Long-Term Outcomes of Cochlear Implantation in Children with Cochlear Nerve Deficiency
Article Information
Anjin Mori1, Akinori Kashio2*, Yusuke Akamatsu2, Erika Ogata2, Yujiro Hoshi1, Yu Matsumoto2, Shinnichi Iwasaki3, Tatsuya Yamasoba2
1Department of Otolaryngology, Faculty of Medicine, The Kindai University, Japan
2Department of Otolaryngology and Head and Neck Surgery, Faculty of Medicine, The University of Tokyo, Japan
3Department of Otolaryngology, Head and Neck Surgery, Nagoya City University Graduate School of Medical Sciences, Japan
*Corresponding Author: Dr. Akinori Kashio, Department of Otolaryngology and Head and Neck Surgery, Faculty of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8655, Japan
Received: 24 August 2020; Accepted: 08 September 2020; Published: 01 November 2020
Citation: Anjin Mori, Akinori Kashio, Yusuke Akamatsu, Erika Ogata, Yujiro Hoshi, Yu Matsumoto, Shinnichi Iwasaki, Tatsuya Yamasoba. Long-Term Outcomes of Cochlear Implantation in Children with Cochlear Nerve Deficiency. Archives of Clinical and Medical Case Reports 4 (2020): 990-1002.
View / Download Pdf Share at FacebookAbstract
Objective: To evaluate long-term auditory outcomes with the continuous use of cochlear implants (CIs) in pediatric patients with cochlear nerve deficiencies (CNDs).
Methods: Six children with CNDs who received CIs (mean age: 38.7 months old) were retrospectively evaluated. Audiological performance was assessed with a mean follow-up of 126 months (range 63-161) using unaided and aided pure-tone average (PTA) thresholds, categories of auditory performance-II (CAP-II) scores, and the perception of Japanese monosyllabic words. CI use was evaluated by retrospective chart review.
Results: Two of the six patients did not achieve sufficient PTA threshold improvement and discontinued CI use more than 7 years after implantation. The remaining four patients demonstrated improvements in PTA thresholds with CIs, while their speech understanding remained poor with CAP-II scores of 5, even in the best cases. They did not develop open-set speech perception, resulting in continued sign language use.
Conclusion: The long-term auditory outcomes of CI use in CND patients are poor, and some patients may discontinue CI use. Continuous support and education in using sign language is highly recommended for children with CNDs undergoing cochlear implantation.
Keywords
Cochlear implantation; Cochlear nerve deficiency; Prelingual deafness; Speech perception
Cochlear implantation articles, Cochlear nerve deficiency articles, Prelingual deafness articles, Speech perception articles
Cochlear implantation articles Cochlear implantation Research articles Cochlear implantation review articles Cochlear implantation PubMed articles Cochlear implantation PubMed Central articles Cochlear implantation 2023 articles Cochlear implantation 2024 articles Cochlear implantation Scopus articles Cochlear implantation impact factor journals Cochlear implantation Scopus journals Cochlear implantation PubMed journals Cochlear implantation medical journals Cochlear implantation free journals Cochlear implantation best journals Cochlear implantation top journals Cochlear implantation free medical journals Cochlear implantation famous journals Cochlear implantation Google Scholar indexed journals Cochlear nerve deficiency articles Cochlear nerve deficiency Research articles Cochlear nerve deficiency review articles Cochlear nerve deficiency PubMed articles Cochlear nerve deficiency PubMed Central articles Cochlear nerve deficiency 2023 articles Cochlear nerve deficiency 2024 articles Cochlear nerve deficiency Scopus articles Cochlear nerve deficiency impact factor journals Cochlear nerve deficiency Scopus journals Cochlear nerve deficiency PubMed journals Cochlear nerve deficiency medical journals Cochlear nerve deficiency free journals Cochlear nerve deficiency best journals Cochlear nerve deficiency top journals Cochlear nerve