Long-Term Observation of Improvement in Liver Fibrosis Index by A Glucagon-Like Peptide-1 Receptor Agonist in A Patient with Type 2 Diabetes: A Case Report
Article Information
Akinori Tokito1, Nobuyuki Koriyama1, Yoshihiko Nishio2
1Department of Diabetes and Endocrine Medicine, National Hospital Organization Kagoshima Medical Center, 8-1 Shiroyama-cho, Kagoshima 892-0853, Japan
2Department of Diabetes and Endocrine Medicine, Kagoshima University Graduate School of Medicine and Dental Sciences, Kagoshima University, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan
*Corresponding Author: Dr. Nobuyuki Koriyama, Department of Diabetes and Endocrine Medicine, National Hospital Organization Kagoshima Medical Center, 8-1 Shiroyama-cho, Kagoshima 892-0853, Japan
Received: 04 March 2020; Accepted: 16 March 2020; Published: 10 April 2020
Citation: Akinori Tokito, Nobuyuki Koriyama, Yoshihiko Nishio. Long-Term Observation of Improvement in Liver Fibrosis Index by A Glucagon-Like Peptide-1 Receptor Agonist in A Patient with Type 2 Diabetes: A Case Report. Archives of Clinical and Medical Case Reports 4 (2020): 292-301.
View / Download Pdf Share at FacebookAbstract
We describe a 51-year-old man with type 2 diabetes and hepatic dysfunction with suspected nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). No liver biopsy was performed. His blood test showed a decrease in platelet count (PLT) of 6.3 × 104/μL. Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were increased, and the AST/ALT ratio (AAR) exceeded 1. FIB4 index, an index of liver fibrosis, was remarkably high at 7.65. On abdominal computed tomography (CT), hepatic parenchyma was visualized as a low absorption area (CT value: 36 HU, L/S ratio <1), suggesting fat accumulation. HbA1c was 8.0%. Treatment was started with liraglutide 0.9 mg/day and changed to exenatide sustained-release formulation 2 mg/week about 1 year later. HbA1c remained around 6%. Both AST and ALT improved to the upper normal limit level, and AAR also decreased in about 2 years. Five years later, PLT increased to 13 × 104/μL and the FIB4 index decreased to 3.30.
In the future, we hope that long-term and prospective studies including histological evaluation and imaging findings of NAFLD/NASH will be conducted, and that the multi-faceted, potential effects of GLP-1RA will be further clarified.
Keywords
Glucagon-like peptide-1 receptor agonist; Nonalcoholic fatty liver disease (NAFLD); Nonalcoholic steatohepatitis (NASH); Type 2 diabetes mellitus; FIB4 index
Glucagon-like peptide-1 receptor agonist articles, Nonalcoholic fatty liver disease (NAFLD) articles, Nonalcoholic steatohepatitis (NASH) articles, Type 2 diabetes mellitus articles, FIB4 index articles
Glucagon-like peptide-1 receptor agonist articles Glucagon-like peptide-1 receptor agonist Research articles Glucagon-like peptide-1 receptor agonist review articles Glucagon-like peptide-1 receptor agonist PubMed articles Glucagon-like peptide-1 receptor agonist PubMed Central articles Glucagon-like peptide-1 receptor agonist 2023 articles Glucagon-like peptide-1 receptor agonist 2024 articles Glucagon-like peptide-1 receptor agonist Scopus articles Glucagon-like peptide-1 receptor agonist impact factor journals Glucagon-like peptide-1 receptor agonist Scopus journals Glucagon-like peptide-1 receptor agonist PubMed journals Glucagon-like peptide-1 receptor agonist medical journals Glucagon-like peptide-1 receptor agonist free journals Glucagon-like peptide-1 receptor agonist best journals Glucagon-like peptide-1 receptor agonist top journals Glucagon-like peptide-1 receptor agonist free medical journals Glucagon-like peptide-1 receptor agonist famous journals Glucagon-like peptide-1 receptor agonist Google Scholar indexed journals Glucagon articles Glucagon Research articles Glucagon review articles Glucagon PubMed articles Glucagon PubMed Central articles Glucagon 2023 articles Glucagon 2024 articles Glucagon Scopus articles Glucagon impact factor journals Glucagon Scopus journals Glucagon PubMed journals Glucagon medical journals Glucagon free journals Glucagon best journals Glucagon top journals Glucagon free medical journals Glucagon famous journals Glucagon Google Scholar indexed journals peptide articles peptide Research articles peptide review articles peptide PubMed articles peptide PubMed Central articles peptide 2023 articles peptide 2024 articles peptide Scopus articles peptide impact factor journals peptide Scopus journals peptide PubMed journals peptide medical journals peptide free journals peptide best journals peptide top journals peptide free medical journals peptide famous journals peptide Google Scholar indexed journals Nonalcoholic fatty liver disease articles Nonalcoholic fatty