Lysine as a Potential Low Molecular Weight Angiogen: its Clinical, Experimental and In-Silico Validation- a Brief Study
Article Information
Debatosh Datta1,2,3,*, Priyanshu Verma1, Anindita Banerjee#, Partha Pratim Mukherjee6 , Debashis Ganguly6 , Sujoy Kar1, Tanima Sengupta4, Nalinava Sengupta5, Sujoy Kumar Samanta1, Enam Murshed Khan3
1Department of Chemical and Biochemical Engineering, IIT Patna, India
2Jagannath Gupta Medical College & Hospital, Budge Budge
3Apollo-Gleneagles Hospital, Kolkata, India
4Epigen, Akershus Universitetssykehus, 1478 Lorenskog, Norway
5iNANOD AS, Oslo, Norway
6Department of Medicine, Calcutta National Medical College
#ICMR National lnstitute of lmmuno-haemarology
*Corresponding Author: Dr Debatosh Datta. Consultant, Apollo-Gleneagles Hospital – Kolkata, India, Jagannath Gupta Institute of Medical Sciences & Hospital , Budge Budge, Ex-Visiting Professor, Department of Chemical & Biochemical Engineering, IIT Patna, India
Received: 15 April 2023; Accepted: 24 April 2023; Published: 29 July 2023
Citation: Debatosh Datta, Priyanshu Verma, Anindita Banerjee, Partha Pratim Mukherjee, Debashis Ganguly, Sujoy Kar, Tanima Sengupta, Nalinava Sengupta, Sujoy Kumar Samanta, Enam Murshed Khan. Lysine as a Potential Low Molecular Weight Angiogen: its Clinical, Experimental and In-Silico Validation- a Brief Study. Journal of Radiology and Clinical Imaging. 6 (2023):
View / Download Pdf Share at FacebookAbstract
Globally, the area of angiogenesis is dominated by investigations on anti-angiogenic agents and processes, due to their role in metastatic cancer treatment although the nearly non-existent area of pro-angiogenesis or inducible controlled angiogenesis in ischemic tissue reperfusion is having much bigger potential demand and far wider clinical footmark. Following clinical failure of VEGF (Vascular endothelial growth factor) as a potential agent for induction of a controlled angiogenic response in ischemic tissues and organs, the progress is reasonably quiet as for new low molecular weight (LMW) angiogen molecules and their clinical applications. Basic amino acid Lysine has been observed to have profound angiogenic property in ischemic tissues, which is controlled, reproducible, time bound and without any accompanying reperfusion-reentry damage. In this study, the basic amino acid Lysine has been suggested as a LMW-angiogen (low mol wt angiogen) and it has been proposed to have a molecular bridging role between VEGF and VEGF receptor (VEGFR). Here, the molecular adhesive hypothesis is being probed and confirmed both in the clinical and lab conditions through induced angiogenic response in tissue repair and in chick chorioallantoic membrane (CAM), respectively; and in dry-docking experiments (in-silico studies)
Keywords
Inducible controlled angiogenesis; Lysine; VEGF; Low Molecular Weight Angiogen(s); Molecular Docking.
Inducible controlled angiogenesis articles; Lysine articles; VEGF articles; Low Molecular Weight Angiogen(s) articles; Molecular Docking articles.