deficiency free medical journals Cochlear nerve deficiency famous journals Cochlear nerve deficiency Google Scholar indexed journals Prelingual deafness articles Prelingual deafness Research articles Prelingual deafness review articles Prelingual deafness PubMed articles Prelingual deafness PubMed Central articles Prelingual deafness 2023 articles Prelingual deafness 2024 articles Prelingual deafness Scopus articles Prelingual deafness impact factor journals Prelingual deafness Scopus journals Prelingual deafness PubMed journals Prelingual deafness medical journals Prelingual deafness free journals Prelingual deafness best journals Prelingual deafness top journals Prelingual deafness free medical journals Prelingual deafness famous journals Prelingual deafness Google Scholar indexed journals Speech perception articles Speech perception Research articles Speech perception review articles Speech perception PubMed articles Speech perception PubMed Central articles Speech perception 2023 articles Speech perception 2024 articles Speech perception Scopus articles Speech perception impact factor journals Speech perception Scopus journals Speech perception PubMed journals Speech perception medical journals Speech perception free journals Speech perception best journals Speech perception top journals Speech perception free medical journals Speech perception famous journals Speech perception Google Scholar indexed journals CND articles CND Research articles CND review articles CND PubMed articles CND PubMed Central articles CND 2023 articles CND 2024 articles CND Scopus articles CND impact factor journals CND Scopus journals CND PubMed journals CND medical journals CND free journals CND best journals CND top journals CND free medical journals CND famous journals CND Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Pathogenesis articles Pathogenesis Research articles Pathogenesis review articles Pathogenesis PubMed articles Pathogenesis PubMed Central articles Pathogenesis 2023 articles Pathogenesis 2024 articles Pathogenesis Scopus articles Pathogenesis impact factor journals Pathogenesis Scopus journals Pathogenesis PubMed journals Pathogenesis medical journals Pathogenesis free journals Pathogenesis best journals Pathogenesis top journals Pathogenesis free medical journals Pathogenesis famous journals Pathogenesis Google Scholar indexed journals PTA articles PTA Research articles PTA review articles PTA PubMed articles PTA PubMed Central articles PTA 2023 articles PTA 2024 articles PTA Scopus articles PTA impact factor journals PTA Scopus journals PTA PubMed journals PTA medical journals PTA free journals PTA best journals PTA top journals PTA free medical journals PTA famous journals PTA Google Scholar indexed journals
Article Details
Abbreviations:
SNHL- Sensorineural hearing loss; CI- Cochlear implant; CND- Cochlear nerve deficiency; CN- Cochlear nerve; MRI- Magnetic resonance imaging; IAC- Internal auditory canal; BCNC- Bony cochlear nerve canal; PTA- pure tone average; CAP- categories of auditory performance
1. Introduction
Cochlear implantation is an effective habilitative option for children with both pre- and post-lingual sensorineural hearing loss (SNHL). Recently, technical advances have allowed for an expansion in cochlear implantation candidacy criteria [1, 2]. Children with inner ear malformations, such as incomplete partition type II and large vestibular aqueduct syndrome, have shown good speech performance with cochlear implant (CI) use, and they are now considered to be good candidates for CIs [3-6]. However, cochlear implantation for children with cochlear nerve deficiencies (CNDs) remain controversial. CND, which was first described by Casselman et al. in 1997 [7], is defined as either an absent or a small cochlear nerve (CN) according to radiographical findings, generally using magnetic resonance imaging (MRI). It has been reported that approximately 20% of children with SNHL have CND [8].
