Longitudinal Performance Validation of Siemens SARS-CoV-2 Spike Protein Serologic Assay
Article Information
Stewart Comer MD MA FCAP*
Cottage Health - Pacific Diagnostics Laboratories, Santa Barbara, CA, USA
*Corresponding Author: Stewart Comer MD FCAP, Cottage Health - Pacific Diagnostics Laboratories, 400 W. Pueblo St. Santa Barbara, CA 93110, USA
Received: 20 October 2021; Accepted: 03 November 2021; Published: 08 November 2021
Citation:
Stewart Comer. Longitudinal Performance Validation of Siemens SARS-CoV-2 Spike Protein Serologic Assay. Archives of Clinical and Medical Case Reports 5 (2021): 807-810.
View / Download Pdf Share at FacebookKeywords
SARS-CoV-2; COVID-19; IgG Antibody assay; Vaccination
SARS-CoV-2 articles SARS-CoV-2 Research articles SARS-CoV-2 review articles SARS-CoV-2 PubMed articles SARS-CoV-2 PubMed Central articles SARS-CoV-2 2023 articles SARS-CoV-2 2024 articles SARS-CoV-2 Scopus articles SARS-CoV-2 impact factor journals SARS-CoV-2 Scopus journals SARS-CoV-2 PubMed journals SARS-CoV-2 medical journals SARS-CoV-2 free journals SARS-CoV-2 best journals SARS-CoV-2 top journals SARS-CoV-2 free medical journals SARS-CoV-2 famous journals SARS-CoV-2 Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals IgG Antibody assay articles IgG Antibody assay Research articles IgG Antibody assay review articles IgG Antibody assay PubMed articles IgG Antibody assay PubMed Central articles IgG Antibody assay 2023 articles IgG Antibody assay 2024 articles IgG Antibody assay Scopus articles IgG Antibody assay impact factor journals IgG Antibody assay Scopus journals IgG Antibody assay PubMed journals IgG Antibody assay medical journals IgG Antibody assay free journals IgG Antibody assay best journals IgG Antibody assay top journals IgG Antibody assay free medical journals IgG Antibody assay famous journals IgG Antibody assay Google Scholar indexed journals lymph node articles lymph node Research articles lymph node review articles lymph node PubMed articles lymph node PubMed Central articles lymph node 2023 articles lymph node 2024 articles lymph node Scopus articles lymph node impact factor journals lymph node Scopus journals lymph node PubMed journals lymph node medical journals lymph node free journals lymph node best journals lymph node top journals lymph node free medical journals lymph node famous journals lymph node Google Scholar indexed journals vasculopathy articles vasculopathy Research articles vasculopathy review articles vasculopathy PubMed articles vasculopathy PubMed Central articles vasculopathy 2023 articles vasculopathy 2024 articles vasculopathy Scopus articles vasculopathy impact factor journals vasculopathy Scopus journals vasculopathy PubMed journals vasculopathy medical journals vasculopathy free journals vasculopathy best journals vasculopathy top journals vasculopathy free medical journals vasculopathy famous journals vasculopathy Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals lymphadenopathy articles lymphadenopathy Research articles lymphadenopathy review articles lymphadenopathy PubMed articles lymphadenopathy PubMed Central articles lymphadenopathy 2023 articles lymphadenopathy 2024 articles lymphadenopathy Scopus articles lymphadenopathy impact factor journals lymphadenopathy Scopus journals lymphadenopathy PubMed journals lymphadenopathy medical journals lymphadenopathy free journals lymphadenopathy best journals lymphadenopathy top journals lymphadenopathy free medical journals lymphadenopathy famous journals lymphadenopathy Google Scholar indexed journals Vaccination articles Vaccination Research articles Vaccination review articles Vaccination PubMed articles Vaccination PubMed Central articles Vaccination 2023 articles Vaccination 2024 articles Vaccination Scopus articles Vaccination impact factor journals Vaccination Scopus journals Vaccination PubMed journals Vaccination medical journals Vaccination free journals Vaccination best journals Vaccination top journals Vaccination free medical journals Vaccination famous journals Vaccination Google Scholar indexed journals Pathogenesis articles Pathogenesis Research articles Pathogenesis review articles Pathogenesis PubMed articles Pathogenesis PubMed Central articles Pathogenesis 2023 articles Pathogenesis 2024 articles Pathogenesis Scopus articles Pathogenesis impact factor journals Pathogenesis Scopus journals Pathogenesis PubMed journals Pathogenesis medical journals Pathogenesis free journals Pathogenesis best journals Pathogenesis top journals Pathogenesis free medical journals Pathogenesis famous journals Pathogenesis Google Scholar indexed journals