Inducible controlled angiogenesis articles Inducible controlled angiogenesis Research articles Inducible controlled angiogenesis review articles Inducible controlled angiogenesis PubMed articles Inducible controlled angiogenesis PubMed Central articles Inducible controlled angiogenesis 2023 articles Inducible controlled angiogenesis 2024 articles Inducible controlled angiogenesis Scopus articles Inducible controlled angiogenesis impact factor journals Inducible controlled angiogenesis Scopus journals Inducible controlled angiogenesis PubMed journals Inducible controlled angiogenesis medical journals Inducible controlled angiogenesis free journals Inducible controlled angiogenesis best journals Inducible controlled angiogenesis top journals Inducible controlled angiogenesis free medical journals Inducible controlled angiogenesis famous journals Inducible controlled angiogenesis Google Scholar indexed journals Lysine articles Lysine Research articles Lysine review articles Lysine PubMed articles Lysine PubMed Central articles Lysine 2023 articles Lysine 2024 articles Lysine Scopus articles Lysine impact factor journals Lysine Scopus journals Lysine PubMed journals Lysine medical journals Lysine free journals Lysine best journals Lysine top journals Lysine free medical journals Lysine famous journals Lysine Google Scholar indexed journals VEGF articles VEGF Research articles VEGF review articles VEGF PubMed articles VEGF PubMed Central articles VEGF 2023 articles VEGF 2024 articles VEGF Scopus articles VEGF impact factor journals VEGF Scopus journals VEGF PubMed journals VEGF medical journals VEGF free journals VEGF best journals VEGF top journals VEGF free medical journals VEGF famous journals VEGF Google Scholar indexed journals Low Molecular Weight Angiogen articles Low Molecular Weight Angiogen Research articles Low Molecular Weight Angiogen review articles Low Molecular Weight Angiogen PubMed articles Low Molecular Weight Angiogen PubMed Central articles Low Molecular Weight Angiogen 2023 articles Low Molecular Weight Angiogen 2024 articles Low Molecular Weight Angiogen Scopus articles Low Molecular Weight Angiogen impact factor journals Low Molecular Weight Angiogen Scopus journals Low Molecular Weight Angiogen PubMed journals Low Molecular Weight Angiogen medical journals Low Molecular Weight Angiogen free journals Low Molecular Weight Angiogen best journals Low Molecular Weight Angiogen top journals Low Molecular Weight Angiogen free medical journals Low Molecular Weight Angiogen famous journals Low Molecular Weight Angiogen Google Scholar indexed journals Molecular Docking articles Molecular Docking Research articles Molecular Docking review articles Molecular Docking PubMed articles Molecular Docking PubMed Central articles Molecular Docking 2023 articles Molecular Docking 2024 articles Molecular Docking Scopus articles Molecular Docking impact factor journals Molecular Docking Scopus journals Molecular Docking PubMed journals Molecular Docking medical journals Molecular Docking free journals Molecular Docking best journals Molecular Docking top journals Molecular Docking free medical journals Molecular Docking famous journals Molecular Docking Google Scholar indexed journals Low molecular weight articles Low molecular weight Research articles Low molecular weight review articles Low molecular weight PubMed articles Low molecular weight PubMed Central articles Low molecular weight 2023 articles Low molecular weight 2024 articles Low molecular weight Scopus articles Low molecular weight impact factor journals Low molecular weight Scopus journals Low molecular weight PubMed journals Low molecular weight medical journals Low molecular weight free journals Low molecular weight best journals Low molecular weight top journals Low molecular weight free medical journals Low molecular weight famous journals Low molecular weight Google Scholar indexed journals chorioallantoic membrane articles chorioallantoic membrane Research articles chorioallantoic membrane review articles chorioallantoic membrane PubMed articles chorioallantoic membrane PubMed Central articles chorioallantoic membrane 2023 articles chorioallantoic membrane 2024 articles chorioallantoic membrane Scopus articles chorioallantoic membrane impact factor journals chorioallantoic membrane Scopus journals chorioallantoic membrane PubMed journals chorioallantoic membrane medical journals chorioallantoic membrane free journals chorioallantoic membrane best journals chorioallantoic membrane top journals chorioallantoic membrane free medical journals chorioallantoic membrane famous journals chorioallantoic membrane Google Scholar indexed journals placental growth factor articles placental growth factor Research articles placental growth factor review articles placental growth factor PubMed articles placental growth factor PubMed Central articles placental growth factor 2023 articles placental growth factor 2024 articles placental growth factor Scopus articles placental growth factor impact factor journals placental growth factor Scopus journals placental growth factor PubMed journals placental growth factor medical journals placental growth factor free journals placental growth factor best journals placental growth factor top journals placental growth factor free medical journals placental growth factor famous journals placental growth factor Google Scholar indexed journals Diabetic Nephropathy articles Diabetic Nephropathy Research articles