The absence of the CN has been assumed to be a contraindication for cochlear implantation because the connection between the cochlea and the brainstem nuclei is lacking in these cases. Several studies have reported poor results of cochlear implantation in CND cases [9, 10]. Zhang et al. (2012) reported that only four of nine children with CND who underwent cochlear implantation showed significant improvements in pure-tone average (PTA) thresholds with CIs but none achieved sufficient speech or perception abilities for oral communication, suggesting that the auditory benefits of CIs for children with CND are limited [9]. Other studies, however, reported that children with CNDs showed exceptionally good auditory improvements after cochlear implantation [11, 12]. For example, Young et al. (2012) reported that three of ten children with CND showed improved speech perception and seven children showed improved auditory detection [12]. According to these previous reports, it is possible for patients with CNDs to recognize environmental sounds with CIs, even if they cannot develop speech perception or oral communication. Detection of environmental sounds, such as a doorbell or a car horn, is a benefit in daily life even the audiological results with CIs are insufficient. It should be noted, however, that little is known about the long-term continuous use of CIs in children with CNDs who enter their adolescence. In the present study, therefore, we evaluated the long-term audiological outcomes and CI use of children with CNDs who had been followed up for more than five years after CI surgery.
2. Materials and Methods
This retrospective study was approved by the Institutional Review Board of the University of Tokyo Hospital (approval no. 2487). We retrospectively reviewed CND patients who underwent cochlear implantation and were followed up for more than five years at the Department of Otolaryngology and Head and Neck Surgery, the University of Tokyo Hospital. Informed consent was obtained from all patients and families in our study.
2.1 Subjects
Between 2001 and 2014, 10 children were diagnosed by using MRI as having bilateral profound hearing loss due to CND. Among them, 6 children who underwent CI surgery and were followed up for more than five years were included in this study. Table 1 shows the clinical features of these children. The mean age at diagnosis was 10.7 months (range: 4-21 months) and the mean age at CI surgery was 38.7 months (range: 28-60 months). The age at surgery of patient 2 was older than that of the other children due to recurrent upper respiratory infections. All the children were radiographically diagnosed as having CN hypoplasia. At surgery, a full electrode insertion into the scala tympani was achieved in all children. CI24M was used in 2 children, CI24RE (ST) in 3, and CI24RE (CA) in one (Cochlear Co., Ltd, NSW, Australia). All children attended follow-up visits beyond elementary school age. The mean follow-up period was 126 months (range: 63-161 months).
FU = Follow-up; CN = Cochlear nerve; BCNC = Bony cochlear nerve canal; IAC = Internal auditory canal; FN = Facial nerve; CHARGE = coloboma, heart defects, atresia [choanal], retardation [mental], genital hypoplasia, and ear abnormalities; CH = Cochlear hypoplasia; IP = Incomplete partition
Table 1: Clinical features and radiological findings.
2.2 Procedures
Evaluation of CNDs was done using MRI, and CNDs were classified as CN aplasia (defined as an absent CN) or CN hypoplasia (defined by the diameter of the CN at the midportion of the internal auditory canal (IAC) being smaller compared to the facial nerve). IAC stenosis was defined when the maximum diameter of the IAC was less than 3.0 mm [17]. Additionally, bony CN canal (BCNC) stenosis was defined when the diameter of the BCNC at the mid-modiolus was 1.5 mm or less in temporal bone CT scans [18]. MRI was performed using a 3.0-T system (Signa 3.0T; GE Medical Systems, Milwaukee, WI) equipped with an 8-channel phased-array head coil. We acquired anatomical MR images using fast imaging employing steady state acquisition (FIESTA) with the following protocol: TR, 4.2 ms; TE, 1.6 ms; slice thickness, 0.4 mm; FOV, 20 cm; matrix size, 512×512; flip angle, 45° voxel size, 0.39 × 0.39 × 0.4 mm; and slice number, 228. High-resolution axial images of the temporal bone were obtained using one of two multi-helical CT scanners: a 4-detector-row CT scanner (Toshiba Aquilon 16; New York, NY) in helical scan mode (0.5-mm slice width, 120 kV, 200 - 300 mA, pitch 3:1) or a 320-detector-row CT scanner (Toshiba Aquilon ONE) in volume scan mode (0.5-mm slice width, 120 kV, 100 mA). The reconstruction spacing was 0.1 mm in both scanners. The raw data were transferred to a CT work-station (Advantage Workstation version 4.0; GE Medical Systems, Milwaukee, WI).