Article Details
1. Introduction
The Siemens Centaur® SARS-CoV-2 IgG Antibody assay, which received Food and Drug Administration Emergency Use Authorization (FDA EUA), serologically detects the presence of circulating IgG antibodies to SARS-CoV-2 in human serum or plasma using the Siemens Centaur® Immunoassay Systems. This new semiquantitative test was validated in February 2021 at the Core facility of Pacific Diagnostics Laboratories (PDL), the largest clinical reference laboratory in coastal California between Los Angeles and San Francisco. It is recognized that this commercially available test correlates well with circulating neutralizing antibody (nAb) titers [1-3] and is capable of assessing specific antibody production without any current claim by the FDA regarding immunity to COVID-19 infection. This was reaffirmed when the FDA issued their safety communication on 19 May 2021; however, many recent studies [4, 5] reported during the summer of 2021 have clearly demonstrated waning levels of circulating antibodies to SARS-CoV-2 over the course of several months post vaccination. This is the rationale for the FDA [6] authorizing a booster dose for select populations at least 6 months after completion of the primary series of Pfizer BioNTech COVID-19 vaccine. At the present time, neutralizing antibodies are the primary and determinative correlate “to protective immunity to SARS-CoV-2 induced either through natural infection or through vaccination” [3]. Commercially available enzyme-linked immunoassays (EIA) similar to this Siemens assay do not directly measure nAb; however, recently published studies [3] have demonstrated that this Siemens EIA demonstrated the highest correlation for commercial assays with the gold-standard Plaque Reduction Neutralization Test (PRNT) and “best agreement” with the newly FDA EUA approved cPass Neutralization Antibody Assay.
2. Methods
This validation study was conducted utilizing a traditional laboratory correlation protocol (as prescribed under CFR Title 42 requirements) that is designed to assess the performance metrics of any new clinical assay in comparison to the FDA Instructions for Use (IFU). In this context, 57 of 57 uninfected laboratory staff, who were tested in March 2020, prior to vaccination, were resulted negative with an index value < 1.00. In February 2021, we validated the new semiquantitative assay and 28 of the original members with no prior COVID-19 infection and who were at least 14 days post 2nd dose of the Pfizer BioNTech (BNT162b2) COVID-19 vaccine, were retested.
3. Results
Of this longitudinal cohort (spanning a time interval of 6-8 months), 25 of the 28 who were tested 2-4 weeks after second vaccine dose, demonstrated maximal response with an index value > 150 (limit of the analytic measurement range instituted by the manufacturer at that time) and then retested at 6 months. 3 of the 28, who had values less than this maximal value, were vaccinated early and thus were actually tested 6-7 weeks after their second dose and thus their retest time interval essentially represents an expanded (7- 8 month) time span. This same group of three were clustered into the lowest tier on the August 2021 retest. These observations clearly appear to demonstrate that circulating antibodies start to decline sometime after the 2-4-week post vaccination period and continue to show larger quantitative decrements as the months progress. Individuals tested during the 7-8-month time frame had noticeably lower index values than index values for those tested at the 6-month interval. Of note, the largest single 6-month decrement was in an individual older than 60 that is similar to the observations published in the large Israeli study [7].