Diabetic Nephropathy review articles Diabetic Nephropathy PubMed articles Diabetic Nephropathy PubMed Central articles Diabetic Nephropathy 2023 articles Diabetic Nephropathy 2024 articles Diabetic Nephropathy Scopus articles Diabetic Nephropathy impact factor journals Diabetic Nephropathy Scopus journals Diabetic Nephropathy PubMed journals Diabetic Nephropathy medical journals Diabetic Nephropathy free journals Diabetic Nephropathy best journals Diabetic Nephropathy top journals Diabetic Nephropathy free medical journals Diabetic Nephropathy famous journals Diabetic Nephropathy Google Scholar indexed journals amino acids articles amino acids Research articles amino acids review articles amino acids PubMed articles amino acids PubMed Central articles amino acids 2023 articles amino acids 2024 articles amino acids Scopus articles amino acids impact factor journals amino acids Scopus journals amino acids PubMed journals amino acids medical journals amino acids free journals amino acids best journals amino acids top journals amino acids free medical journals amino acids famous journals amino acids Google Scholar indexed journals
Article Details
1. Introduction
Controlled reperfusion of ischemic tissues is an active area of investigation both as an alternate approach of therapy and as the fundamental platform of in-situ cellular expansion in healing of wounds and restoration of blood supply in ischemic tissues. VEGF is the lead mitogen for endothelial cells. In human subjects, VEGF family consists of five different iso-forms - VEGF-A, VEGF-B, VEGF-C, VEGF-D and PlGF (placental growth factor} [1]. It plays a central role in the initiation of angiogenesis through regeneration and growth of new blood vessels at capillary level. In vascular exchange beds, angiogenesis, which is an extremely conserved process across species, forms network of capillary supply lines for healthy cells to provide required nutrients and gas, signaling molecules and for removal of carbon dioxide and other metabolic end products[1-6]. As mentioned, angiogenesis is a highly conserved natural process and endogenously controlled by the body’s own regulatory system. This system is considerably complex and precise, which involve both endogenous inhibitors and activators [6] Degree of angiogenesis supports natural growth phase in the early part of life. However, with aging-normal angiogenic process gets slow and inadequate for required growth at any given instance and particularly in situations of very high demand for tissue repair and significant tissue regeneration. In-vivo angiogenesis is promoted through the production of VEGF by endothelial cells in relative ischaemia followed by its binding to the dedicated receptors VEGFR in the immediate vicinity. VEGFR family consists of two non-protein kinases (neuropilin-1 and 2) and three receptor tyrosine kinases (VEGFR-1, VEGFR-2 and VEGFR-3)[1,4-6]. VEGFR-1 binds to VEGF-A, VEGF- B and PlGF; VEGFR-2 binds to VEGF-A, VEGF-C and VEGF-D; VEGFR-3 binds to VEGF-C and VEGF-D. VEGFR-1 and VEGFR-2 receptors are mainly found on the blood vascular endothelium and responsible for angiogenesis. However, VEGFR-3 receptors are mainly found on the lymphatic endothelium and responsible for Lymphangiogenesis [2-4]. In situations of relative ischaemia, depending on the degree, ischaemic zone endothelial cells elaborate excess titres of VEGF as a means of controlled ischaemia reperfusion [7,8]. Since, tumor angiogenesis or uncontrolled non-hierarchical angiogenesis play central role in proliferation of cancerous cells, the area of angiogenesis is mainly governed by studies on the inhibition of the process [9-14]. In addition, the absence of a good small molecule candidate as an effective mediator of inducible angiogenic process the field of induced controlled augmented angiogenesis is substantially on a low key in the current global research trend. Following clinical limitation of use of VEGF as a therapeutic agent, the area of controlled angiogenesis is evolving rather slowly because of the absence of a non-toxic low-molecular- weight angiogen molecule compared to rapid progresses made in the other half of angiogenesis research i.e. suppression of it [15]. In this study, the potential of basic amino acid Lysine as a LMW-angiogen (low mol wt angiogen) has been examined with the help of histopathological and CAM (Chorio-Allantoic-Membrane) experiments. Molecular docking or in-silico docking has been employed to check and compare the binding affinities of VEGF-A and VEGF-A receptor with and without Lysine . In addition, experimentally observed results have been further validated through the in-silico docking studies of VEGF-A and VEGF- A receptor in the presence of Lysine.
2. Methods and materials
2.1 Histopathological assay
Histopathological assay involved a surface wound treatment with bare 24-hourly topical application of hydrogel based Lysine HCl formulation (250 mg/g) to observe inducible angiogenesis in wound bed, if any. Human study was undertaken under all prescribed rules governing clinical evaluation studies, both at the controller general of drugs as well as the local institutional ethical committee levels. In addition, informed and explained patient consent was part of this clinical study. Following topical application for 5 days under moistened wound conditions, routine histopathology studies were carried out with tissue samples obtained from the peripheral margin of wound beds through punch biopsy.