liver disease Research articles Nonalcoholic fatty liver disease review articles Nonalcoholic fatty liver disease PubMed articles Nonalcoholic fatty liver disease PubMed Central articles Nonalcoholic fatty liver disease 2023 articles Nonalcoholic fatty liver disease 2024 articles Nonalcoholic fatty liver disease Scopus articles Nonalcoholic fatty liver disease impact factor journals Nonalcoholic fatty liver disease Scopus journals Nonalcoholic fatty liver disease PubMed journals Nonalcoholic fatty liver disease medical journals Nonalcoholic fatty liver disease free journals Nonalcoholic fatty liver disease best journals Nonalcoholic fatty liver disease top journals Nonalcoholic fatty liver disease free medical journals Nonalcoholic fatty liver disease famous journals Nonalcoholic fatty liver disease Google Scholar indexed journals liver disease articles liver disease Research articles liver disease review articles liver disease PubMed articles liver disease PubMed Central articles liver disease 2023 articles liver disease 2024 articles liver disease Scopus articles liver disease impact factor journals liver disease Scopus journals liver disease PubMed journals liver disease medical journals liver disease free journals liver disease best journals liver disease top journals liver disease free medical journals liver disease famous journals liver disease Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Nonalcoholic steatohepatitis articles Nonalcoholic steatohepatitis Research articles Nonalcoholic steatohepatitis review articles Nonalcoholic steatohepatitis PubMed articles Nonalcoholic steatohepatitis PubMed Central articles Nonalcoholic steatohepatitis 2023 articles Nonalcoholic steatohepatitis 2024 articles Nonalcoholic steatohepatitis Scopus articles Nonalcoholic steatohepatitis impact factor journals Nonalcoholic steatohepatitis Scopus journals Nonalcoholic steatohepatitis PubMed journals Nonalcoholic steatohepatitis medical journals Nonalcoholic steatohepatitis free journals Nonalcoholic steatohepatitis best journals Nonalcoholic steatohepatitis top journals Nonalcoholic steatohepatitis free medical journals Nonalcoholic steatohepatitis famous journals Nonalcoholic steatohepatitis Google Scholar indexed journals Type 2 diabetes mellitus articles Type 2 diabetes mellitus Research articles Type 2 diabetes mellitus review articles Type 2 diabetes mellitus PubMed articles Type 2 diabetes mellitus PubMed Central articles Type 2 diabetes mellitus 2023 articles Type 2 diabetes mellitus 2024 articles Type 2 diabetes mellitus Scopus articles Type 2 diabetes mellitus impact factor journals Type 2 diabetes mellitus Scopus journals Type 2 diabetes mellitus PubMed journals Type 2 diabetes mellitus medical journals Type 2 diabetes mellitus free journals Type 2 diabetes mellitus best journals Type 2 diabetes mellitus top journals Type 2 diabetes mellitus free medical journals Type 2 diabetes mellitus famous journals Type 2 diabetes mellitus Google Scholar indexed journals FIB4 index articles FIB4 index Research articles FIB4 index review articles FIB4 index PubMed articles FIB4 index PubMed Central articles FIB4 index 2023 articles FIB4 index 2024 articles FIB4 index Scopus articles FIB4 index impact factor journals FIB4 index Scopus journals FIB4 index PubMed journals FIB4 index medical journals FIB4 index free journals FIB4 index best journals FIB4 index top journals FIB4 index free medical journals FIB4 index famous journals FIB4 index Google Scholar indexed journals Surgery articles Surgery Research articles Surgery review articles Surgery PubMed articles Surgery PubMed Central articles Surgery 2023 articles Surgery 2024 articles Surgery Scopus articles Surgery impact factor journals Surgery Scopus journals Surgery PubMed journals Surgery medical journals Surgery free journals Surgery best journals Surgery top journals Surgery free medical journals Surgery famous journals Surgery Google Scholar indexed journals
Article Details
1. Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) is increasing in Japan, along with an increase in obesity and metabolic syndrome [1]. In NAFLD, ectopic fat accumulated in the liver and skeletal muscle leads to insulin resistance, resulting in impaired glucose tolerance and an increase in free fatty acids in the blood, both of which further promote hepatic steatosis [2]. Patients with NAFLD are 3.51 times more likely to develop type 2 diabetes compared to a control group [3], while 50-60% of patients with type 2 diabetes have a complication of NAFLD [4]. Furthermore, NAFLD associated with type 2 diabetes tends to progress to nonalcoholic steatohepatitis (NASH) [5]. Therefore, NAFLD complications must always be kept in mind in patients with type 2 diabetes.