Evaluations of audiological performance included unaided pure-tone average (PTA) thresholds, aided pre- and post-operative PTA thresholds, post-operative PTA thresholds with CI, categories of auditory performance-II (CAP-II), post-operative auditory-only speech perception, and post-operative auditory-and-visual speech perception using Japanese monosyllabic words. The CAP score is an index which is designed to evaluate auditory perception of children with hearing impairments in everyday situations at home and school. This originally consisted of eight performance categories arranged in order of increasing difficulty, ranging from 0 (no awareness of environmental sounds or voice) to 7 (use of telephone with a known speaker). The CAP-II scores added two new categories representing more complex skills: 8 (following group conversation in a reverberant room or where there is some interfering noise, such as a classroom or restaurant) and 9 (use of telephone with an unknown speaker in an unpredictable context) (Table 2). Both CAP and CAP-II have been shown to be reliable scaling systems [19, 20]. Post-operative monosyllabic speech perception testing was administered by live-voice under both auditory-only and auditory-and-visual condition with either type-57 or type-67 monosyllable speech perception test batteries (Association of Audiology, Japan). CAP-II scores and post-operative speech perception scores in the present study were evaluated by two experienced rehabilitation professionals. Each child’s primary mode of communication was also assessed both pre- and post-operatively. Post-operative evaluations were conducted more than five years after CI surgery. CI use was assessed by retrospective chart review.
|
Category |
|
|
0 |
No awareness of environmental sounds or voice |
|
1 |
Awareness of environmental sounds |
|
2 |
Response to speech sounds |
|
3 |
Identification of environmental sounds |
|
4 |
Discrimination of speech sounds without lip-reading |
|
5 |
Understanding of common phrases without lip-reading |
|
6 |
Understanding of common phrases without lip-reading |
|
7 |
Use of telephone with known speaker |
|
8 |
Follows group conversation in a reverberant room or where there is some interfering noise, such as a classroom or restaurant |
|
9 |
Use of telephone with an unknown speaker in unpredictable context. |
Table 2: Categories of Auditory Performance - II (CAP-II).
3. Results
3.1 Clinical features
Table 1 shows the clinical features of the six children with CND. All the children had CN hypoplasia on their surgical side. Among them, four children had BCNC stenosis (patients 3, 4, 5, and 6) and three children had IAC stenosis (patients 2, 4 and 6) (Table 1). One child (patient 2) had also cochlear hypoplasia (CH) type III, while the other children had no cochlear abnormalities on their surgical side. All except one (patient 1) showed abnormal inner ear structures including the vestibule and semicircular canals on temporal bone CT imaging. Three patients had a form of syndromic SNHL, such as Goldenhar syndrome (patient 2), CHARGE syndrome (patient 5), and a cleft lip and palate (patient 6). None of the children had any evidence of mental retardation.
3.2 Cochlear implant surgery
The surgical side was determined based upon the findings of pre-operative audiological assessments, equilibrium function tests, and radiological evaluations. Four children were implanted on the right and two on the left. Patients 2, 4 and 6 had CN hypoplasia on one side and CN aplasia on the opposite side and therefore the CI surgery was performed on the hypoplastic side. Patient 2 had CH type III on one side and CH type II, which has a potential risk of cerebrospinal fluid gusher, on the other side. Patient 1 had IAC stenosis on one side and the implantation was performed on the other side with a normal-sized IAC. Patient 3 had incomplete partition type II and CN aplasia on the left side and normal cochlea and CN hypoplasia on the right side, thus the cochlear implantation was performed on the right side (Figure 1). Patient 4 had incomplete partition type II in the left ear and a normal cochlea in the right ear, so CI surgery was done on the normal side.