2.2 CAM experiment
Chorio-Allantoic-Membrane (CAM) model was utilized to have confirmatory visual evidence of inducible angiogenesis as described earlier [14,16-18]. Briefly, CAM of 9-days-old chicken embryos had been used in two groups: experimental group versus a control group, each group had 5 embryos. Control CAMs were installed with aqueous vehicle (2 ml of water) only, whereas experimental group were supplemented with 2 ml of an aqueous solution of Lysine HCl (Ajinomoto Co., Japan). Both the sets of embryos were photographed after the 5 days incubation at 37 °C (to visualize the sprouting vessels).
2.3 In-silico docking analysis
The present section is mainly focused on in-silico study of binding affinity of VEGF-A to VEGF-A receptor in the presence of basic amino acid Lysine. Docking tools - Z-DOCK [19] and PATCHDOCK [20] followed by FIREDOCK [21,22] have been used for the analyses. Required protein molecules’ pdb files have been selected from the PDB database [23]. Molecules with PDB IDs – 1VPF (VEGF-A) and 4KZN (VEGF-A receptor) have been used respectively for the docking studies. The Lysine molecule’s sdf file has been downloaded from the PubChem server [24] with further conversion to pdb file with the help of an offline tool - Open Babel [25]. Prediction studies have been performed for the same combination to predict the most probable binding site(s) on Z-DOCK platform. However, for stability analysis, only FIREDOCK results have been used. Chimera 1.10.2[26] and and Swiss –PDB Viewer 4.1.0 [ 27] have been used for the visualization of models generated through different docking tools. This study is mainly based on a hypothesis – basic amino acid Lysine possibly acts as a molecular binder between the most potent in-vivo angiogenic peptide VEGF and its receptor, thereby augmenting the binding stability between the two resulting in an enhanced biological response (Fig.1)[28].
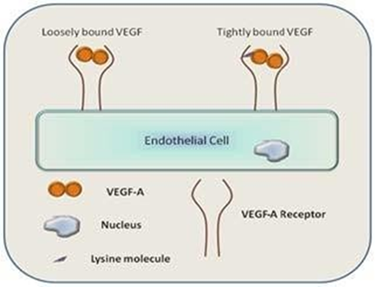
Figure 1: Schematic representation of the hypothesis: VEGF-A and VEGF-A receptor binding on the endothelial cell surface in the presence of Lysine molecule.
This facilitated binding probably enhances the in-vivo physiological level of angiogenic response which in turn helps in improved controlled reperfusion of ischaemic tissues anywhere , which otherwise is a remarkably slow
process. To examine tbe hypothesis, the present design included two modes of approach in in-silico binding studies:
- Lysine being coupled to VEGF-A receptor (4KZN) to generate the complex–VEGF- A receptor-Lysine. This complex is then presented for binding with VEGF-A (1VPF).
- Lysine is first coupled to VEGF-A (1VPF) to generate the complex–VEGF-A-Lysine.
This complex peptide is then presented for binding studies with VEGF-A receptor ( 4KZN)
In protocol (1) above, VEGF-A receptor structure was scanned for distribution of surface negative charges along the entire length of the peptide. This was done in anticipation of a possible electrostatic interaction between added Lysine and the receptor peptide (Fig. 2a). Similarly, in pre-binding added lysine complexing protocol (2) described above, VEGF-A molecule was screened for negative charge distribution along the entire length of the peptide molecule (Fig. 2d).
