23], [31], [38]. Alexander disease is a rare leukodystrophy disorder caused by mutations in the GFAP gene leading to the accumulation of Rosenthal fibers within the brain. Patients typically display symptoms by age 2. Clinical features include megalencephaly, psychomotor retardation and seizures. Typical neuroimaging findings include extensive white matter abnormalities with frontal lobe predominance [23], [24], [31], [39]. Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is an autosomal recessive disorder characterized by cerebral white matter edema. Patients with MLC develop severely progressive macrocephaly, sometimes exceeding 4-6 standard deviations above normal. Neuroimaging reveals diffuse white matter abnormalities, edema, and subcortical cysts predominantly in the anterior temporal lobe [23], [40].
8. Conclusion
Macrocephaly is a common clinical finding in infants and may result from a wide variety of clinical entities, ranging from benign to pathologic. Infants are particularly prone to macrocephaly due to their underdeveloped calvarial bones and open sutures, which allow for significant expansion of the cranium. Radiologists and clinicians must be familiar with the major classes of conditions that can manifest with macrocephaly to facilitate timely diagnosis and ensure optimal treatment outcomes.
References:
- Tan P, Mankad K, Gonçalves F G, et al. “Macrocephaly: Solving the Diagnostic Dilemma,” Top. Magn. Reson. Imaging vol. 27 (2018): 197.
- Neuberger N, Stence V, Maloney JA, et al, “Imaging of Macrocephaly,” Clin. Perinatol 49 (2022): 715-734
- Orman G, Benson JE, Kweldam CF, et al. Neonatal head ultrasonography today: a powerful imaging tool! J Neuroimaging 25 (2015): 31-55.
- Medina LS, Frawley K, Zurakowski D, et al. “Children with Macrocrania: Clinical and Imaging Predictors of Disorders Requiring Surgery,” 2001.
- Orrù E, Calloni SF, Tekes A, et al. “The Child With Macrocephaly: Differential Diagnosis and Neuroimaging Findings,” Am. J. Roentgenol Apr. 210 (2018) : 848-859
- Accogli A. “Diagnostic Approach to Macrocephaly in Children,” Front. Pediatr 9 (2022): 794069
- Jones SG, Samanta D. “Macrocephaly,” in StatPearls, Treasure Island (FL): StatPearls Publishing, 2023.
- Thomas N, Kolbe AB, Binkovitz LA, et al. “Asymptomatic macrocephaly: to scan or not to scan,” Pediatr. Radiol 51 (2021): 811-821
- Sampson MA, Berg AD, Huber JN, et al. “Necessity of Intracranial Imaging in Infants and Children With Macrocephaly,” Pediatr. Neurol 93 (2019): 21-26.
- Smith R, Leonidas JC, Maytal J. “The value of head ultrasound in infants with macrocephaly,” Pediatr. Radiol 28 (1998): 143-146
- Alvarez H, Garcia-Monaco R, Rodesch G, et al .Vein of Galen aneursymal malformations. Neuroimaging Clin N Am 17 (2007):189-206
- Huang J, Sarma A, Little S, et al. “Systematic Approach to Pediatric Macrocephaly,” RadioGraphics 43 (2023): 220159
- Karimy JK, Reeves BC, Damisah E, et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat Rev Neurol 16 (2020):285-296.
- Holste KG, Xia F, Ye F, et al. Mechanisms of neuroinflammation in hydrocephalus after intraventricular hemorrhage: a review. Fluids Barriers CNS 19, 28 (2022).
- McAllister JP, Guerra MM, Ruiz LC, et al. Ventricular Zone Disruption in Human Neonates With Intraventricular Hemorrhage. J Neuropathol Exp Neurol 76 (2017): 358-375.
- Greitz D, Greitz T, Hindmarsh T. A new view on the CSF-circulation with the potential for pharmacological treatment of childhood hydrocephalus. Acta Paediatr 86 (1997): 125-132
- Greitz D. The hydrodynamic hypothesis versus the bulk flow hypothesis. Neurosurg Rev (2004): 299-300.