3.3 Audiological outcomes
The results of pre- and post-operative audiological assessments are shown in Table 3 and Figures 2 and 3. In all 6 children, even with the use of hearing aids, the pre-operative auditory-only and auditory-and-visual speech perception scores were both 0%, and CAP-II scores were also all 0. Post-operatively, auditory-only speech perception scores ranged from 0 to 22% (median: 9%) and four children showed marked improvement in hearing thresholds with the use of CIs (patients 1, 3, 4 and 5). Three of these children showed improvement of auditory-only speech perception scores and achieved CAP-II scores of 5 (patients 1, 3, and 5).
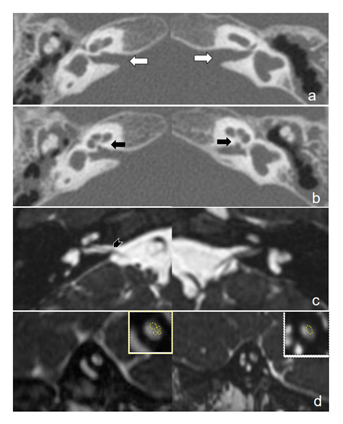
Figure 1: Temporal bone CT and MRI of patient number 3. a: Axial CT scans of the temporal bones on both sides. The size of the internal auditory canal is normal (white arrows). b: Axial CT scan of the bony cochlear nerve canals on both sides are stenotic (black arrows). c: Axial MRI scans using the fast imaging employing steady state acquisition. The right cochlear nerve can be observed in the internal auditory canal and left cochlear nerve is absent (black arrowhead). d: Sagittal MRI scans using the fast imaging employing steady state acquisition. The right upper images are enlarged views of both sides of the internal auditory canals at the midportion. The right cochlear nerve is half the size of the facial nerve. The left cochlear nerve cannot be observed.
During the post-operative follow-up of more than five years, only two of the six children (patients 3 and 5) developed speech communication, albeit limited, with the use of sign language. One child (patient 1) exhibited improved speech perception after CI surgery, but was not able to acquire speech communication skills. The other three children did not show improvements in auditory perception after CI surgery and did not acquire communication skills during the follow-up (patients 2, 4 and 6). Patient 4 developed some recognition of environmental sounds. Interestingly, three children (patients 1, 3, and 5) demonstrated marked improvements in post-operative PTA thresholds on their non-implanted side with the use of hearing aids. During the follow up, patients 2 and 6 discontinued use of their CIs seven years and thirteen years after the implantation, respectively. Four children continued using CIs, although all of them needed sign language for their daily communication and chose schools for special needs education.
|
No. |
Pre-operative CAP score |
Post-operative CAP score |
A SPS (%) |
A-V SPS (%) |
LFU Mode of communication |
CI device |
Usage of devices |
|
1 |
0 |
5 |
22 |
40 |
Sign |
CI24M |
CI + HA |
|
2 |
0 |
0 |
0 |
6 |
Sign |
CI24REST |
None |
|
3 |
0 |
5 |
18 |
38 |
Speech and sign |
Freedom CA |
CI + HA |
|
4 |
0 |
1 |
0 |
6 |
Sign |
CI24REST |
CI + HA |
|
5 |
0 |
5 |
22 |
68 |
Speech and sign |
CI24REST |
CI + HA |
|
6 |
0 |
0 |
0 |
20 |
Sign |
CI24M |
HA |
A SPS = Post-operative auditory only speech perception score; A-V SPS = Post-operative auditory and visual speech perception score; LFU = last follow-up; CI = Cochlear implant; HA = Hearing aid.
Table 3: Audiological results.
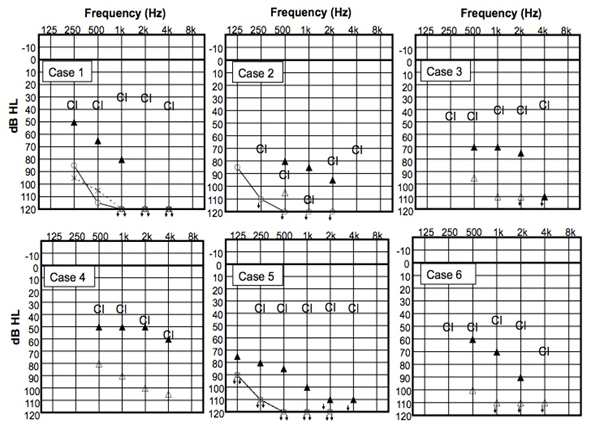
Figure 2: Pre-operative audiometry and cochlear implant thresholds.