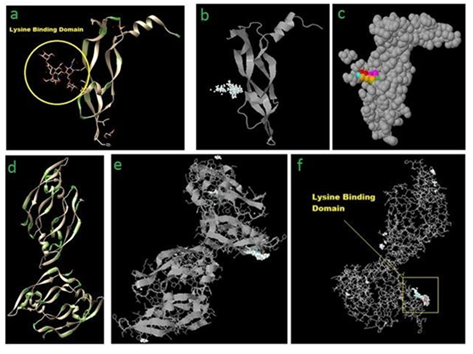
Figure 2: (a) VEGF-A receptor (4KZN) showing the most probable lysine binding domain as well as negatively charged amino acid(s) in the vicinity (mainly aspartic acid) marked as green islands. Z-DOCK results for 4KZN (VEGF-A receptor) and Lysine (as a ligand) showing top 500 possible Lysine binding sites (b), and top 5 Lysine binding sites (c) on VEGF-A receptor. (d) VEGF-A (1VPF) ribbon structure showing uniform distribution of negatively charged amino acids along the entire length of the chains. Z-DOCK results for 1VPF (VEGF-A) and Lysine (as an additional ligand) showing ribbon structure (e), and wire-frame structure (f) of VEGF-A (1VPF) with top 500 preferential binding locations of added Lysine residue (white spheres) and top 5 locations (colored lines).
3. Results and Discussion
3.1 Histopathological assay
Lysine induced remarkably enhanced angiogenic response in surface tissue repair of extensive bedsore was observed within approx. two weeks' time (Fig.3a). Low and high power field optical microscopic representations of the tissue obtained from the margin of the wound bed through punch biopsy showed hierarchical angiogenic response in the wound bed (Fig. 3b, c ). This clinical evidence of topical Lysine HCI gel mediated induction of an extensive angiogenic response in a profusely ischaemic wound bed possibly conforms to the hypothesis of the enhanced angiogenic response capability of the natural amino acid Lysine in ischemic tissues in a much enhanced biological concentration window compared to metabolic concentrations required in protein metabolism . Nearly all amino acids are having differential concentration windows in their metabolic and biological roles.
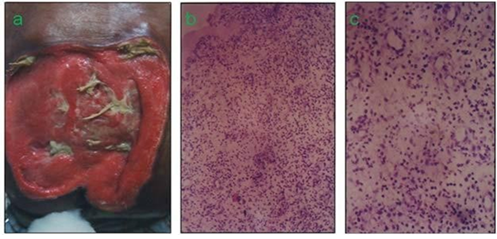
Figure 3: Extensive bedsore in a clinical study (patient was ready for graft with two-weeks’ topical application of a Lysine hydrogel formulation 250 mg/g). (b) Low-power field optical microscopic representation of the tissue from the margin of the wound obtained through punch biopsy. (c) High power field photograph of the tissue showing the hierarchical angiogenic response in the wound bed.
3.2 CAM experiment
Ability of the amino acid as a potent angiogen was evaluated in in-vitro angiogenesis model. Chicken embryo CAMs were supplemented with Lysine HCl in aqueous form (250 mg/ml). A representative photograph showed sharp quantitative contrast in angiogenic response in chicken embryo CAMs with and without Lysine in terms of growth of new capillaries (Fig. 4a, b). Substantive difference between the natural and lysine induced angiogenic response is obvious in the figures.
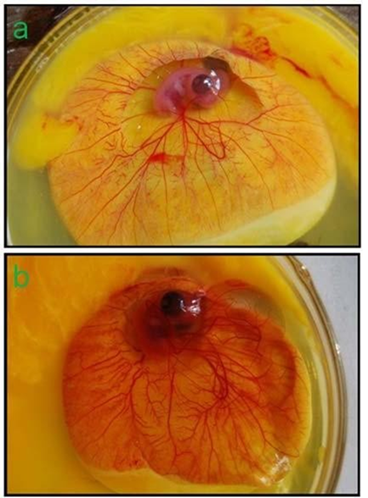
Figure 4: CAM of 9 days old chicken embryo incubated for 5 days (14th day CAM): (a) Physiological angiogenesis or control CAM; (b) Lysine HCl induced extensive angiogenic response or experimental CAM.
3.3 In-silico docking analysis
Z-DOCK result for 4KZN (VEGF-A receptor) and Lysine (as a ligand) shows a specific location on VEGF-A receptor as the most probable binding site for added Lysine (Fig. 2b, c). Interestingly, although negative charge distribution among the entire body of the peptide was uniform (Fig. 2a,d), added external lysine binding had a preferential binding site (Fig. 2b, c). Again, as observed in VEGF-A receptor-Lysine complexing study, VEGF-A showed a very dedicated binding site for the added single Lysine moiety, although unlike in VEGF-A receptor, VEGF-A showed few additional distributed comparatively insignificant locations of added Lysine binding sites also (Fig. 2e,f). It is worth noting that the preferential and other lysine binding sites' distribution in the peptide are all being on either ends or on the tip of projected part of the peptide.