The primary treatment of NAFLD is weight loss via diet and exercise, but noncompliance to this regimen is high. Therefore, the effects of various drugs on improving NAFLD have been examined, although no established drug treatment has been identified. Multiple large randomized controlled trials (RCTs) have shown that pioglitazone (Pio), an insulin sensitizer, improves liver function and histology of NAFLD within 6 months after administration [6-8]. On the other hand, only about 40-60% of patients experience a therapeutic response, and long-term administration is associated with obesity, edema, heart failure, fracture, and bladder cancer [9]. Recently, the usefulness of glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter 2 inhibitors for the treatment of NAFLD has been reported [10]. In clinical practice, these 2 additional treatment options increase the number of drugs available for NAFLD, but the effect of these agents on the improvement of liver fibrosis and long-term prognosis are unknown. Few reports show improvements in NAFLD using a once-weekly GLP-1 receptor agonist (GLP-1RA) [11, 12].
This case report describes a patient treated with a once-weekly GLP-1RA who showed continuous improvement in liver function and liver fibrosis, indices of NAFLD or NASH associated with type 2 diabetes. Although liver biopsy was not performed, this case suggests a potential hepatoprotective effect of a GLP-1RA over a long period.
2. Case Report
A 51-year-old man with a family history of type 2 diabetes, occasional drinking, and smoking 20 cigarettes a day underwent surgery for lung cancer at age 47. He was diagnosed with type 2 diabetes and suspected NAFLD/NASH at a gastroenterology department he visited before presenting to our hospital. No liver biopsy was performed. Multiple oral hypoglycemic agents (OHA) were started, and at age 48 years, basal insulin supporting oral therapy (BOT) was introduced. However, HbA1c was >8%. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were gradually increased, and it was recommended that he see a specialist to help with blood glucose control. In June 2012, he was referred to our outpatient clinic.
The patient was 174.0 cm tall and weighed 90.4 kg (body mass index, 29.8 kg/m2). Blood pressure was 108/71 mmHg, and heart rate was regular at 71 beats/min. His cardiopulmonary examination showed normal results, and no abnormal abdominal findings were identified. A blood test showed a clear decrease in platelet count (PLT) of 6.3 × 104/μL, and a coagulation test showed a slight prolongation of prothrombin time. Serum levels of both AST and ALT were increased, and the AST/ALT ratio (AAR) exceeded 1. Cholinesterase (ChE) and serum protein levels were also low, and FIB4 index (age × AST [IU/L]/PLT [109/L] × √ALT [IU/L]) [13], an index of liver fibrosis, was remarkably high at 7.65 [<1.3]. Both hepatitis B virus antigen and hepatitis C virus antibody were negative (Table 1). Abdominal ultrasonography and abdominal computed tomography (CT) (Figure 1) obtained by the previous physician showed that the liver surface was slightly irregular and the margins were dull, suggesting chronic hepatitis. Hepatic parenchyma was visualized as a low absorption area (CT value: 36 HU, L/S ratio <1), suggesting fat accumulation. Splenomegaly was mild, and no ascites was noted (Figure 1). HbA1c was 8.0%, but fasting serum C-peptide immunoreactivity was 3.4 ng/mL, C-peptide immunoreactivity index was 1.92, and endogenous insulin secretion ability was maintained (Table 1). No microangiopathy was observed.