? Unaided PTA of the right side, × Unaided PTA of the left side, ? pre-operative unaided PTA of the both side in a free sound field, ? pre-operative PTA with HA in a free sound field, CI - post-operative PTA with CI
CI = Cochlear implant, PTA = Pure-tone audiometry
Figure 3: Pre-operative audiometry and post-operative hearing aid thresholds in the non-implanted ear.
? Unaided PTA of the right side, × Unaided PTA of the left side, ? pre-operative unaided PTA of the both side in a free sound field, ? pre-operative PTA with HA in a free sound field, HA - post-operative PTA with HA on the non-implanted ear HA = Hearing aid, PTA = Pure-tone audiometry
4. Discussion
In the present study, we retrospectively reviewed the long-term audiological outcomes of six children with CN hypoplasia who underwent CI surgery. Post-operatively, four of the six children showed an improvement in PTA thresholds and three of them showed an improvement in CAP-II scores. However, they did not develop sufficient open speech perception abilities for oral communication during the follow up period. The other two children did not obtain sufficient hearing with CI and discontinued CI use long after surgery. Auditory brainstem implantation was not possible for these patients as it has not been covered by health insurance in Japan.
Cochlear implantation for CND children has been controversial because the presence of the cochlear nerve is essential for the success of CIs. Some studies have reported poor outcomes of cochlear implantation in CND children [9, 10], while other studies have shown relatively good audiological outcomes for such cases [11-14]. Young et al. reported that three of ten children with CND were able to develop open-set speech perception and spoken language and one achieved closed-set speech perception with CI use [12]. In another study, one of five CND children developed spoken language and another developed closed-set speech perception after CI surgery [14]. Another child also exhibited improvements in closed-set speech perception with CI and hearing aids [11]. The discrepancies among studies might be explained by the following several reasons. The first possibility is differences of the age at CI surgery. It has been reported that children tend to exhibit greater speech intelligibility with earlier CI [23-25]. Subjects in the present study underwent cochlear implantation at older age compared to previous studies. The oldest mean age at implantation among previous reports for children with CND was 30.0 months (range: 13-62 months) [12], while our subjects received CIs at the mean age of 38.7 months (range: 28-60 months). The second possible reason is the accuracy of evaluating the CN. A significant correlation between IAC and BCNC diameters and auditory performance including CAP scores has been reported [26]. However, the exact diameters of hypoplastic CNs have been reported to be impossible to measure in previous studies [9-11, 14, 15], as in the current study. We judged the presence of CN hypoplasia by comparing its diameter to that of the facial nerve because of the limited resolution of MRI. Further improvements in the resolution of MRI may enable more precise diagnosis and classification of hypoplastic CNs leading to better predictions of the audiological outcomes after CI surgery in the future.
It has been reported that the most common reason for discontinued use of CIs is poor hearing benefits [21]. Continuity of CI use can be a good indicator of the benefit of CIs, especially for the poor performer. All the patients in this study were observed for more than five years to evaluate the benefit of CIs. They all used their CI initially, but two children voluntarily discontinued use of their CI in adolescence, in spite of their parents’ encouragement, because of an absence of hearing benefits. In these children, their parents did not observe any awareness of environmental sound. Patients who cannot achieve environmental sound detection will likely perceive no benefit from using their CI and thus discontinue use, in spite of the efforts of their parents or care givers. In the children who showed some improvement in PTA thresholds, all continued to use their CI even though one patient (patient 4) could only achieve the detection of environmental sounds. This result indicates that those patients able to achieve any kind of sound detection will regard their CI as a useful tool, even if it doesn’t enable them to communicate.