3.4 In-silico binding studies of pre-Lysine bound VEGF-A and VEGF-A receptor
Complex-A, i.e. the best obtained structure of lysine bound VEGF-A receptor (4KZN + Lysine, 1:1), binding stability with VEGF-A (1VPF) had been shown and compared based on the global energy changes in interactions between native VEGF-A receptor–VEGF-A (4KZN + 1VPF) and Lysine modified (Complex-A + 1VPF) forms. Change in global energy of binding had been taken as a definite parameter of binding stability; lower the energy more the stability. In Complex-A and VEGF-A (1VPF) binding, energy profile shows more unstable binding (Table 1: Step 1 vs Step 4) compared to the Complex-B, i.e. the best obtained structure of Lysine bound VEGF-A (1VPF + Lysine, 1:1), and VEGF-A receptor (4KZN) binding (Table 1: Step 1 vs Step 5) where further lowering of global energy profiles of the final combine clearly shows an enhanced stability of binding (nearly 40 % lowering indicates a definite shift towards much enhanced stability of the ligand–receptor complex).
|
Step |
Receptor Molecule |
Ligand |
Comments and/or Results |
|
1 |
4KZN (VEGF-A receptor) |
1VPF (VEGF-A) |
Global Energy = -9.41; Score = 16252 |
|
2 |
4KZN (VEGF-A receptor) |
Lysine (in pdb format) |
Best structure has been selected and named as Complex-A |
|
3 |
1VPF (VEGF-A) |
Lysine (in pdb format) |
Best structure has been selected and named as Complex-B |
|
4 |
Complex-A |
1VPF (VEGF-A) |
Global Energy = 2.66; Score =17132 |
|
5 |
4KZN (VEGF-A receptor) |
Complex-B |
Best result with Global Energy = -13.14; Score = 17864 |
Table 1: Overall profile of docking experiments
Added Lysine (1:1) with VEGF-A and VEGF-A receptor gives more stable complex of the peptide-and-receptor (Fig. 5), thereby inducing enhanced angiogenic response as seen clinically and experimentally (Fig. 3, 4), where added lysine molecule with its functional groups –NH2 (2) and –COOH (1) possibly helps in stabilizing -a probable loose-fit between the ligand and the receptor.
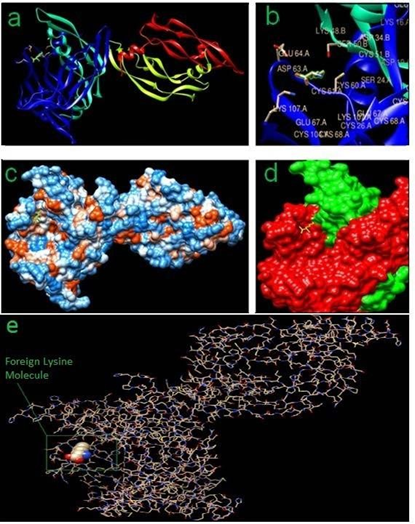
Figure 5: In-silico docking results showing the exact location of -foreign Lysine molecule: (a) Ribbon View; (b) Stereo diagram (Foreign Lysine is highlighted with green color); (c) Hydrophobic surface view; (d) Foreign Lysine in yellow color acting as a binder between VEGF-A (red) and VEGF-A receptor (green); (e) All atoms stick view.
4. Conclusion
Angiogenesis is a widely conserved process across species which forms the foundations for embryogenesis, organogenesis and all in-situ repair processes like wound repair & tissue regeneration, exchange-bed renewal in all ischaemic diseases like Diabetes and its complications such as Nephropathy, Vasculopathy, Ischaemic Paralytic Stroke, CAD, Placental Insufficiency & IUGR , Chemotherapy etc. to name a few. Although the process and its deficiencies play such an important role as the foundation anomaly of all these divergent conditions, it becomes a fundamentally slow and essentially ineffective process with aging. Embryogenic stage demands an extremely high rate of cell expansion as a basis of organogenesis and overall growth in-utero. The relative anoxic environment may possibly play a key role in the expression level of the key angiogen(s) in foetal tissues and organs. Post birth, this inbuilt stimulus gets removed with possible gradual loss of expression of the peptide and its receptor gene(s) overall on a sustained basis. This possibly explains a very slow collateral generation in ischaemic myocardium with advancing age. A detailed population study would possibly open up answers to differential clinical progresses in patients with ischaemic conditions like Paralytic Stroke, CAD etc., including giving directions to prognostic aspects (objective ischemia assessment) of ongoing therapeutic approaches in all ischemic conditions (e.g. Diabetes and its complications). Ischaemia assessment as a prognostic marker in all ischaemic diseases like Diabetes and its complications may well be a new investigative direction which is summarily absent in clinical medicine now , anywhere .