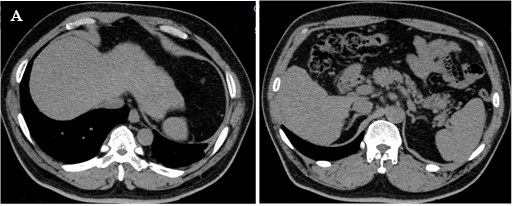
Figure 1: Abdominal computed tomography. The surface of the liver is slightly irregular and the margin is dulled, and the liver parenchyma is depicted as a low-absorption zone (CT value: 36 HU, Liver / Spleen (L / S) ratio <1).
|
Peripheral blood |
Biochemistry |
||||
|
WBC |
3550 / μL |
TP |
6.2g/dL |
Na |
137 mEq/L |
|
RBC |
399 × 104 / μL |
Alb |
3.1g/dL |
K |
4.2 mEq/L |
|
Hb |
13.6 g/dL |
TTT |
6.9MU |
Cl |
108 mEq/L |
|
Ht |
40.9% |
ZTT |
18.0KU |
T-chol |
229 mg/dL |
|
PLT |
6.3 × 104 / μL |
T-Bil |
1.8mg/dL |
LDL-chol |
112 mg/dL |
|
AST |
72IU/L |
HDL-chol |
40 mg/dL |
||
|
Coagulation-related |
ALT |
58IU/L |
TG |
277 mg/dL |
|
|
PT |
14.1 sec |
LDH |
229IU/L |
Ferritin |
291 ng/mL |
|
PT-% |
75.4% |
ChE |
189IU/L |
||
|
PT-INR |
1.17 |
ALP |
410IU/L |
Glucose metabolism-related |
|
|
γGTP |
274IU/L |
FPG |
177 mg/dL |
||
|
Infection-related |
Amy |
90IU/L |
HbA1c |
8.0% |
|
|
HBsAg |
(-) |
BUN |
11.1mg/dL |
F-sCPR |
3.4 ng/mL |
|
HBcAb |
(-) |
Cre |
0.67mg/dL |
2hr-sCPR |
5.3 ng/mL |
WBC, white blood cells; RBC, red blood cells; Hb, hemoglobin; Ht, hematocrit; PLT, platelets; PT, prothrombin time; INR, international normalized ratio; HBsAg, hepatitis B virus antigen; HBcAb, hepatitis B virus antibody; TP, total protein; Alb, albumin; TTT, thymol turbidity test; ZTT, zinc sulfate turbidity test; T-Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ChE, cholinesterase; ALP, alkaline phosphatase; γ-GTP, γ-glutamyltransferase; Amy, amylase; BUN, blood urea nitrogen; Cr, creatinine; T-chol, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; F-sCPR, fasting serum C-peptide immunoreactivity; 2hr-sCPR, 2 hours postprandial serum C-peptide immunoreactivity.
Table 1: Laboratory data on admission.
The clinical course of the patient is shown in Figure 2. In a previous BOT, 20 units insulin glargine (Gla) was subcutaneously administered once daily in the morning and 3 OHA used together: glimepiride (Glim) 6 mg, metformin (Met) 500 mg, and sitagliptin (Sita) 50 mg. Gla was discontinued and Glim was reduced from 6 mg to 3 mg because he was obese and had sufficient endogenous insulin secretion. Pio was started at 7.5 mg and was increased to 15 mg two months later, and Sita was changed to a once-daily GLP-1RA, liraglutide (Lira) 0.9 mg/day. Glycemic control improved steadily, and after about 1 year, Glim was gradually reduced and discontinued, and the GLP-1RA was changed to a once-weekly exenatide sustained-release formulation (ExLA) 2 mg/week at the wish of the patient. Since then, blood glucose control was good, and HbA1c remained around 6%. His body weight temporarily increased after the treatment change, but after stopping Pio in 2014, it decreased to around 90 kg, the same as before starting the GLP-1RA, and stabilized. In terms of liver function, Both AST and ALT improved to the upper normal limit level, and AAR also decreased in about 2 years. PLT and ChE showed a time-dependent recovery trend up to 5 years after the start of treatment. In particular, PLT increased from 6.3 × 104/μL before the treatment change to 10 × 104/μL in 2015 and increased to 13 × 104/μL in 2017. As a result, the FIB4 index decreased continuously and markedly, from 7.65 before treatment to 3.30 after 5 years. No adverse events due to the GLP-1RA were observed during the treatment period.