Although this sample size is small, the 6-month average index value decrement was large enough to be considered highly statistically significant (p < 0.001) as illustrated in Figure 1. None of the 28 individuals dipped below the index value of 1.00, which is qualitatively resulted as negative. Specifically, this means that the level of circulating Spike (S1 RBD) antibody cannot be analytically distinguished from the cohort of 57 uninfected individuals tested as part of the original PDL validation studies, performed prior to the release of vaccine, who demonstrated index values < 1.00 consistent with the performance characteristics as submitted to the FDA. The Siemens SARS-CoV-2 IgG Spike (S1 RBD) Antibody performed on the Siemens Centaur® must generate an index value ≥ 4.8 in order to meet the FDA requirement to be categorized as a high-titer COVID-19 Convalescent Plasma donor [2]. Although none of 28 individuals dropped below an index value of 1.00, 6 of the 28 (21%) after 6 months and 3 of 3 (100%) after 7 months demonstrated index values < 4.8, which is a numerical cutoff recognized as an immunity transference milestone.
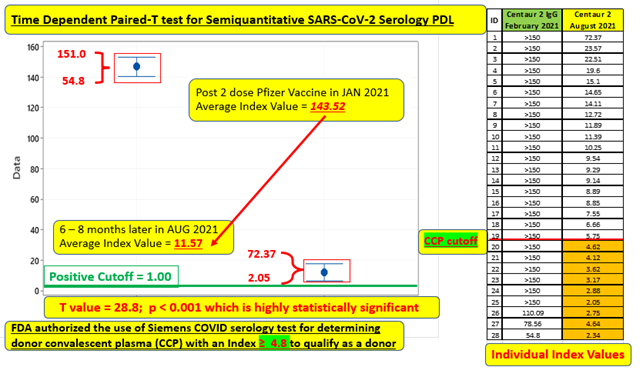
Figure 1: Time Dependent Paired-T test of average index value for 28-member cohort 6-8 months post vaccination.
4. Conclusion
The intent of this longitudinal validation study was to corroborate the temporal pattern in waning circulating antibodies as recently reported in multiple studies [3, 4, 6, 7] using a commercially available assay, which has been recognized [3] as having demonstrated excellent agreement with FDA-EUA approved SARS-CoV-2 nAb and PRNT assays. Although limited in sample size, the decrement is highly statistically significant and the time interval of our longitudinal validation was 180-225 days, which exceeds those time intervals of 70, 146 and 150 days previously reported [4, 7, 8]. Our time interval and eventual results tends to parallel the decay interval as recently reported in an expansive and comprehensive predictive study [5] that specifically evaluated multiple vaccine and convalescent studies and mathematically modeled “the decay of neutralization titers over the first 250 days after immunization.”
Acknowledgements
The PDL Clinical Laboratory Scientists, particularly Paola Rubio MBA, MLS (ASCP)CM, DLM CM, MB CM who provided invaluable support for this longitudinal laboratory validation study.
Conflict of Interest Disclosures
None
Funding/Sponsor
There is no specific funding beyond the normal and customary costs associated with performing required laboratory validation studies, which is supported by the performing laboratory (Cottage Health - Pacific Diagnostics Laboratories).
References
- US Food and Drug Administration (FDA). Coronavirus Disease (COVID-19) Emergency Use Authorizations for Medical Devices - In Vitro Diagnostics EUAs for SARS-CoV-2, on US FDA (2020).
- Zhen W, Smith E, Manji R, et al. Clinical Evaluation of Three Sample-To-Answer Platforms for the Detection of SARS-CoV-2. J Clin Microbiol (2020).
- Stein R. Study Raises Questions About False Negatives from Quick COVID-19 Test, p In National Public Radio (NPR). (2020).
- Hanson KE, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19 (IDSA) (2020).
- Kim C, Ahmed JA, Eidex RB, et al. Comparison of Nasopharyngeal and Oropharyngeal Swabs for the Diagnosis of Eight Respiratory Viruses by Real-Time Reverse Transcription-PCR Assays. PLoS ONE 6 (2011): e21610.
- Interim study by Tu YP and O’Leary TJ. Sample Collection and Molecular Diagnosis of SARS-CoV-2 Infection presented at the Association of Molecular Pathology webinar (2020).
- Basu A, et al. Performance of the rapid Nucleic Acid Amplification by Abbott ID NOW COVID-19 in nasopharyngeal swabs transported in viral media and dry nasal swabs, in a New York City academic institution. bioRxiv/Cold Spring Harbor Laboratory (2020).
- Puranik A, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta prevalence. medRxiv (2021).