The audiological outcomes of cochlear implantation for children with CND in the present study were much poorer compared to children with normal CNs and inner ear structures. It has been reported that the median CAP scores of children who underwent CI surgery without any inner ear malformation was 7 at 24 months post-implantation [22]. In our study, even patients (patient 3 and 5) who gained the greatest benefit obtained a CAP-II score of only 5 and were not able to develop open-set speech perception, resulting in proficient speech communication in only limited situations. All CND children in the present study continued to use sign language after cochlear implantation. These results were in line with those in previous studies that showed limited auditory benefits of CI use in children with CND [9, 10]. Therefore, special education programs with sign language instruction must be considered for children with CND even those using CIs.
In the present study, three of six children who underwent cochlear implantation showed improvement in PTA thresholds in the contralateral ear during the follow-up period. Ribari et al. reported that 18 of 49 children who underwent unilateral CI surgery revealed improvements in the audiometric thresholds in the contralateral ears using hearing aids [27]. They speculated that it might be caused by trophic influence on the efferent olivocochlear system in the regeneration of the inner hair cells’ afferents or the effect of chronic unilateral electrical stimulation on regeneration of the auditory pathways. According to these results, CIs for CND children can contribute to speech communication as well as giving an increased awareness of sound, leading to an increase in the children’s quality of life.
5. Conclusions
We reported the long-term outcomes of six children with CND who underwent cochlear implantation. Two of the six patients did not achieve sufficient PTA threshold improvement and discontinued the use of their CI more than 7 years after implantation. The other four children demonstrated some improvements in PTA threshold with continuous CI use, but their speech understanding remained poor. When considering the candidacy of a patient with CND for cochlear implantation, careful counseling about these limited benefits should be provided to the families and caregivers pre-operatively. Also, continuous support and education in using sign language is highly recommended.
Declaration of Interest
None
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Dettman S, Sadeghi-Barzalighi A, Ambett R, et al. Cochlear implants in forty-eight children with cochlear and/or vestibular abnormality. Audiol. Neurootol 16 (2011): 222-232.
- Heman-Ackah SE, Ronald T, Haynes DS, et al. Pediatric cochlear implantation candidacy evaluation, medical and surgical considerations and expanding criteria. Otolaryngol. Clin. North. Am. 45 (2012): 41-67.
- Papsin BC. Cochlear implantation in children with anomalous cochleovestibular anatomy, Laryngoscope. 115 (2005): 1-26.
- Lee KH, Lee J, Isaacson B, et al. Cochlear implantation in children with enlarged vestibular aqueduct. Laryngoscope. 120 (2010): 1675-1681.
- Patel ND, Ascha MS, Manzoor NF, et al. Morphology and cochlear implantation in enlarged vestibular aqueduct. Am. J. Otolaryngol. 39 (2018): 657-663.
- Chen X, Liu B, Liu S, et al, The development of auditory skills in infants with isolated large vestibular aqueduct syndrome after cochlear implantation. Int. J. Pediatr. Otorhinolaryngol. 75 (2011): 943-947.
- Casselman JW, Offeciers FE, Govaerts PJ, et al, Aplasia and hypoplasia of the vestibulocochlear nerve: diagnosis with MR imaging. Radiology. 202 (1997): 773-781.
- Wu CM, Lee LA, Chen CK, et al. Impact of cochlear nerve deficiency determined using 3-dimensional magnetic resonance imaging on hearing outcome in children with cochlear implants. Otol. Neurotol. 36 (2015): 14-21.
- Zhang Z, Li Y, Hu L, et al. Cochlear implantation in children with cochlear nerve deficiency: a report of nine cases. Int. J. Pediatr. Otorhinolaryngol. 76 (2012): 1188-1195.