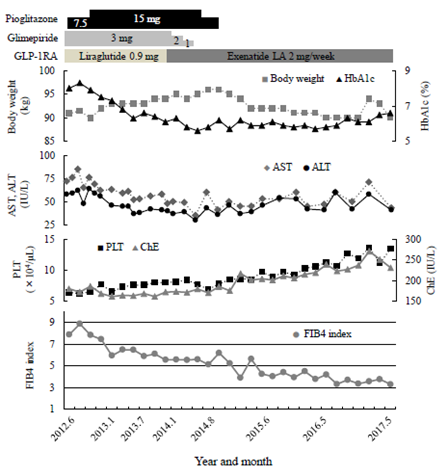
Figure 2: Clinical course of the patient. GLP-1RA, Glucagon-like peptide-1 receptor agonist; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLT, platelet count; ChE, cholinesterase.
3. Discussion
Administration of long-acting GLP-1RAs (Lira, ExLA) to improve blood glucose control in this patient with type 2 diabetes and hepatic dysfunction resulted in continuous improvement of liver function and liver fibrosis index over about 5 years. This patient was obese at age 45 at the start of therapy with no history of excessive alcohol consumption. Viral hepatitis was ruled out, and blood sampling data showed AAR > 1, marked PLT reduction, hypoproteinemia, and high serum ferritin. Furthermore, the FIB4 index significantly exceeded the cutoff value of 3.25 (specificity 95%), which strongly suggests hepatic fibrosis [14]. Abdominal ultrasound and CT revealed a chronic hepatitis pattern and intrahepatic fat accumulation. These findings suggest that the cause of liver dysfunction in this case was likely NAFLD/NASH. Although liver biopsy is considered the gold standard for histological diagnosis of NAFLD/NASH, it was not performed in this patient. Notably, it is not realistic to perform a liver biopsy in all NAFLD cases. In addition, objectivity regarding liver biopsy findings is poor due to differences in diagnosis between pathologists and sampling errors [15]. In recent years, nonalcoholic fatty liver (NAFL), which is equivalent to conventional simple fatty liver, and NASH have come to be regarded as representing different stages of the same disease rather than different diseases. Therefore, attention has been paid to the evaluation of hepatic fibrosis using a serum marker, a scoring system, or a diagnostic image, which are noninvasive procedures and can be performed repeatedly. Among these assessments, FIB4 index, NAFLD fibrous score, and BARD score [an easily calculated composite score based on the results of forced entry logistic regression analysis (BMI >28=1 point, AAR of >0.8=2 points, DM=1 point)] [16] use specific scoring systems, and their usefulness has been verified [17]. Kakuta et al. examined the usefulness of 7 scoring methods in 576 Japanese patients with NAFLD diagnosed by liver biopsy and found that the FIB4 index was the most reliable index of liver fibrosis [14]. It was reported that a cutoff of 1.45 could detect severe fibrosis cases (stage 3 and 4) with a sensitivity of 90% and a specificity of 64% (specificity increased to 95% at a cutoff of 3.25) [14]. In this case, combination therapy with Pio was successful for the treatment of NAFLD/NASH at first, but was discontinued after 19 months due to weight gain. After discontinuation of Pio, body weight stabilized at just over 90 kg, as was seen at the first consultation. About 1 year after the start of the GLP-1RA, the glycemic control status improved to HbA1c 6%, and remained stable even after the change to the once-weekly sustained-release GLP-1RA. On the other hand, PLT, ChE, and FIB4 index continued to improve even after weight and glycemic control stabilized. One of the hepatoprotective effects of GLP-1RA that does not depend on body weight or blood glucose may be an improvement in insulin resistance. Miki et al. demonstrated that the GLP-1 receptor (GLP-1R) is expressed on adipocytes and muscle cells, and that GLP-1 enhances glucose uptake by insulin into rat adipocytes [18]. If insulin resistance is improved, lipolysis from fat cells is suppressed. As a result, blood fatty acids and fat supply and accumulation in the liver are also reduced. In addition, Gupta et al. reported that Exendin-4 has an inhibitory effect on hepatic steatosis by directly activating 3-phosphoinositide-dependent protein kinase-1 (PDK-1), protein kinase B (AKT), and protein kinase C (PKC) –ζ of the insulin signaling system via the GLP-1R in the fated human hepatoma cell line, HuH-7 [19]. On the other hand, various direct effects of GLP-1 on the liver, including suppression of inflammation and oxidative stress, reduction of hepatic fatty acid synthesis and β-oxidation, reduction of intrahepatic fat accumulation by enhancement of autophagy, or suppression of hepatic cell death (apoptosis/necrosis) through reduction of endoplasmic reticulum stress have been reported in experimental studies (Figure 3) [20, 21]. These mechanisms may contribute to the improvement on NAFLD/NASH seen with a GLP-1RA.