Also the level of VEGF-A and VEGFR may well be differentially expressed in various races, communities and even in individuals in a given community and geographical location, based on given genetic profiles. Certain clinical condition like Sudden Cardiac Death may well be explained by near total absence of collateral formation in myocardium with advancing age. Collateral generation and the absence of it can possibly be explained on this molecular expression & interaction status. Contributing to overall less and differential expression(s) of angiogenic peptide(s) including VEGF-A in individuals, another contributory factor to the inefficiency of physiological angiogenic response could be weak binding or nearly no binding of the peptide and its receptor. The proposed loose-fit model may partly explain the overall lack of binding stability leading to the final inefficient biological expression by way of less induction of angiogenic response under normal physiological conditions in ischaemic tissues.
This raises a couple of related questions:
- Whether titre of VEGF-A (or/and iso-forms) in general circulation or in a particular ischaemic tissue zone gives an idea about the degree of ischaemia? And whether it alone or in combination with a few other Hypoxia induced peptide(s) can indicate definite direction towards objective assessment of a given ischaemic condition, either as an objective diagnostic or prognostic parameter or both. It may as well possibly be a parameter of predictive assessment of ongoing therapy in any of the ischaemic diseases with long drawn complications, like Diabetic Nephropathy, Diabetic Paralytic Stroke, CAD etc. as explained above. This new direction can possibly give advanced indication(s) of setting-in of ischaemia even without any obvious expressions of it or clinical presentations at a given instance (predictive diagnostics).
- Whether a possible new therapeutic angiogenic mode can be visualized by way of the natural amino acid molecule (and its synthetic analogue(s) where in-silico docking work is in progress), despite the molecule per se being non-angiogenic and only possibly helps stabilize the VEGF-VEGFR complex to get its augmented response so long as the two complimentary peptides are in the vicinity in response to an ischaemic stimulus, thereby making the induction process completely controllable. Basic amino acids (Lysine, Arginine and Histidine) are all vasoactive in addition to their routine metabolic roles. Arginine, in-vivo, acts as the source of NO as one of the most potent vasodilators of existing capillary beds anywhere (29). Histidine remains locally vasoactive in some tissues - being converted to Histamine.
Vasoactivity of Lysine possibly lies in its ability to form new capillary beds in ischemic tissues and organs as shown in Fig. 3 and 4. Physiological angiogenesis being slow and functionally ineffective in nearly all ischaemic conditions needs augmentation for it to be therapeutically meaningful and a significant mean of controlled reperfusion (30). Present study raises a possibility of examination of the natural amino acid and its defined synthetic analogue(s) as possible putative agents in clinical induction of time-bound controlled angiogenic responses in ischaemic tissues and organs as an alternate mode of therapeutic approach without any risk of reperfusion injury.
- Can we have a new direction - Vaccine in Non-Communicable Diseases - like Diabetes, Paralytic Ischemic Stroke, Coronary Artery Disease and the likes?
Acknowledgments
The authors would like to acknowledge the help of Dr. D. Banerjee during the dry experiments.
Compliance with ethical standards
Clinical studies had been conducted as per the institutional ethical committee guidelines and with informed individual patient consent.
Conflict of interest:
None
Funding:
None
References:
- Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13 (1999):
- Ferrara N & Davis-Smyth T. The Biology of Vascular Endothelial Growth Factor. Endocr Rev18 (1997): 4.
- Ferrara N, Gerber H P & LeCouter J. The biology of VEGF and its receptors. Nat Med 9 (2003): 669.
- Tammela T, Enholm, B, Alitalo K, et al.The biology of vascular endothelial growth factors. Cardiovasc Res 65 (2005): 550.