- Colletti L, Zoccante L, Nonverbal cognitive abilities and auditory performance in children fitted with auditory brainstem implants: preliminary report. Laryngoscope. 118 (2008): 1443-1448.
- Zanetti D, Guida M, Barezzani MG, et al, Favorable outcome of cochlear implant in VIIIth nerve deficiency. Otol. Neurotol. 27 (2006): 815-823.
- Young NM, Kim FM, Ryan ME, et al. Pediatric cochlear implantation of children with eighth nerve deficiency. Int. J. Pediatr. Otorhinolaryngol. 76 (2012): 1442-1448.
- Warren FM, Wiggins RH, Pitt C, et al. Apparent cochlear nerve aplasia: to implant or not to implant? Otol. Neurotol. 31 (2010): 1088-1094.
- Vincenti V, Ormitti F, Ventura E, et al. Cochlear implantation in children with cochlear nerve deficiency. Int. J. Pediatr. Otorhinolaryngol. 78 (2014): 912-917.
- Kutz JW, Lee KH, Isaacson B, et al. Cochlear implantation in children with cochlear nerve absence or deficiency. Otol. Neurotol. 32 (2011): 956-961.
- Ehrmann-Müller D, Kühna H, Matthiesb C, et al. Outcomes after cochlear implant provision in children with cochlear nerve hypoplasia or aplasia. Int. J. Pediatr. Otorhinolaryngol. 112 (2018): 132-140.
- Song JJ, Choi HG, Oh SH, et al. Unilateral sensorineural hearing loss in children: the importance of temporal bone computed tomography and audiometric follow-up. Otol. Neurotol. 30 (2009): 604-608.
- Miyasaka M, Nosaka S, Morimoto N, et al. CT and MR imaging for pediatric cochlear implantation: emphasis on the relationship between the cochlear nerve canal and the cochlear nerve. Pediatr. Radiol. 40 (2010): 509-516.
- Archbold S, Lutman ME, Marshall D. Categories of auditory performance. Ann. Otol. Rhinol. Laryngol. Suppl. 166 (1995): 312-314.
- Gilmour L. The inter-rater reliability of categories of auditory performance-II (CAP-II):, In Masters Thesis, University of Southampton, Institute of Sound and Vibration Research (2010):.
- Contrera KJ, Choi JS,, Blake CR, et al. Rates of long-term cochlear implant use in children. Otol. Neurotol. 35 (2014): 426-430.
- Zhou H, Chen Z, Shi H, et al. Categories of auditory performance and speech intelligibility ratings of early-implanted children without speech training, PLoS. ONE. 8 (2013): e53852.
- Schafer E, Utrup A. The effect of age of cochlear implantation on speech intelligibility to others. Journal of Educational, Pediatric & (Re) Habilitative Audiology. 22 (2016): 1-11.
- Dunn CC, Walker EA, Oleson J, et al. Longitudinal speech perception and language performance in pediatric cochlear implant users: The effect of age at implantation. Ear. Hear. 35 (2014): 148-160.
- Ramos-Macías A, Borkoski-Barreiro S, Falcón-González JC, et al. Results in cochlear implanted children before 5 years of age. A long term follow up. Int. J. Pediatr. Otorhinolaryngol 78 (2014): 2183-2189.
- Wei X, Li Y, Chen B, et al, Predicting auditory outcomes from radiological imaging in cochlear implant patients with cochlear nerve deficiency. Otol. Neurotol. 38 (2017): 685-693.
- Ribári O, Küstel M, Szirmai A, et al. Cochlear implantation influences contralateral hearing and vestibular responsiveness. Acta. Otolaryngol. 119 (1999): 225-228.
- Peng KA, Kuan EC, Hagan S, et al. Cochlear nerve aplasia and hypoplasia: predictors of cochlear implant success. Otolaryngol. Head. Neck. Surg. 157 (2017): 392-400.
- Holt RF, Kirk KI. Speech and language development in cognitively delayed children with cochlear implants. Ear. Hear. 26 (2005): 132-148.