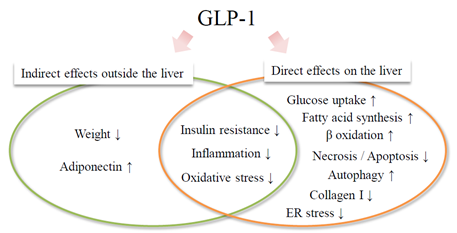
Figure 3: Various mechanisms of action of GLP-1 receptor agonists on NAFLD / NASH. NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis NASH; ER, endoplasmic reticulum.
This patient received both Lira and ExLA, long-acting GLP-1RAs. GLP-1RAs have a variety of extrapancreatic effects such as weight loss via appetite suppression, as well as blood glucose-dependent insulin secretion, glucagon suppression, and blood glucose improvement through a delay in gastric emptying. [22]. Recently, it has been reported that some GLP-1RAs suppress cardiovascular events and renal function deterioration [23-26]. Many clinical studies have suggested the usefulness for liver protection in NAFLD/NASH [27]. In the LEAN-J study conducted in Japan, 0.9 mg of Lira was administered to 10 patients with type 2 diabetes with NAFLD/NASH who had not received any drug treatment for 96 weeks, and liver tissue findings before and after administration were compared. Seven patients showed improvement in inflammatory findings, and 6 patients showed a reduction in liver fibrosis [28]. In the first multicenter RCT, the LEAN study, obese NASH patients were randomly assigned to Lira 1.8 mg or a control group and observed for 48 weeks [29]. Liver tissue findings associated with NASH were significantly improved in the Lira group compared to the control group (relative risk 4.3, P = 0.019), and this improvement in tissue findings was independent of changes in body weight and HbA1c [29]. Multiple meta-analyses have also concluded that GLP-1RAs significantly improve liver tissue findings and liver function in NAFLD/NASH patients with or without diabetic complications [30, 31]. However, the mechanism of the hepatoprotective effect of GLP-1RAs has not yet been fully elucidated, and there are no reports on long-term prognosis.
There is no evidence assessing long-term drug effects on NAFLD or effects on endpoints such as progression to cirrhosis and improved prognosis. In terms of Pio, the only hypoglycemic drug recommended in Japan for treatment of NAFLD/NASH, 3 large-scale RCTs have been published [6-8], but all reported that the therapeutic effect lasted at most for 2 years. There are also reports that there was no additional effect after 3 years of extended administration [32]. In this case, long-term administration of a GLP-1RA continuously improved the FIB4 index for at least 5 years. Mesquita et al. performed in vitro experiments using human and rat hepatic stellate cells (HSCs) and in vivo experiments using cirrhosis model rats, and clarified the antifibrotic effect of Lira. That is, HSCs were inactivated by Lira, decreasing nuclear factor-kappa B/sex-determining region Y-box 9 activity in a GLP-1R–independent manner. As a result, liver fibrosis was improved by suppressing collagen I production, and portal vein pressure was also reduced by improving microvascular function [33]. Although further clinical validation is needed, it is expected that GLP-1RAs may have a long-term antifibrotic effect even in cases where liver fibrosis has already progressed. It is also possible that these agents may improve prognosis.
In this case, treatment was started with Lira 0.9 mg/day, a once-daily GLP-1RA, and changed to once-weekly ExLA 2 mg/week about 1 year later. ChE and the FIB4 index continuously improved. To date, few reports have verified the clinical effect of a once-weekly GLP-1RA on NAFLD/NASH. Seko et al. revealed an improvement in liver function and liver hardness evaluated using elastography by retrospectively examining 15 NAFLD patients with type 2 diabetes who received the once-weekly drug duraglutide (Dura) 0.75 mg/week for 12 weeks [11]. Post-hoc testing of the recently reported Phase 3 AWARD trial of Dura also suggests that administration of Dura for 6 months may improve liver dysfunction associated with NAFLD [12]. However, it should be noted that the administration period was short in each case, and that HbA1c and body weight were simultaneously improved with liver function.