- Olsson A K, Dimberg A, Kreuger J, et al. VEGF receptor signalling — in control of vascular function. Nat Rev Mol Cell Biol 7 (2006): 359.
- Roskoski Jr R. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol 62 (2007): 179.
- Laham R J, Sellke F W, Edelman E R, et al. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery results of a phase I randomized, double-blind, placebo- controlled trial. Circulation100 (1999): 1865.
- Laham R J, Rezaee M, Post M, et al. Intrapericardial delivery of Fibroblast Growth Factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia, J Pharmacol Exp Ther 292 (2000): 795.
- Ghoshal D. Anti-angiogenic activity of BIM (bio-immunomodulator), Indian J Exp Biol 40 (2002): 5.
- Roy S & Maity P. Effect of glutamine analogue-acivicin on tumor induced angiogenesis in Ehrlich ascites carcinoma. Indian J Exp Biol 43 (2005): 407.
- Luo H, Rankin G O, Liu L, Daddysman M K, et al. Kaempferol Inhibits Angiogenesis and VEGF Expression Through Both HIF Dependent and Independent Pathways in Human Ovarian Cancer Cells. Nutr Cancer 61 (2009): 554.
- Bhat T A, Moon J S, Lee S, et al. Inhibition of angiogenic attributes by decursin in endothelial cells and ex vivo rat aortic ring angiogenesis model, Indian J Exp Biol 49 (2011): 848.
- Ding G, Chen X, Zhu J, et al. A murine–human chimeric IgG antibody against vascular endothelial growth factor receptor 2 inhibits angiogenesis in vitro. Cytotechnology 66 (2014): 395.
- Kyadari M, Fatma T, Velpandian T, et al. Antiangiogenic and antiproliferative assessment of cyanobacteria. Indian J Exp Biol 52 (2014): 835.
- Ferrara N & Adamis A P. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov 15 (2016): 385.
- Ribatti D. Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. Int Rev Cell Mol Biol 270 (2008): 181.
- Devi M S & Sudhakaran P R. Angiogenic response of advanced glycation end products (AGEs) involves PPAR γ. Indian J Biochem Biophys 49 (2012): 18.
- Cimpean A M, Ribatti D & Raica M. The chick embryo chorioallantoic membrane as a model to study tumor metastasis. Angiogenesis 11 (2008): 311.
- Pierce B G, Wiehe K, Hwang H, et al. ZDOCK Server: Interactive Docking Prediction of Protein-Protein Complexes and Symmetric Multimers. Bioinformatics 30 (2014): 1771.
- Schneidman-Duhovny D, Inbar Y, Nussinov R, et al. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucl Acids Res 33 (2005): 363.
- Andrusier N, Nussinov R & Wolfson H J. FireDock: Fast Interaction Refinement in Molecular Docking. Proteins 69 (2007): 139.
- Mashiach E, Schneidman-Duhovny D, Andrusier N, et al. FireDock: a web server for fast interaction refinement in molecular docking. Nucl Acids Res 36 (2008): 229.
- Berman H M, Westbrook J, Feng Z, et al. The Protein Data Bank. Nucl Acids Res 28 (2000): 235.
- Kim S, Thiessen P A, Bolton E E, et al. PubChem Substance and Compound databases. Nucl Acids Res 44 (2016): 1202.
- O’Boyle N M, Banck M, James C A, et al. Open Babel: An open chemical toolbox. J Cheminform 3 (2011) 33.
- Pettersen E F, Goddard T D, Huang C C, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25 (2004): 1605.
- Guex N & Peitsch M C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 18 (1997): 2714.
- Datta D, Bhinge A & Chandran V. Lysine: Is it worth more? Cytotechnology 36 (2001): 3.
- Bode-Böger S M, Böger R H, Alfke H, et al. L-Arginine Induces Nitric Oxide–Dependent Vasodilation in Patients With Critical Limb Ischemia- A Randomized, Controlled Study. Circulation 93 (1996): 85.
- Gupta R, Agarwal A, Agarwal S, et al. Reversal Of Acute Human Brain Ischemic Injury By Lysine Induced Therapeutic Angiogenesis: Preliminary Results Of A Pilot Study. The Internet Journal of Neurology 4 (2004): 1.