- Johnston M, Papaiconomou C. Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci 2002: 227-330.
- Weller RO, Djuanda E, Yow HY, et al. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 117 (2009): 1-14.
- Kim JY, Nam Y, Kim S, et al. MRI-visible Perivascular Spaces in the Neonatal Brain. Radiology. 307 (2023): 221314.
- Huisman TAGM. Unraveling the Mystery of the Perivascular Spaces and Glymphatic System of the Neonatal Central Nervous System. Radiology 307 (2023): 223009.
- Huisman TAGM. Unraveling the Mystery of the Perivascular Spaces and Glymphatic System of the Neonatal Central Nervous System. Radiology 307 (2023): 223009.
- Hergan F, Huisman TA. “Melting brain” as complication of a vein of Galen aneurysmal malformation diagnosed by fetal MRI. Clinical Obstetrics, Gynecology and Reproductive Medicine. Printed on line
- Accogli A. “Diagnostic Approach to Macrocephaly in Children,” Front. Pediatr., vol. 9, 2022, Accessed: Aug. 11, 2023.
- KHOSROSHAHI N, NIKKHAH N. “Benign Enlargement of Subarachnoid Space in Infancy: ‘A Review with Emphasis on Diagnostic Work-Up,’” Iran. J. Child Neurol 12 (2018): 7-15.
- Ludwig S, Warman “Shaken baby syndrome: A review of 20 cases,” Ann. Emerg. Med 13 (1984): 104-107
- Chamnanvanakij S, Rollins N, Perlman JM. “Subdural hematoma in term infants,” Pediatr. Neurol 26 (2002): 301-304
- Pauli RM. “Achondroplasia: a comprehensive clinical review,” Orphanet J. Rare Dis 14( 2019): 1.
- McDonald EJ, De Jesus O. “Achondroplasia,” in StatPearls, Treasure Island (FL): StatPearls
- Kim SR, Han YS, “Craniometaphyseal Dysplasia,” Arch. Plast. Surg 40 (2013): 157-159
- Pavone P. “A clinical review on megalencephaly,” Medicine (Baltimore) 96 (2017): 6814
- Mirzaa GM, Poduri A.“Megalencephaly and hemimegalencephaly: breakthroughs in molecular etiology,” Am. J. Med. Genet. C Semin. Med. Genet., vol. 166C (2014): 156-172
- Wang MX et al., “Neurofibromatosis from Head to Toe: What the Radiologist Needs to Know,” RadioGraphics, vol. 42 (2022): 1123-1144
- Mirzaa GM, Poduri A. “Megalencephaly and hemimegalencephaly: Breakthroughs in molecular etiology,” Am. J. Med. Genet. C Semin. Med. Genet., vol. 166 (2014): 156-172
- Cutting LE. “Megalencephaly in NF1: Predominantly white matter contribution and mitigation by ADHD,” Neurology, vol. 59, no. 9, pp. 1388–1394, Nov. 2002: 1388-1394
- Griffiths PD, Gardner SA, Smith M, et al., “Hemimegalencephaly and focal megalencephaly in tuberous sclerosis complex.,” Am. J. Neuroradiol 19 (1998): 1935–1938.
- Bokhari MR, Samanta D, Bokhari SRA. “Canavan Disease,” in StatPearls, Treasure Island (FL): StatPearls Publishing, 2023.
- KARIMZADEH P. “GM2-Gangliosidosis (Sandhoff and Tay Sachs disease): Diagnosis and Neuroimaging Findings (An Iranian Pediatric Case Series),” Iran. J. Child Neurol., 8 (2014):55-60
- Ozkaya H. “Juvenile Alexander Disease: a Case Report,” Eurasian J. Med. 44 (2012): 46-50
- Hamilton EMC. “Megalencephalic leukoencephalopathy with subcortical cysts: Characterization of disease variants,” Neurology, vol. 90, no. 16, pp. e 90 (2018): 1395-1403
- Wilson MH. Monro-Kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab. 36 (2016):1338-1350.