The limitation of this case report is that liver biopsy was not performed. In addition, as it was a retrospective observation, only AST, ALT, PLT, ChE, and FIB4 index findings were available. Therefore, no changes in hyaluronic acid, type IV collagen 7s, ferritin, high sensitive C-reactive protein, or coagulation factors could be confirmed. This is a topic for further study, including follow-up diagnostic imaging. In addition, the involvement of diet and exercise therapy in hepatoprotection during the observation period cannot be ruled out, although there were no significant changes in these lifestyle factors during the observation period, based on patient interviews and changes in body weight. Similarly, there were no changes in blood pressure or lipid management.
A GLP-1RA, and in particular, a once-weekly, long-acting GLP-1RA, is convenient and tolerable, and may be important factors in terms of patient quality of life. In the future, we hope that long-term and prospective studies including histological evaluation and imaging findings of NAFLD/NASH will be conducted, and that the multi-faceted potential effects of GLP-1RAs will be further clarified.
4. Conclusion
We reported a case of NAFLD or NASH associated with type 2 diabetes, in which a continuous improvement in liver function and liver fibrosis index with a once-weekly GLP-1RA was observed for at least 5 years.
Acknowledgments
The authors thank the patient for permission to publish this manuscript. The authors also thank Dr. Hidetaka Moriya of Moriya Hospital who referred this patient to our hospital.
Conflicts of Interest
Dr. Yoshihiko Nishio received a personal lecture fee from Novo Nordisk Pharma Ltd.
References
- Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 143 (2005): 722-728.
- Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 14 (2017): 32-42.
- Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 43 (2011): 617-649.
- Jimba S, Nakagami T, Takahashi M, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med 22 (2005): 1141-1145.
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 12 (1990): 1106-1110.
- Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355 (2006): 2297-2307.
- Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135 (2008): 1176-1784.
- Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362 (2010): 1675-1685.
- Cusi K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions. Diabetologia 59 (2016): 1112-1120.
- Cholankeril R, Patel V, Perumpail BJ, et al. Anti-Diabetic Medications for the Pharmacologic Management of NAFLD. Diseases 6 (2018): pii: E93.
- Seko Y, Sumida Y, Tanaka S, et al. Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res 47 (2017): 1206-1211.
- Cusi K, Sattar N, Garcia-Perez LE, et al. Dulaglutide decreases plasma aminotransferases in people with Type 2 diabetes in a pattern consistent with liver fat reduction: a post hoc analysis of the AWARD programme. Diabet Med 35 (2018)): 1434-1439.
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43 (2006): 1317-1325.
- Sumida Y, Yoneda M, Hyogo H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC gastroenterol 12 (2012): 10.1186/1471-230X-12-2.
- Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 53 (2010): 372-384.
- Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 57 (2008): 1441-1447.
- Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 30 (1999): 1356-1362.
- Miki H, Namba M, Nishimura T, et al. Glucagon-like peptide-1(7-36)amide enhances insulin-stimulated glucose uptake and decreases intracellular cAMP content in isolated rat adipocytes. Biochim Biophys Acta 1312 (1996): 132-136.
- Gupta NA, Mells J, Dunham RM, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 51 (2010): 1584-1592.
- Lee WY. New Potential Targets of Glucagon-Like Peptide 1 Receptor Agonists in Pancreatic beta-Cells and Hepatocytes. Endocrinol Metab 32 (2017): 1-5.
- Liu J, Wang G, Jia Y, et al. GLP-1 receptor agonists: effects on the progression of non-alcoholic fatty liver disease. Diabet Metab Res Rev 31 (2015): 329-335.
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87 (2007): 1409-1439.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 375 (2016): 311-322.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 375 (2016): 1834-1844.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394 (2019): 121-130.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394 (2019): 131-138.
- Dhir G, Cusi K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-alcoholic fatty liver disease: a novel therapeutic option. J Investig Med 66 (2018): 7-10.
- Eguchi Y, Kitajima Y, Hyogo H, et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res 45 (2015): 269-278.
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387 (2016): 679-690.
- Dong Y, Lv Q, Li S, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 41 (2017): 284-295.
- Carbone LJ, Angus PW, Yeomans ND. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 31 (2016): 23-31.
- Mahady SE, Webster AC, Walker S, et al. The role of thiazolidinediones in non-alcoholic steatohepatitis - a systematic review and meta analysis. J Hepatol 55 (2011): 1383-1390.
- de Mesquita FC, Guixe-Muntet S, Fernandez-Iglesias A, et al. Liraglutide improves liver microvascular dysfunction in cirrhosis: Evidence from translational studies. Sci Rep 7 (2017): 3255.
